Enhancing Patient Safety in Spain: Streamlining Adverse Event Detection in Occupational Healthcare Records
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rocco, C.; Garrido, A. Seguridad del paciente y cultura de seguridad. Rev. Méd. Clín. Condes 2017, 28, 785–795. [Google Scholar] [CrossRef]
- Larizgoitia, I.; Bouesseau, M.-C.; Kelley, E. WHO Efforts to Promote Reporting of Adverse Events and Global Learning. J. Public Health Res. 2013, 2, e29. [Google Scholar] [CrossRef] [PubMed]
- Lapuente, F.P. La Seguridad del Paciente: Un Problema Importante y Actual. 2011. Available online: http://gestionclinica.sespa.es/pdf/jornadas2012/t9_lectura2.pdf (accessed on 19 May 2023).
- Patient Safety. Who.int s/f. Available online: https://www.who.int/es/news-room/fact-sheets/detail/patient-safety (accessed on 19 May 2023).
- Figura 1. Relación de estudios realizados para conocer la incidencia de eventos adversos en hospitales. In Estrategia de Seguridad del Paciente del Sistema Nacional de Salud; Ministerio de Sanidad, Servicios Sociales e Igualdad: Madrid, Spain, 2016; Gob.es s/f. Available online: https://seguridaddelpaciente.sanidad.gob.es/docs/Estrategia_Seguridad_del_Paciente_2015-2020.pdf (accessed on 14 August 2023).
- Ministry of Health & Consumer Affairs, Government of Spain. APEAS Study. Patient Safety in Primary Health Care; Government of Spain: Madrid, Spain, 2008.
- Aranaz-Andrés, J.M.; Aibar, C.; Limón, R.; Mira, J.J.; Vitaller, J.; Agra, Y.; Terol, E. A study of the prevalence of adverse events in primary healthcare in Spain. Eur. J. Public Health 2012, 22, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Aranaz-Andrés, J.M.; Aibar-Remon, C.; Vitaller-Burillo, J.; Requena-Puche, J.; Terol-Garcia, E.; Kelley, E.; de Castro, M.G.-V.; The ENEAS Work Group. Impact and preventability of adverse events in Spanish public hospitals: Results of the Spanish National Study of Adverse Events (ENEAS). Int. J. Qual. Heal. Care 2009, 21, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health & Consumer Affairs, Government of Spain. Estudio Nacional sobre los Efectos Adversos ligados a la Hospitalización (ENEAS 2005); Government of Spain: Madrid, Spain, 2006.
- AMAT. Mutuas Colaboradoras con la Seguridad Social. Comprometidos con la Salud de los Trabajadores y la Competitividad de las Empresas; AMAT: 2019. Available online: https://amat.es/wp-content/uploads/2021/03/que-son-las-mutuas.pdf (accessed on 14 June 2023).
- Sociedad Española de Calidad Asistencial. Calidadasistencial.es s/f. Available online: https://calidadasistencial.es/ (accessed on 22 December 2023).
- 2021 Memoria. Barcelona: MC MUTUAL. 2022. Available online: https://www.mc-mutual.com/estaticos/Memorias/resources/memoria_2022_es/mobile/index.html (accessed on 20 August 2023).
- Agra, Y. Estrategia de Seguridad del Paciente del Sistema Nacional de Salud Período 2015–2020; Ministerio de Sanidad, Servicios Sociales e Igualdad: Madrid, Spain, 2016. Available online: https://seguridaddelpaciente.es/resources/documentos/2015/Estrategia%20Seguridad%20del%20Paciente%202015-2020.pdf?cdnv=2 (accessed on 22 August 2023).
- Ministerio de Sanidad, Política Social e Igualdad. Desarrollo de la Estrategia Nacional en Seguridad del Paciente 2005–2011; Oficina de Planificación Sanitaria y Calidad, Agencia de Calidad del SNS, Madrid, Spain; 2011. Available online: https://www.sanidad.gob.es/gl/organizacion/sns/planCalidadSNS/docs/estrategia_sp_sns_2005_2011.pdf (accessed on 22 July 2023).
- Bender, J.A.; Kulju, S.; Soncrant, C. Combined Proactive Risk Assessment: Unifying Proactive and Reactive Risk Assessment Techniques In Health Care. Jt. Comm. J. Qual. Patient Saf. 2022, 48, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Mora-Capín, A.; Ignacio-Cerro, C.; Díaz-Redondo, A.; Vázquez-López, P.; Marañón-Pardillo, R. Impact of risk mapping as a strategy for monitoring and improving patient safety in paediatric emergency care. An. Pediatr. (Engl. Ed.) 2022, 97, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Committee on Patient Safety and Quality Improvement. Committee Opinion No. 681: Disclosure and Discussion of Adverse Events. Obstet. Gynecol. 2016, 128, e257–e261. [Google Scholar] [CrossRef] [PubMed]
- Pysyk, C.L.; Filteau, L.; Baxter, A. Quality and patient safety committee structure and activities in an academic department of anesthesiology: A narrative description. Can. J. Anaesth. 2020, 67, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Health Quality & Safety Commission. Learning from Adverse Events: Adverse Events Reported to the Health Quality & Safety Commission 1 July 2018 to 30 June 2019 | Te Ako i Ngā Pāpono Kōaro: Ngā Pāpono Kōaro i Pūrongorongotia ki te Kupu Taurangi Hauora o Aotearoa i te 1 o Hōngongoi 2018 ki te 30 o Pipiri 2019; Health Quality & Safety Commission (NZ): Wellington, New Zealand, 2019. Available online: https://www.hqsc.govt.nz/assets/Our-work/System-safety/Adverse-events/Publications-resources/Learning-from-adverse-events2019-web-final.pdf (accessed on 2 May 2023).
- Ogrinc, G.S.; Headrick, L.A.; Barton, A.J.; Dolansky, M.A.; Madigosky, W.S.; Miltner, R.S.; Hall, A.G. Fundamentals of Health Care Improvement: A Guide to Improving Your Patients’ Care, 4th ed.; Joint Commission Resources: Oakbrook Terrace, IL, USA; Institute for Healthcare Improvement: Boston, MA, USA, 2022. [Google Scholar]
- Gens-Barberà, M.; Rey-Reñones, C.; Hernández-Vidal, N.; Vidal-Esteve, E.; Mengíbar-García, Y.; Hospital-Guardiola, I.; Palacios-Llamazares, L.; Satué-Gracia, E.M.; Oya-Girona, E.M.; Bejarano-Romero, F.; et al. Effectiveness of New Tools to Define an Up-to-Date Patient Safety Risk Map: A Primary Care Study Protocol. Int. J. Environ. Res. Public Health 2021, 18, 8612. [Google Scholar] [CrossRef] [PubMed]
- Ortner Sancho, J.; Manzanera López, R.; Grau Balcells, N.; Moya Alcocer, D.J.; Farrús Esteban, X.; Martínez Martínez, J.M. Uso del Trigger Tool para la detección de incidentes y eventos adversos en una mutua colaboradora con la Seguridad Social. Arch. Prev. Riesgos Laborales 2020, 23, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Ortner, J.; Vives, A.; Moya, D.; Torres, M.; Grau, N.; Farrús, X.; Manzanera, R.; Mira, J.J. Frequency of outpatient care adverse events in an Occupational Mutual Insurance Company in Spain. J. Healthc. Qual. Res. 2021, 36, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Ortner, J.; Vives, A.; Moya, D.; Torres, M.; Grau, N.; Farrús, X.; Manzanera, R.; Mira, J. Utilización del Trigger Tool para detectar incidentes de seguridad en una mutua colaboradora de la Seguridad Social en España. J. Healthc. Qual. Res. 2021, 37, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Ortner, J.; Moya, D.; Manzanera, R.; Torres, M.; Vives, A.; Farrus, X.; Grau, N.; Mira, J.J. Adverse events in the global healthcare practice of an Occupational Mutual Insurance Company in Spain. Work 2023, 76, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Dhand, N.K.; Khatkar, M.S.; Statulator: An Online Statistical Calculator. Sample Size Calculator for Estimating a Single Proportion. 2014. Available online: http://statulator.com/SampleSize/ss1P.html (accessed on 21 December 2023).
- Otero, M.J.; Codina, C.; Tamés, M.J.; Pérez, M. Errores de medicación: Estandarización de la terminología y clasificación. Resultados de la Beca Ruiz-Jarabo 2000. Farm Hosp. 2003, 27, 137–149. [Google Scholar]
- Griffin, F.; Resar, R. IHI Global Trigger Tool for Measuring Adverse Events, 2nd ed.; Institute for Healthcare Improvement: Cambridge, MA, USA, 2009; Available online: http://www.ihi.org:80/resources/Pages/IHIWhitePapers/IHIGlobalTriggerToolWhitePaper.aspx (accessed on 20 August 2023).
- Hibbert, P.D.; Molloy, C.J.; Hooper, T.D.; Wiles, L.K.; Runciman, W.B.; Lachman, P.; Muething, S.E.; Braithwaite, J. The application of the Global Trigger Tool: A systematic review. Int. J. Qual. Health Care 2016, 28, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Sathiyakumar, V.; Thakore, R.V.; Greenberg, S.E.; Whiting, P.S.; Molina, C.S.; Obremskey, W.T.; Sethi, M.K. Adverse events in orthopaedics: Is trauma more risky? An analysis of the NSQIP data. J. Orthop. Trauma 2015, 29, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Esmail, A. Measuring and Monitoring Safety: A Primary Care Perspective; The Health Foundation: London, UK, 2013; Available online: https://www.health.org.uk/publications/measuring-and-monitoring-safety-a-primary-care-perspective (accessed on 19 July 2023).
- Bates, D.W.; Levine, D.M.; Salmasian, H.; Syrowatka, A.; Shahian, D.M.; Lipsitz, S.; Zebrowski, J.P.; Myers, L.C.; Logan, M.S.; Roy, C.G.; et al. The Safety of Inpatient Health Care. N. Engl. J. Med. 2023, 388, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Berwick, D.M. Constancy of Purpose for Improving Patient Safety—Missing in Action. N. Engl. J. Med. 2023, 388, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Al-Mugheed, K.; Bayraktar, N.; Al-Bsheish, M.; AlSyouf, A.; Jarrar, M.; AlBaker, W.; Aldhmadi, B.K. Patient Safety Attitudes among Doctors and Nurses: Associations with Workload, Adverse Events, Experience. Healthcare 2022, 10, 631. [Google Scholar] [CrossRef] [PubMed]
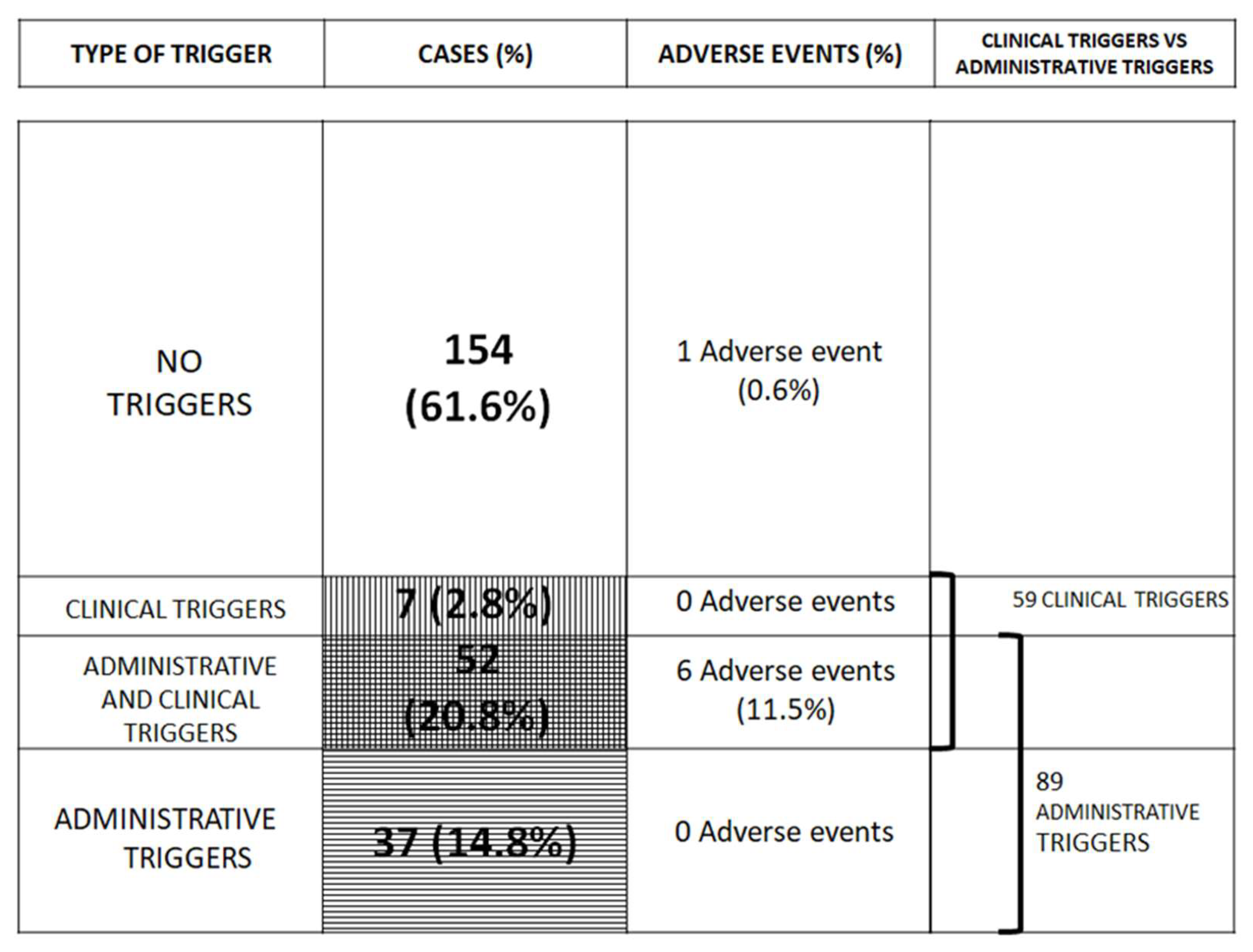
| Cases | % Total Cases | ||
|---|---|---|---|
| Days of sick leave (Range 1–286) | Cases | 250 | |
| Mean | 31 | ||
| 36.7 | |||
| Age in years (Range 19–63) | Cases | 250 | |
| Mean (Standard deviation) | 44 (10.9) | ||
| Gender | Men | 172 | 68.8% |
| Women | 78 | 31.2% | |
| TOTAL | 250 | 100% | |
| Sector | Agriculture/Fishing | 18 | 7.2% |
| Commerce | 33 | 13.2% | |
| Construction | 26 | 10.4% | |
| Industry | 73 | 29.2% | |
| Services | 96 | 38.4% | |
| Others | 4 | 1.6% | |
| TOTAL | 250 | 100% | |
| Pathology | Contusions/Bruises | 64 | 25.6% |
| Fractures/Sprains | 41 | 16.4% | |
| Spine | 41 | 16.4% | |
| Inflammatory | 49 | 19.6% | |
| Wounds | 39 | 15.6% | |
| Others | 16 | 6.4% | |
| TOTAL | 250 | 100% | |
| Final decision | Without sick leave | 76 | 30.4% |
| Sick leave | 121 | 48.4% | |
| Referred to the National Health Service | 53 | 21.2% | |
| TOTAL | 250 | 100% | |
| Number | % Total Group Cases | ||
|---|---|---|---|
| Administrative triggers | >7 days of sick leave | 94 | 37.6% |
| >3 clinical attendances | 140 | 56.0% | |
| Cases with both | 89 | 35.6% | |
| Cases with none | 105 | 42.0% | |
| TOTAL CASES | 250 | 100% | |
| Clinical triggers | Cases without clinical triggers | 191 | 76.4% |
| Cases with one clinical triggers | 44 | 17.6% | |
| Cases with two or more clinical triggers | 15 | 6.0% | |
| Cases with clinical triggers | 59 | 23.6% | |
| TOTAL CASES | 250 | 100% | |
| CLINICAL TRIGGERS | 76 | 100% | |
| Medication | 42 | 55.3% | |
| Surgery | 11 | 14.5% | |
| Treatment change | 6 | 7.9% | |
| Post-discharge attendance | 11 | 14.5% | |
| Sick leave after two attendances | 6 | 7.9% | |
| TOTAL | 76 | 100% | |
| Adverse events | Postoperative | 3 | 42.9% |
| Medication | 2 | 28.6% | |
| Diagnostic delay | 2 | 28.6% | |
| TOTAL | 7 | 100% | |
| Severity | Severity C | 2 | 28.6% |
| Severity D | 3 | 42.9% | |
| Severity E | 1 | 14.3% | |
| Severity F | 1 | 14.3% | |
| TOTAL | 7 | 100% | |
| Preventability | Preventability 2 | 2 | 28.6% |
| Preventability 3 | 1 | 14.3% | |
| Preventability 4 | 1 | 14.3% | |
| Preventability 5 | 1 | 14.3% | |
| Preventability 6 | 2 | 28.6% | |
| TOTAL | 7 | 100% | |
| Previous Study * | Actual Study ** | |||||
|---|---|---|---|---|---|---|
| Complex Patients | Intermediate Patients | Minor Complexity Patients | Complex Patients | Intermediate Patients | Minor Complexity Patients | |
| Clinical records | 240 | 60 | 60 | 89 | 32 | 129 |
| Adverse events | 26 | 1 | 1 | 6 | 0 | 1 |
| Adverse event/Clinical record | 0.11 | 0.02 | 0.02 | 0.07 | 0 | 0.01 |
| Confidence Interval | 7.2–15% | 0–4.8% | 0–4.8% | 1.7–12.3% | 0.0% | 0–2.7% |
| Gender | Age | Sector | Diagnosis | Type of Adverse Event | Severity (A-I) | Preventability (1 a 6) |
|---|---|---|---|---|---|---|
| Men | 58 | Commerce | Radius fracture | Postoperative | D | 5 |
| Men | 29 | Construction | Hand open wound | Postoperative | D | 2 |
| Women | 42 | Commerce | Neck sprain | Medication | C | 3 |
| Men | 56 | Industry | Ankle fracture | Diagnostic delay | E | 6 |
| Men | 43 | Industry | Vertigo | Diagnostic delay | C | 6 |
| Men | 44 | Industry | Elbow dislocation | Postoperative | F | 4 |
| Men | 46 | Industry | Second degree burn | Medication | D | 2 |
| Cases without Sick Leave | Cases with Sick Leave | Cases Referred to the National Health Service | Mean of Days of Sick Leave | SD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | % | Cases | % | Cases | % | Total | % | p | |||
| Gender | |||||||||||
| Men | 52 | 30% | 79 | 46% | 41 | 24% | 172 | 68.8% | 0.296 | 34 | 41.6 |
| Women | 24 | 31% | 42 | 54% | 12 | 15% | 78 | 31.2% | 25 | 33.4 | |
| Age (range of years) | |||||||||||
| (19–40) | 28 | 33% | 37 | 44% | 20 | 24% | 85 | 34.0% | 0.350 | 6 | 10.4 |
| (40–49) | 27 | 32% | 43 | 52% | 12 | 15% | 82 | 32.8% | 23 | 55.3 | |
| (49–63) | 21 | 25% | 41 | 49% | 21 | 25% | 83 | 33.2% | 17 | 26.5 | |
| Sector * | |||||||||||
| Agriculture/Fishing | 4 | 22% | 9 | 50% | 5 | 28% | 18 | 7.2% | 0.263 | 32 | 22.5 |
| Commerce | 13 | 39% | 14 | 42% | 6 | 18% | 33 | 13.2% | 33 | 48.4 | |
| Construction | 4 | 15% | 17 | 65% | 5 | 19% | 26 | 10.4% | 28 | 26.0 | |
| Industry | 16 | 22% | 39 | 53% | 18 | 25% | 73 | 29.2% | 33 | 40.8 | |
| Services | 35 | 36% | 42 | 44% | 19 | 20% | 96 | 38.4% | 29 | 33.1 | |
| Pathology | |||||||||||
| Contusions/Bruises | 23 | 36% | 32 | 50% | 9 | 14% | 64 | 25.6% | <0.001 | 28 | 27.9 |
| Fractures/Sprains | 8 | 20% | 29 | 71% | 4 | 10% | 41 | 16.4% | 51 | 59.2 | |
| Spine | 7 | 17% | 17 | 41% | 17 | 41% | 41 | 16.4% | 17 | 15.5 | |
| Inflammatory | 12 | 34% | 17 | 49% | 20 | 57% | 49 | 19.6% | 17 | 11.1 | |
| Wounds | 20 | 51% | 19 | 49% | 0 | 0% | 39 | 15.6% | 14 | 10.6 | |
| Others | 6 | 25% | 7 | 29% | 3 | 13% | 16 | 6.4% | 56 | 61.6 | |
| TOTAL | 76 | 30% | 121 | 48% | 53 | 21% | 250 | 31 | |||
| Administrative Triggers | Clinical Triggers | Both Triggers | No Triggers | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Prev | IC 95% Prev | p | Cases | Prev | IC 95% Prev | p | Cases | Prev | IC 95% Prev | p | Cases | Prev | IC 95% Prev | p | |
| Gender | 1.000 | 0.108 | 0.042 | 0.785 | ||||||||||||
| Men | 63 | 37% | (29–44%) | 45 | 26% | (20–33%) | 34 | 20% | (14–27%) | 98 | 57% | (49–64%) | ||||
| Women | 29 | 37% | (26–49%) | 13 | 17% | (9–27%) | 7 | 9% | (4–18%) | 43 | 55% | (43–66%) | ||||
| Age (range of years) | 0.036 | 0.006 | 0.073 | 0.001 | ||||||||||||
| (19–40) | 22 | 26% | (17–37%) | 10 | 12% | (6–21%) | 8 | 9% | (4–18%) | 61 | 72% | (61–81%) | ||||
| (40–49) | 35 | 43% | (32–54%) | 25 | 30% | (21–42%) | 15 | 18% | (11–28%) | 37 | 45% | (34–57%) | ||||
| (49–63) | 35 | 42% | (31–54%) | 23 | 28% | (18–39%) | 18 | 22% | (13–32%) | 43 | 52% | (41–63%) | ||||
| Sector * | 0.116 | 0.254 | 0.143 | 0.266 | ||||||||||||
| Agriculture/Fishing | 8 | 44% | (22–69%) | 4 | 22% | (6–48%) | 3 | 17% | (4–41%) | 9 | 50% | (26–74%) | ||||
| Commerce | 10 | 30% | (16–49%) | 4 | 12% | (3–28%) | 3 | 9% | (2–24%) | 22 | 67% | (48–82%) | ||||
| Construction | 15 | 58% | (37–77%) | 8 | 31% | (14–52%) | 8 | 31% | (14–52%) | 11 | 42% | (23–63%) | ||||
| Industry | 29 | 40% | (28–52%) | 22 | 30% | (20–42%) | 15 | 21% | (12–32%) | 37 | 51% | (39–63%) | ||||
| Services | 30 | 31% | (22–42%) | 20 | 21% | (13–30%) | 12 | 13% | (7–21%) | 58 | 60% | (50–70%) | ||||
| Pathology | 0.032 | <0.001 | 0.008 | 0.004 | ||||||||||||
| Contusions/Bruises | 23 | 36% | (24–49%) | 6 | 9% | (4–26%) | 5 | 8% | (3–17%) | 40 | 63% | (50–74%) | ||||
| Fractures/Sprains | 24 | 59% | (42–74%) | 16 | 39% | (24–55%) | 13 | 32% | (18–48%) | 14 | 34% | (20–51%) | ||||
| Spine | 12 | 29% | (16–46%) | 5 | 12% | (4–19%) | 3 | 7% | (2–20%) | 27 | 66% | (49–80%) | ||||
| Inflammatory | 12 | 24% | (13–39%) | 9 | 18% | (9–32%) | 7 | 14% | (6–27%) | 35 | 71% | (32–65%) | ||||
| Wounds | 14 | 36% | (21–53%) | 14 | 36% | (21–53%) | 9 | 23% | (11–39%) | 20 | 51% | (17–43%) | ||||
| Others | 7 | 44% | (20–70%) | 8 | 50% | (25–75%) | 4 | 25% | (7–52%) | 5 | 31% | (41–89%) | ||||
| Final decision | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||||
| Cases without sick leave | 1 | 1% | (0–7%) | 9 | 12% | (6–21%) | 0 | 0% | (0–5%) | 66 | 87% | (77–94%) | ||||
| Cases with sick leave | 90 | 74% | (66–82%) | 45 | 37% | (29–46%) | 41 | 34% | (26–43%) | 27 | 22% | (15–31%) | ||||
| Cases referred to the Public Health Service | 1 | 2% | (0–10%) | 4 | 8% | (2–18%) | 0 | 0% | (0–7%) | 48 | 91% | (79–97%) | ||||
| TOTAL | 92 | 37% | (31–43%) | 58 | 23% | (18–29%) | 41 | 16% | (12–22%) | 141 | 56% | (50–63%) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya, D.; Manzanera, R.; Ortner, J.; Torres, M.; Serfaty, J.C.; Sauri, C.; Jimenez, L.; Mira, J.J. Enhancing Patient Safety in Spain: Streamlining Adverse Event Detection in Occupational Healthcare Records. Safety 2024, 10, 13. https://doi.org/10.3390/safety10010013
Moya D, Manzanera R, Ortner J, Torres M, Serfaty JC, Sauri C, Jimenez L, Mira JJ. Enhancing Patient Safety in Spain: Streamlining Adverse Event Detection in Occupational Healthcare Records. Safety. 2024; 10(1):13. https://doi.org/10.3390/safety10010013
Chicago/Turabian StyleMoya, Diego, Rafael Manzanera, Jordi Ortner, Marta Torres, Joan Carles Serfaty, Carme Sauri, Lourdes Jimenez, and Jose Joaquin Mira. 2024. "Enhancing Patient Safety in Spain: Streamlining Adverse Event Detection in Occupational Healthcare Records" Safety 10, no. 1: 13. https://doi.org/10.3390/safety10010013
APA StyleMoya, D., Manzanera, R., Ortner, J., Torres, M., Serfaty, J. C., Sauri, C., Jimenez, L., & Mira, J. J. (2024). Enhancing Patient Safety in Spain: Streamlining Adverse Event Detection in Occupational Healthcare Records. Safety, 10(1), 13. https://doi.org/10.3390/safety10010013







