Oral Glutamine Supplement Reduces Subjective Fatigue Ratings during Repeated Bouts of Firefighting Simulations
Abstract
:1. Introduction
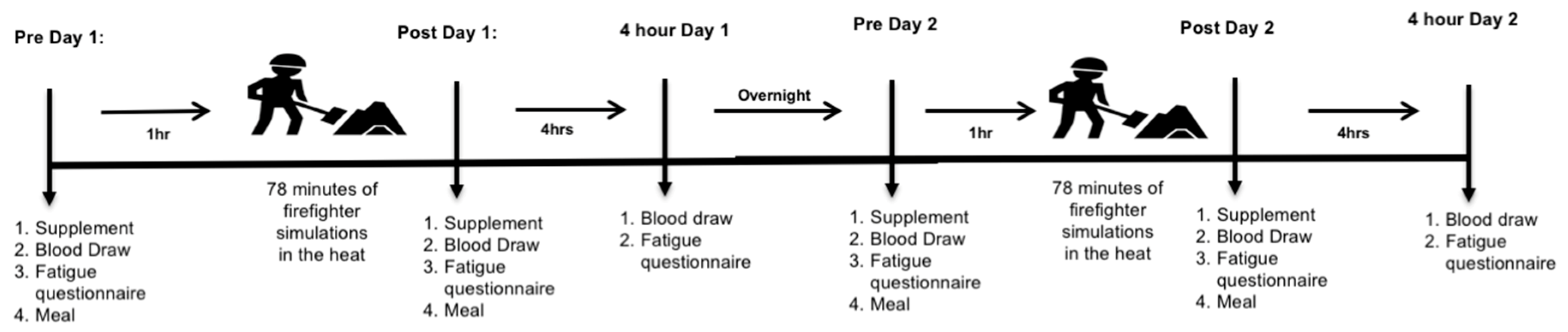
2. Subjects and Methods
3. Biological Measurements
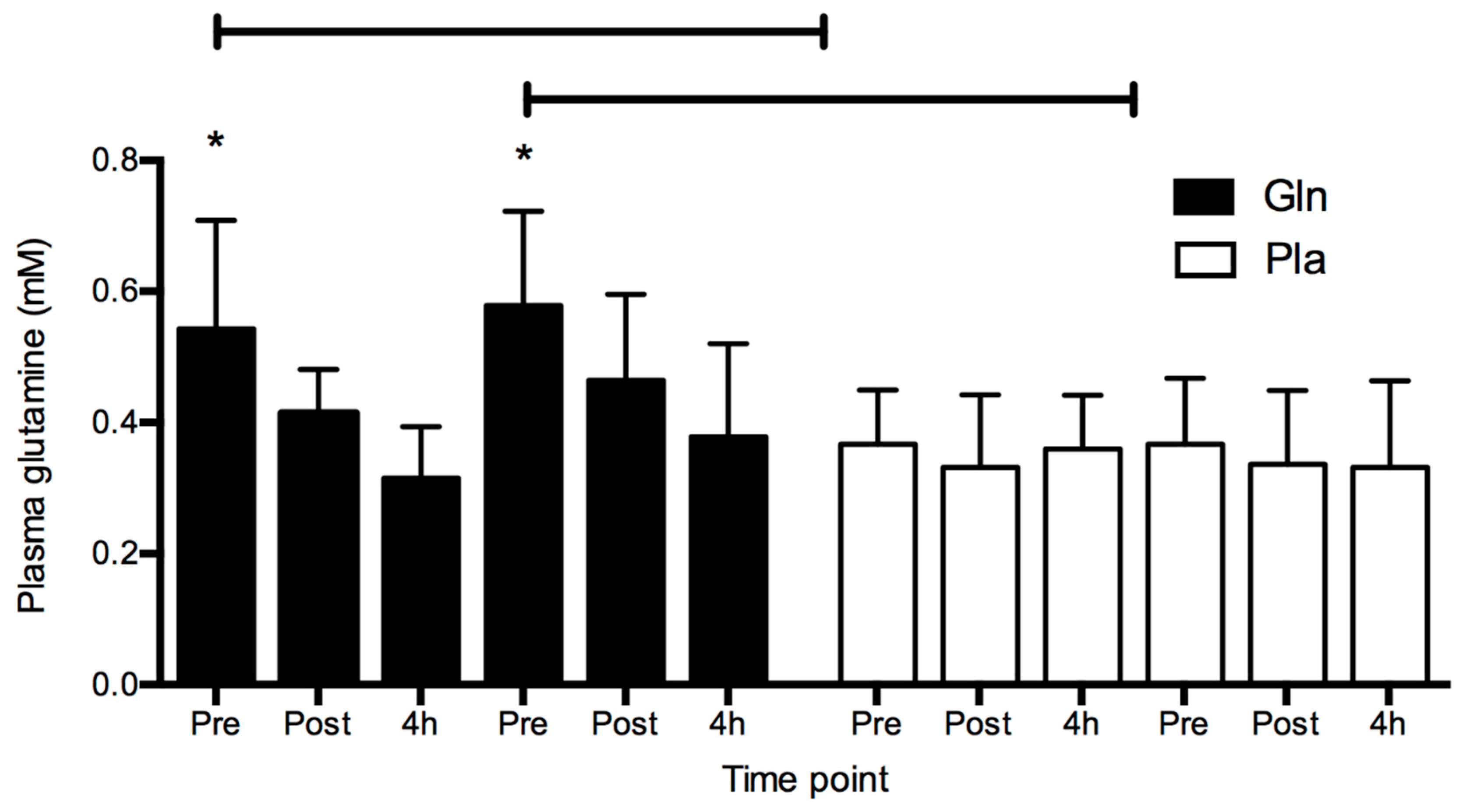
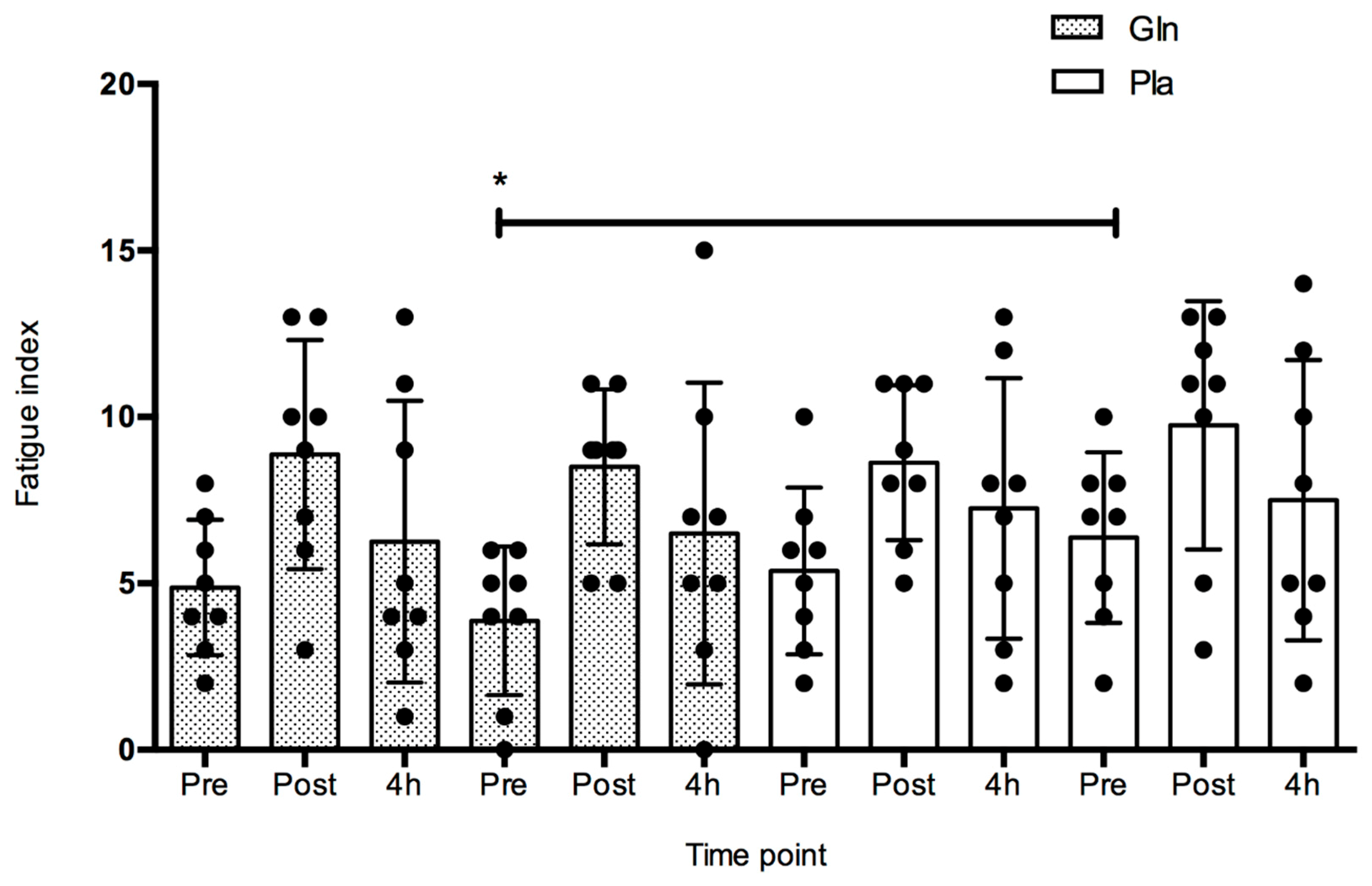
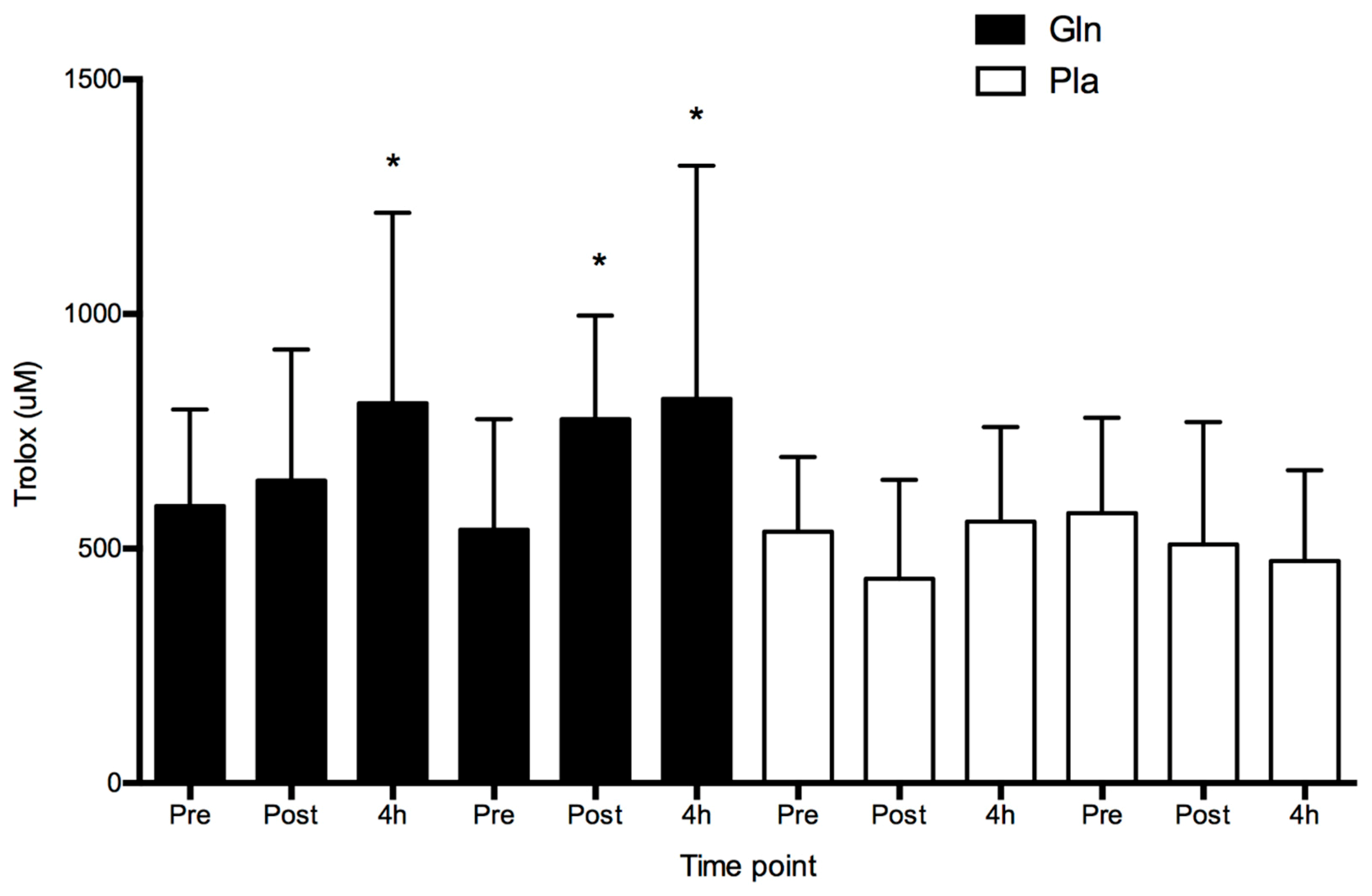
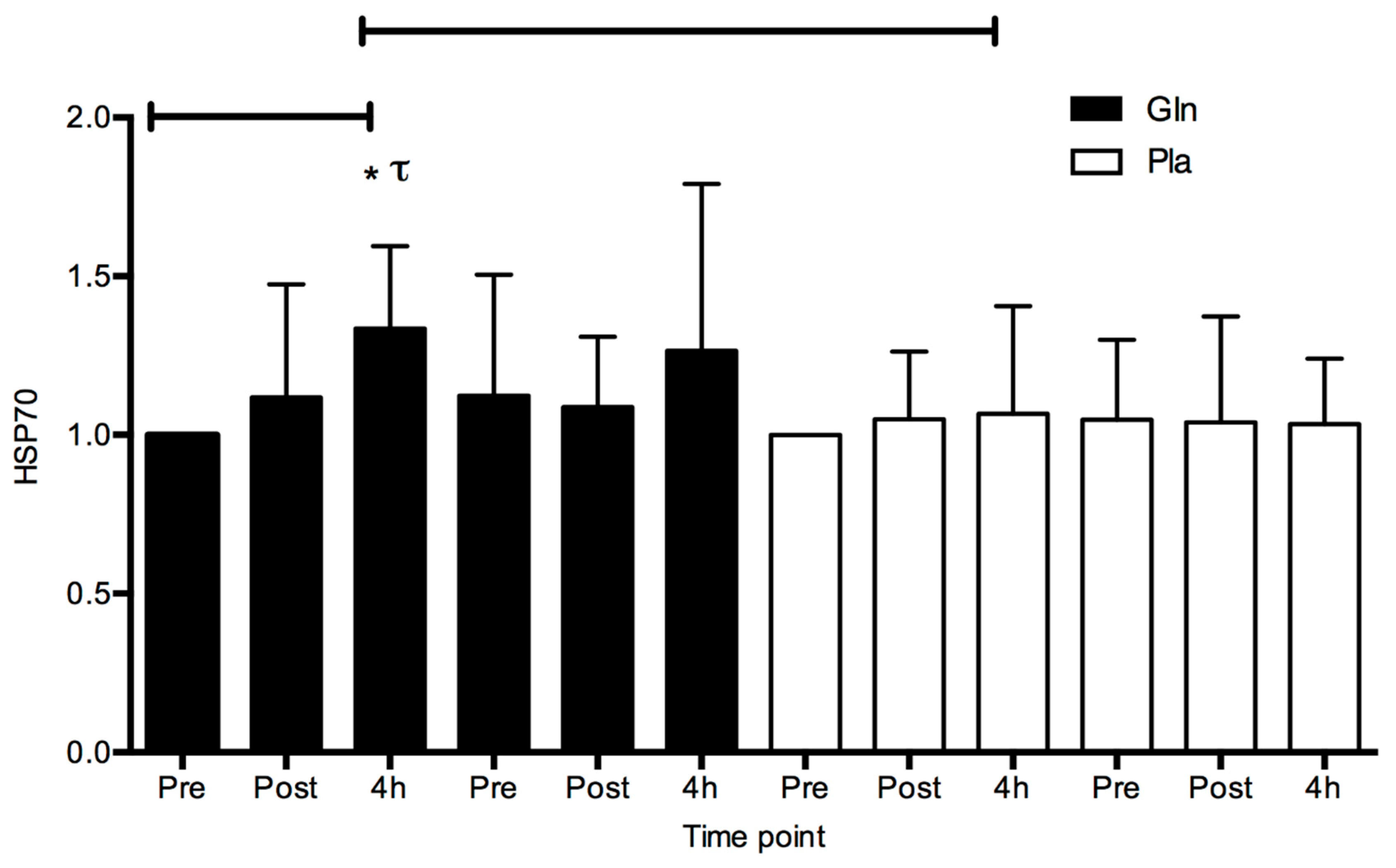
4. Results
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Tiesinga, L.J.; Dassen, T.W.N.; Halfens, R.J.G. Fatigue: A Summary of the Definitions, Dimensions, and Indicators. Int. J. Nurs. Terminol. Classif. 1996, 7, 51–62. [Google Scholar] [CrossRef]
- Xiang, J.; Bi, P.; Pisaniello, D.; Hansen, A.; Sullivan, T. Association between high temperature and work-related injuries in Adelaide, South Australia, 2001–2010. Occup. Environ. Med. 2014, 71, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Grooms, B.D.; Straley, D. Exposure injury: Examining heat- and cold-related illnesses and injuries. Osteopat. Fam. Physician 2013, 5, 200–207. [Google Scholar] [CrossRef]
- Samn, S.W.; Perelli, L.P. Estimating aircrew fatigue: A technique with application to airlift operations. In School of Aerospace Medicine; Brooks AFB: San Antonio, TX, USA, 1982. [Google Scholar]
- Epstein, Y.; Druyan, A.; Heled, Y. Heat Injury Prevention—A Military Perspective. J. Strength Cond. Res. 2012, 26, S82–S86. [Google Scholar] [CrossRef] [PubMed]
- Cuddy, J.S.; Ruby, B.C. High Work Output Combined with High Ambient Temperatures Caused Heat Exhaustion in a Wildland Firefighter Despite High Fluid Intake. Wilderness Environ. Med. 2011, 22, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Purchio, T. Descriptive Analysis of Injuries Sustained by Wildland Firefighters. Master’s Thesis, University of Montana, Missoula, MT, USA, 2017. [Google Scholar]
- Pryor, J.L.; Johnson, E.C.; Roberts, W.O.; Pryor, R.R. Application of evidence-based recommendations for heat acclimation: Individual and team sport perspectives. Temperature 2018, 6, 37–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuennen, M.; Gillum, T.; Dokladny, K.; Bedrick, E.; Schneider, S.; Moseley, P. Thermotolerance and heat acclimation may share a common mechanism in humans. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R524–R533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatouros, I.G.; Jamurtas, A.Z.; Villiotou, V.; Pouliopoulou, S.; Fotinakis, P.; Taxildaris, K.; Deliconstantinos, G. Oxidative Stress Responses in Older Men during Endurance Training and Detraining. Med. Sci. Sports Exerc. 2004, 36, 2065–2072. [Google Scholar] [CrossRef] [Green Version]
- Vallyathan, V.; Shi, X. The role of oxygen free radicals in occupational and environmental lung diseases. Environ. Health Perspect. 1997, 105, 165–177. [Google Scholar]
- Ho, E.; Galougahi, K.K.; Liu, C.-C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Butler, C.; Marsh, S.; Domitrovich, J.W.; Helmkamp, J. Wildland firefighter deaths in the United States: A comparison of existing surveillance systems. J. Occup. Environ. Hyg. 2017, 14, 258–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castell, L.M. Glutamine Supplementation in Vitro and in Vivo, in Exercise and in Immunodepression. Sports Med. 2003, 33, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Castell, L.M.; Newsholme, E.A. The effects of oral glutamine supplementation on athletes after prolonged, exhaustive exercise. Nutrition 1997, 13, 738–742. [Google Scholar] [CrossRef]
- Neu, J.; Shenoy, V.; Chakrabarti, R. Glutamine nutrition and metabolism: Where do we go from here? FASEB J. 1996, 10, 829–837. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Williams, D.R.; Emerson, N.S.; Hoffman, M.W.; Wells, A.J.; McVeigh, D.M.; McCormack, W.P.; Mangine, G.T.; Gonzalez, A.M.; Fragala, M.S. L-alanyl-L-glutamine ingestion maintains performance during a competitive basketball game. J. Int. Soc. Sports Nutr. 2012, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Naclerio, F.; Larumbe-Zabala, E.; Cooper, R.; Allgrove, J.; Earnest, C.P. A Multi-Ingredient Containing Carbohydrate, Proteins L-Glutamine and L-Carnitine Attenuates Fatigue Perception with No Effect on Performance, Muscle Damage or Immunity in Soccer Players. PLoS ONE 2015, 10, e0125188. [Google Scholar] [CrossRef] [PubMed]
- Olubodun, J.O.; Zulkifli, I.; Farjam, A.S.; Hair-Bejo, M.; Kasim, A. Glutamine and glutamic acid supplementation enhances performance of broiler chickens under the hot and humid tropical condition. Ital. J. Anim. Sci. 2015, 14, 3263. [Google Scholar] [CrossRef]
- Zuhl, M.; Dokladny, K.; Mermier, C.; Schneider, S.; Salgado, R.; Moseley, P. The effects of acute oral glutamine supplementation on exercise-induced gastrointestinal permeability and heat shock protein expression in peripheral blood mononuclear cells. Cell Stress Chaperones 2015, 20, 85–93. [Google Scholar] [CrossRef]
- Zuhl, M.N.; Lanphere, K.R.; Kravitz, L.; Mermier, C.M.; Schneider, S.; Dokladny, K.; Moseley, P.L. Effects of oral glutamine supplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. J. Appl. Physiol. 2013, 116, 183–191. [Google Scholar] [CrossRef]
- Hamiel, C.R.; Pinto, S.; Hau, A.; Wischmeyer, P.E. Glutamine enhances heat shock protein 70 expression via increased hexosamine biosynthetic pathway activity. Am. J. Physiol. 2009, 297, C1509–C1519. [Google Scholar] [CrossRef] [Green Version]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Musch, M.W.; Madonna, M.B.; Thisted, R.; Chang, E.B. Glutamine protects intestinal epithelial cells: Role of inducible HSP70. Am. J. Physiol. Liver Physiol. 1997, 272, G879–G884. [Google Scholar] [CrossRef]
- Ziegler, T.R.; Ogden, L.G.; Singleton, K.D.; Luo, M.; Fernandez-Estivariz, C.; Griffith, D.P.; Galloway, J.R.; Wischmeyer, P.E. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensiv. Care Med. 2005, 31, 1079–1086. [Google Scholar] [CrossRef]
- Gabai, V.L.; Meriin, A.B.; A Yaglom, J.; Volloch, V.Z.; Sherman, M.Y. Role of Hsp70 in regulation of stress-kinase JNK: Implications in apoptosis and aging. FEBS Lett. 1998, 438, 1–4. [Google Scholar] [CrossRef]
- McAnulty, S.R.; McAnulty, L.; Pascoe, D.D.; Gropper, S.S.; Keith, R.E.; Morrow, J.D.; Gladden, L.B. Hyperthermia Increases Exercise-induced Oxidative Stress. Int. J. Sports Med. 2005, 26, 188–192. [Google Scholar] [CrossRef]
- Nakhostin-Roohi, B.; Javanamani, R. The Effect of Glutamine Supplementation on Exercise-Induced Oxidative Stress. J. Adv. Agric. Technol. 2015, 2. [Google Scholar] [CrossRef]
- Szczygiel, T.; Moore, M.; Pettit-Mee, R.; Slezsak, A.; Zuhl, M. Low dose glutamine supplementation preserves the biological stress response among US university students. J. Hum. Nutr. Food Sci. 2017, 5, 1114. [Google Scholar]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Balilionis, G.; Casaru, C.; Geary, C.; Schumacker, R.E.; Neggers, Y.H.; Curtner-Smith, M.D.; Richardson, M.T.; Bishop, P.A.; Green, J.M. Effects of caffeine and menthol on cognition and mood during simulated firefighting in the heat. Appl. Ergon. 2014, 45, 510–514. [Google Scholar] [CrossRef]
- Zhang, Y.; Balilionis, G.; Casaru, C.M.; Schumacker, R.E.; Neggers, Y.H.; Curtner-Smith, M.D.; Richardson, M.T.; Green, J.M.; Bisshop, P.A. Effect of menthol on respiratory and perceptual responses to exercise in firefighter protective gear. Monten. J. Sports Sci. Med. 2015, 4, 29–34. [Google Scholar]
- Gibson, A.L.; Wagner, D.; Heyward, V. Advanced Fitness Assessment and Exercise Prescription, 8E; Human Kinetics: Champaign, IL, USA, 2018. [Google Scholar]
- Buono, M.J.; Heaney, J.H.; Canine, K.M. Acclimation to humid heat lowers resting core temperature. Am. J. Physiol. Integr. Comp. Physiol. 1998, 274, R1295–R1299. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Maresh, C.M.; Castellani, J.W.; Bergeron, M.F.; Kenefick, R.W.; Lagasse, K.E.; Riebe, D. Urinary Indices of Hydration Status. Int. J. Sport Nutr. 1994, 4, 265–279. [Google Scholar] [CrossRef]
- Dill, D.B.; Costill, D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974, 37, 247–248. [Google Scholar] [CrossRef]
- Skubitz, K.M.; Anderson, P.M. Oral Glutamine to Reduce Stomatitis. Google Patents US-RE39485-E1, 1995. [Google Scholar]
- Borg, G.A.J. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Keen, M.L.; Miller, K.C.; Zuhl, M.N. Thermoregulatory and Perceptual Effects of a Percooling Garment Worn Underneath an American Football Uniform. J. Strength Cond. Res. 2017, 31, 2983–2991. [Google Scholar] [CrossRef]
- Costill, D.L.; Fink, W.J. Plasma volume changes following exercise and thermal dehydration. J. Appl. Physiol. 1974, 37, 521–525. [Google Scholar] [CrossRef]
- Antonio, J.; Sanders, M.S.; Kalman, D.; Woodgate, D.; Street, C. The Effects of High-Dose Glutamine Ingestion on Weightlifting Performance. J. Strength Cond. Res. 2002, 16, 157–160. [Google Scholar]
- Petry, É.R.; Cruzat, V.F.; Heck, T.G.; Leite, J.S.M.; de Bittencourt, P.I.H., Jr.; Tirapegui, J. Alanyl-glutamine and glutamine plus alanine supplements improve skeletal redox status in trained rats: Involvement of heat shock protein pathways. Life Sci. 2014, 94, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Milner, C.; Campbell, R. Polymorphic analysis of the three MHC-linked HSP70 genes. Immunogenetics 1992, 36, 357–362. [Google Scholar] [CrossRef]
- Wang, Z.-Z.; Wang, C.-L.; Wu, T.-C.; Pan, H.-N.; Wang, S.-K.; Jiang, J.-D. Autoantibody response to heat shock protein 70 in patients with heatstroke. Am. J. Med. 2001, 111, 654–657. [Google Scholar] [CrossRef]
- Wu, T.; Ma, J.; Chen, S.; Sun, Y.; Xiao, C.; Gao, Y.; Wang, R.; Poudrier, J.; Dargis, M.; Currie, R.W.; et al. Association of plasma antibodies against the inducible Hsp70 with hypertension and harsh working conditions. Cell Stress Chaperones 2001, 6, 394–401. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Kahana, M.; Wolfson, R.; Ren, H.; Musch, M.M.; Chang, E.B. Glutamine induces heat shock protein and protects against endotoxin shock in the rat. J. Appl. Physiol. 2001, 90, 2403–2410. [Google Scholar] [CrossRef] [Green Version]
- Wischmeyer, P.E.; Riehm, J.; Singleton, K.D.; Ren, H.; Musch, M.W.; Kahana, M.; Chang, E.B. Glutamine attenuates tumor necrosis factor-α release and enhances heat shock protein 72 in human peripheral blood mononuclear cells. Nutrition 2003, 19, 1–6. [Google Scholar] [CrossRef]
- Gong, J.; Jing, L. Glutamine induces heat shock protein 70 expression via O-GlcNAc modification and subsequent increased expression and transcriptional activity of heat shock factor-1. Minerva Anestesiol. 2011, 77, 488–495. [Google Scholar]
- Morrison, A.L.; Dinges, M.; Singleton, K.D.; Odoms, K.; Wong, H.R.; Wischmeyer, P.E. Glutamine’s protection against cellular injury is dependent on heat shock factor-1. Am. J. Physiol. Physiol. 2006, 290, C1625–C1632. [Google Scholar] [CrossRef]
- Pruna, G.J.; Hoffman, J.R.; McCormack, W.P.; Jajtner, A.R.; Townsend, J.R.; Bohner, J.D.; La Monica, M.B.; Wells, A.J.; Stout, J.R.; Fragala, M.S.; et al. Effect of acute L-Alanyl-L-Glutamine and electrolyte ingestion on cognitive function and reaction time following endurance exercise. Eur. J. Sport Sci. 2016, 16, 72–79. [Google Scholar] [CrossRef]
- Nava, R.C.; Zuhl, M.N.; Moriarty, T.A.; Amorim, F.T.; Bourbeau, K.C.; Welch, A.M.; McCormick, J.J.; King, K.E.; Mermier, C.M. The Effect of Acute Glutamine Supplementation on Markers of Inflammation and Fatigue during Consecutive Days of Simulated Wildland Firefighting. J. Occup. Environ. Med. 2019, 61, e33–e42. [Google Scholar] [CrossRef]
- Hejl, A.M.; Adetona, O.; Diaz-Sanchez, D.; Carter, J.D.; Commodore, A.A.; Rathbun, S.L.; Naeher, L.P. Inflammatory Effects of Woodsmoke Exposure Among Wildland Firefighters Working at Prescribed Burns at the Savannah River Site, SC. J. Occup. Environ. Hyg. 2013, 10, 173–180. [Google Scholar] [CrossRef]
- Ramezani, A.A.; Rayyani, E.; Bahreini, M.; Mansoori, A. The effect of glutamine supplementation on athletic performance, body composition, and immune function: A systematic review and a meta-analysis of clinical trials. Clin. Nutr. 2018, 36, 1076–1091. [Google Scholar] [CrossRef]
- Akbarnezhad, A.; Ravasi, A.; Aminian, R.T.; Nourmohammadi, I. The effect of creatine and glutamine supplements on athletic performance in elite wrestlers after one acute period of weight losing. HARAKAT 2006, 27, 173–188. [Google Scholar]
- Kusano, C.; Ferrari, B. Total antioxidant capacity: A biomarker in biomedical and nutritional studies. J. Cell Mol. Biol. 2008, 7, 1–15. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazúr, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Boil. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Barclay, J.K.; Hansel, M. Free radicals may contribute to oxidative skeletal muscle fatigue. Can. J. Physiol. Pharmacol. 1991, 69, 279–284. [Google Scholar] [CrossRef]
- Reid, M.B. Free radicals and muscle fatigue: Of ROS, canaries, and the IOC. Free. Radic. Boil. Med. 2008, 44, 169–179. [Google Scholar] [CrossRef]
- Shichiri, M.; Harada, N.; Ishida, N.; Komaba, L.K.; Iwaki, S.; Hagihara, Y.; Niki, E.; Yoshida, Y. Oxidative stress is involved in fatigue induced by overnight deskwork as assessed by increase in plasma tocopherylhydroqinone and hydroxycholesterol. Boil. Psychol. 2013, 94, 527–533. [Google Scholar] [CrossRef]
- Medved, I.; Brown, M.J.; Bjorksten, A.R.; Murphy, K.T.; Petersen, A.C.; Sostaric, S.; Gong, X.; McKenna, M. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J. Appl. Physiol. 2004, 97, 1477–1485. [Google Scholar] [CrossRef]
- Jówko, E.; Sadowski, J.; Długołęcka, B.; Gierczuk, D.; Opaszowski, B.; Cieśliński, I. Effects of Rhodiola rosea supplementation on mental performance, physical capacity, and oxidative stress biomarkers in healthy men. J. Sport Health Sci. 2018, 7, 473–480. [Google Scholar] [CrossRef]
- Granger, D.N.; E Höllwarth, M.; A Parks, D. Ischemia-reperfusion injury: Role of oxygen-derived free radicals. Acta Physiol. Scand. Suppl. 1986, 548, 47–63. [Google Scholar]
- Salomão, A.B.; Aguilar-Nascimento, J.E.; Percario, S.; Sano, V.; Marques, N.R.; Dias, C.C.G.D.O. Intestinal intraluminal injection of glutamine increases trolox total equivalent antioxidant capacity (TEAC) in hepatic ischemia-reperfusion. Acta Cir. Bras. 2006, 21, 69–73. [Google Scholar] [CrossRef]
- Souba, W.W.; Smith, R.J.; Wilmore, D.W. Glutamine Metabolism by the Intestinal Tract. J. Parenter. Enter. Nutr. 1985, 9, 608–617. [Google Scholar] [CrossRef]
- Ruby, B.C.; Shriver, T.C.; Zderic, T.W.; Sharkey, B.J.; Burks, C.; Tysk, S. Total energy expenditure during arduous wildfire suppression. Med. Sci. Sports Exerc. 2002, 34, 1048–1054. [Google Scholar] [CrossRef]
- Cuddy, J.S.; Sol, J.A.; Hailes, W.S.; Ruby, B.C. Work Patterns Dictate Energy Demands and Thermal Strain during Wildland Firefighting. Wilderness Environ. Med. 2015, 26, 221–226. [Google Scholar] [CrossRef]
- Lui, B.; Cuddy, J.S.; Hailes, W.S.; Ruby, B.C. Seasonal heat acclimatization in wildland firefighters. J. Therm. Biol. 2014, 45, 134–140. [Google Scholar] [CrossRef]
- Britton, C.; Lynch, C.F.; Ramirez, M.; Torner, J.; Buresh, C.; Peek-Asa, C. Epidemiology of injuries to wildland firefighters. Am. J. Emerg. Med. 2013, 31, 339–345. [Google Scholar] [CrossRef]
- Tawatsupa, B.; Yiengprugsawan, V.; Kjellstrom, T.; Berecki-Gisolf, J.; Seubsman, S.-A.; Sleigh, A. Association between Heat Stress and Occupational Injury among Thai Workers: Findings of the Thai Cohort Study. Ind. Health 2013, 51, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Flouris, A.D.; McGinn, R.; Poirier, M.P.; Louie, J.C.; Ioannou, L.G.; Tsoutsoubi, L.; Sigal, R.J.; Boulay, P.; Hardcastle, S.G.; Kenny, G.P. Screening criteria for increased susceptibility to heat stress during work or leisure in hot environments in healthy individuals aged 31–70 years. Temperature 2018, 5, 86–99. [Google Scholar] [CrossRef]
- Notley, S.R.; Meade, R.D.; D’Souza, A.W.; McGarr, G.W.; Kenny, G.P. Cumulative effects of successive workdays in the heat on thermoregulatory function in the aging worker. Temperature 2018, 5, 293–295. [Google Scholar] [CrossRef] [Green Version]
- Raimundo, A.M.; Figueiredo, A.R.A. Personal protective clothing and safety of firefighters near a high intensity fire front. Fire Saf. J. 2009, 44, 514–521. [Google Scholar] [CrossRef]
- Gonzalez-Olabarria, J.R.; Reynolds, K.M.; Larrañaga, A.; Garcia-Gonzalo, J.; Busquets, E.; Pique, M. Strategic and tactical planning to improve suppression efforts against large forest fires in the Catalonia region of Spain. For. Ecol. Manag. 2019, 432, 612–622. [Google Scholar] [CrossRef]
- Walsh, N.P.; Gleeson, M.; Pyne, D.B.; Nieman, D.C.; Dhabhar, F.S.; Shephard, R.J.; Oliver, S.J.; Bermon, S.; Kajeniene, A. Position statement. Part two: Maintaining immune health. Exerc. Immunol. Rev. 2011, 17, 64–103. [Google Scholar]
| Sex (male, female) | Age (years) | Body Mass (kg) | Height (cm) | Body Fat (%) | Maximal Aerobic Capacity (mL/kg/min) |
|---|---|---|---|---|---|
| male n = 5 female n = 3 | 24 ± 1.0 | 75.46 ± 8.6 | 174.13 ± 6.9 | 18.22 ± 10.3 | 52.61 ± 6.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, M.; Moriarty, T.A.; Connolly, G.; Mermier, C.; Amorim, F.; Miller, K.; Zuhl, M. Oral Glutamine Supplement Reduces Subjective Fatigue Ratings during Repeated Bouts of Firefighting Simulations. Safety 2019, 5, 38. https://doi.org/10.3390/safety5020038
Moore M, Moriarty TA, Connolly G, Mermier C, Amorim F, Miller K, Zuhl M. Oral Glutamine Supplement Reduces Subjective Fatigue Ratings during Repeated Bouts of Firefighting Simulations. Safety. 2019; 5(2):38. https://doi.org/10.3390/safety5020038
Chicago/Turabian StyleMoore, Mary, Terence A. Moriarty, Gavin Connolly, Christine Mermier, Fabiano Amorim, Kevin Miller, and Micah Zuhl. 2019. "Oral Glutamine Supplement Reduces Subjective Fatigue Ratings during Repeated Bouts of Firefighting Simulations" Safety 5, no. 2: 38. https://doi.org/10.3390/safety5020038
APA StyleMoore, M., Moriarty, T. A., Connolly, G., Mermier, C., Amorim, F., Miller, K., & Zuhl, M. (2019). Oral Glutamine Supplement Reduces Subjective Fatigue Ratings during Repeated Bouts of Firefighting Simulations. Safety, 5(2), 38. https://doi.org/10.3390/safety5020038






