Evaluation of the Efficacy of IALUSET VITAL® Cream in Helping the Improvement of the Atopic Dermatitis Symptoms in Adults: A Randomized, Double Blind, Vehicle-Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
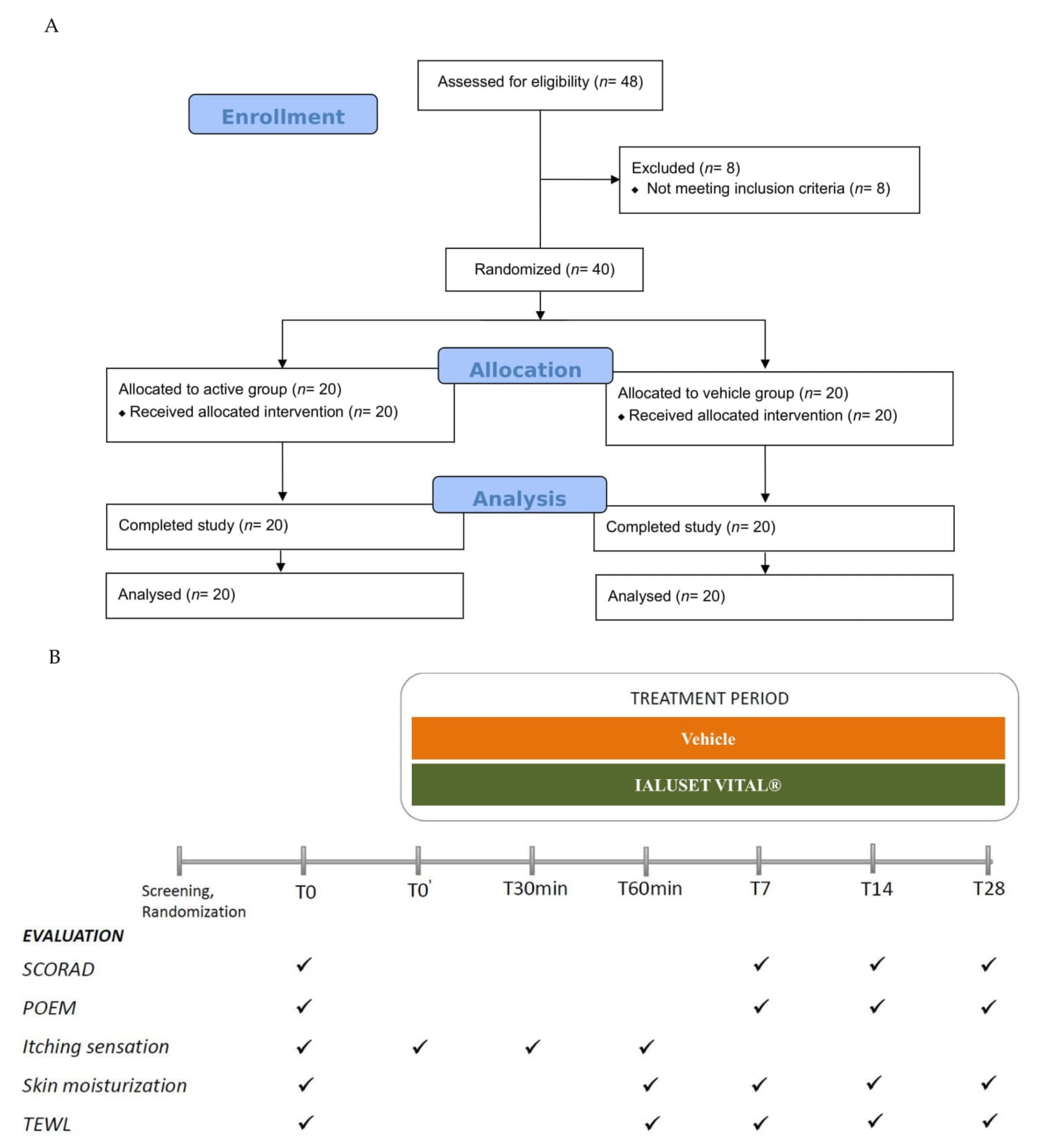
2.2. Study Population, Randomization, and Treatment
2.3. Study Outcomes
2.4. Statistical Analysis and Interpretation of Results
2.5. Reference and Test Cream
3. Results
3.1. Clinical Study: Patient Enrolment and Disposition
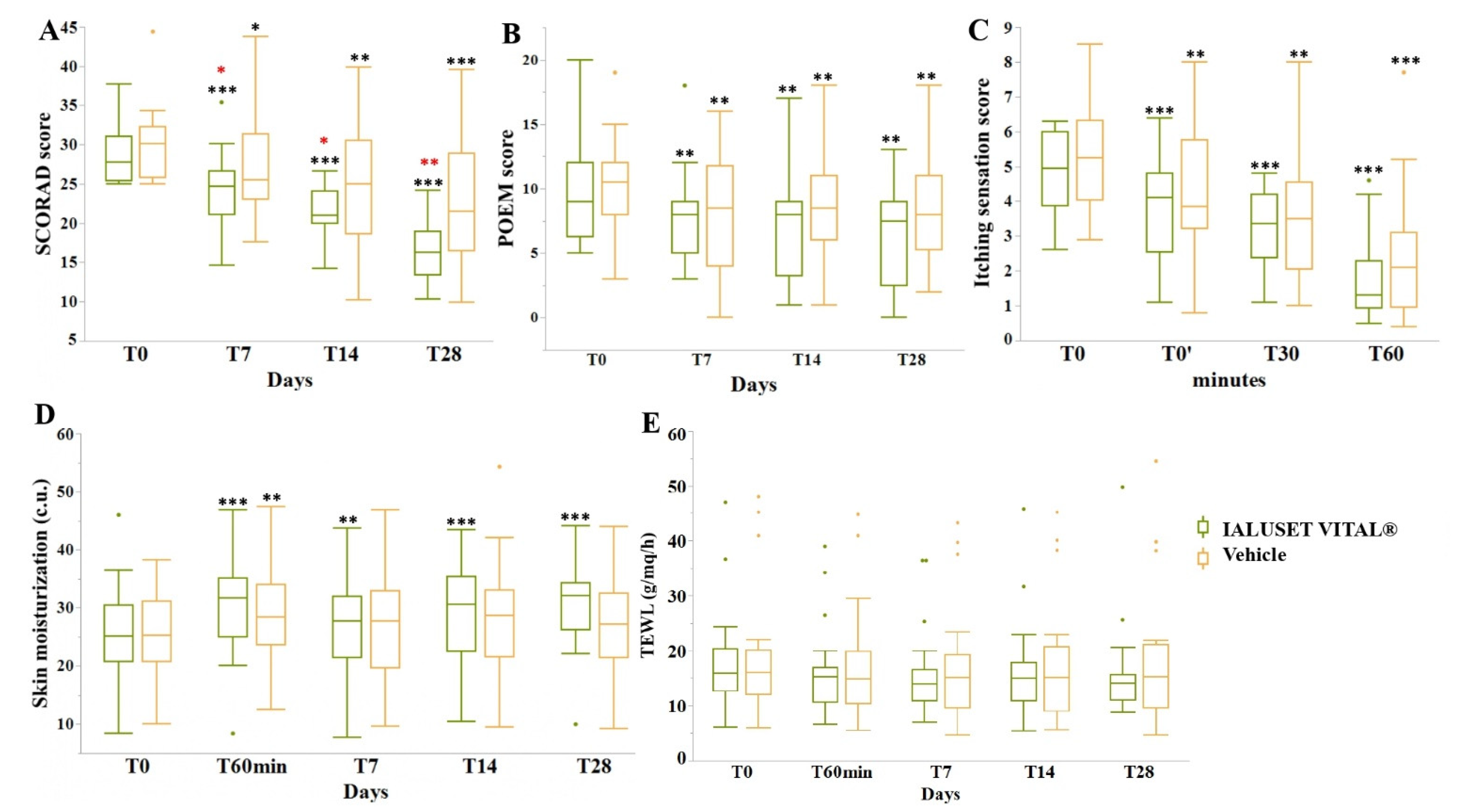
3.2. Efficacy Endpoints
3.3. Tolerance and Safety
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nutten, S. Atopic Dermatitis: Global Epidemiology and Risk Factors. Ann. Nutr. Metab. 2015, 66 (Suppl. 1), 8–16. [Google Scholar] [CrossRef]
- Ständer, S.; Metz, M.; Ramos, M.; Maurer, M.; Schoepke, N.; Tsianakas, A.; Zeidler, C.; Luger, T.A. Anti-pruritic Effect of Sertaconazole 2% Cream in Atopic Dermatitis Subjects: A Prospective, Randomized, Double-blind, Vehicle-controlled, Multi-centre Clinical Trial of Efficacy, Safety and Local Tolerability. Acta Derm. Venereol. 2014, 96, 792–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunello, L. Atopic dermatitis. Nat. Rev. Dis. Primer 2018, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Novak, N. Atopic dermatitis and filaggrin. Curr. Opin. Immunol. 2016, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Nomura, T.; Ohguchi, Y.; Mizuno, O.; Suzuki, S.; Tsujiuchi, H.; Hamajima, N.; McLean, W.H.I.; Shimizu, H.; Akiyama, M. Comprehensive screening for a complete set of Japanese-population-specific filaggrin gene mutations. Allergy 2014, 69, 537–540. [Google Scholar] [CrossRef]
- Ishikawa, J.; Narita, H.; Kondo, N.; Hotta, M.; Takagi, Y.; Masukawa, Y.; Kitahara, T.; Takema, Y.; Koyano, S.; Yamazaki, S.; et al. Changes in the Ceramide Profile of Atopic Dermatitis Patients. J. Investig. Dermatol. 2010, 130, 2511–2514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, N.; Simon, D. Atopic dermatitis—From new pathophysiologic insights to individualized therapy. Allergy 2011, 66, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Hajar, T.; Gontijo, J.R.V.; Hanifin, J.M. New and developing therapies for atopic dermatitis. Bras. Dermatol. 2018, 93, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinol 2012, 4, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Reed, R.K.; Lilja, K.; Laurent, T.C. Hyaluronan in the rat with special reference to the skin. Acta Physiol. Scand. 1988, 134, 405–411. [Google Scholar] [CrossRef]
- Maytin, E.V.; Chung, H.H.; Seetharaman, V.M. Hyaluronan participates in the epidermal response to disruption of the permeability barrier in vivo. Am. J. Pathol. 2004, 165, 1331–1341. [Google Scholar] [CrossRef] [Green Version]
- Passi, A.; Sadeghi, P.; Kawamura, H.; Anand, S.; Sato, N.; White, L.E.; Hascall, V.C.; Maytin, E.V. Hyaluronan suppresses epidermal differentiation in organotypic cultures of rat keratinocytes. Exp. Cell Res. 2004, 296, 123–134. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.W.; Ramez, M.; Gilad, E.; Singleton, P.A.; Man, M.-Q.; Crumrine, D.A.; Elias, P.M.; Feingold, K.R. Hyaluronan–CD44 Interaction Stimulates Keratinocyte Differentiation, Lamellar Body Formation/Secretion, and Permeability Barrier Homeostasis. J. Investig. Dermatol. 2006, 126, 1356–1365. [Google Scholar] [CrossRef] [Green Version]
- Bourguignon, L.Y.W.; Wong, G.; Xia, W.; Man, M.-Q.; Holleran, W.M.; Elias, P.M. Selective matrix (hyaluronan) interaction with CD44 and RhoGTPase signaling promotes keratinocyte functions and overcomes age-related epidermal dysfunction. J. Derm. Sci. 2013, 72, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Hebert, A.; Pacha, O. Treating atopic dermatitis: Safety, efficacy, and patient acceptability of a ceramide hyaluronic acid emollient foam. Clin. Cosmet. Investig. Dermatol. 2012, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draelos, Z.D. A clinical evaluation of the comparable efficacy of hyaluronic acid-based foam and ceramide-containing emulsion cream in the treatment of mild-to-moderate atopic dermatitis: Barrier restoration therapy in atopic dermatitis. J. Cosmet. Dermatol. 2011, 10, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Reuter, J.; Wölfle, U.; Weckesser, S.; Schempp, C. Which plant for which skin disease? Part 1: Atopic dermatitis, psoriasis, acne, condyloma and herpes simplex. J. Dtsch. Dermatol. Ges. 2010, 8, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Canaviri, C.; María, A. Medicina Tradicional y la Medicina Occidental en el Manejo de la Tuberculosis Municipio Caranavi Primer Semestre 2006. Ph.D. Dissertation, Universidad Mayor de San André, La Paz, Bolivia, 2007; p. 78. [Google Scholar]
- Matic, I.; Revandkar, A.; Chen, J.; Bisio, A.; Dall’Acqua, S.; Cocetta, V.; Brun, P.; Mancino, G.; Milanese, M.; Mattei, M.; et al. Identification of Salvia haenkei as gerosuppressant agent by using an integrated senescence-screening assay. Aging 2016, 8, 3223–3236. [Google Scholar] [CrossRef] [Green Version]
- Cestone, E.; Bellia, G.; Nobile, V.; Giori, A.M.; Alimonti, A.; Montopoli, M. Evaluation of the anti-ageing efficacy of Hilow Haenkenium cream in healthy woman. Aesthetic. Med. 2020, 6, 9. [Google Scholar]
- Alimonti, A.; Giori, A.M.; Montopoli, M.; Cadau, J. Use of a Vegetal Extract as an Active Agent in Tissue Re-Epithelizing and Cicatrizing Processes [Internet]. WO2019121425 (A1). Available online: https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=20190627&DB=&locale=en_EP&CC=WO&NR=2019121425A1&KC=A1&ND=4 (accessed on 21 April 2020).
- Alimonti, A.; Giori, A.M.; Montopoli, M.; Cadau, J. Use of a Vegetal Extract as an Active Agent in the Treatment of Dermatological Diseases [Internet]. WO2019121427 (A1). Available online: https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=20190627&DB=&locale=en_EP&CC=WO&NR=2019121427A1&KC=A1&ND=5 (accessed on 21 April 2020).
- Stalder, J.F.; Taïeb, A.; Atherton, D.J.; Bieber, P.; Bonifazi, E.; Broberg, A.; Calza, A.; Coleman, R.; De Prost, Y.; Stalder, J.F.; et al. Severity scoring of atopic dermatitis: The SCORAD index: Consensus report of the european task force on atopic dermatitis. Dermatology 1993, 186, 23–31. [Google Scholar]
- Charman, C.R.; Venn, A.J.; Williams, H.C. The patient-oriented eczema measure: Development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch. Dermatol. 2004, 140, 1513–1519. [Google Scholar] [CrossRef]
- Chen, W.Y.; Abatangelo, G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999, 7, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, A.; Nomura, T.; Rerknimitr, P.; Seidel, J.A.; Honda, T.; Kabashima, K. The interplay between genetic and environmental factors in the pathogenesis of atopic dermatitis. Immunol. Rev. 2017, 278, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Neuman, M.G.; Nanau, R.M.; Oruña, L.; Coto, G. In vitro anti-inflammatory effects of hyaluronic acid in ethanol-induced damage in skin cells. J. Pharm. Pharm. Sci. 2011, 14, 425–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Lee, Y.-S.; Hahn, J.-H.; Choe, J.; Kwon, H.J.; Ro, J.Y.; Jeoung, D. Hyaluronic acid targets CD44 and inhibits FcepsilonRI signaling involving PKCdelta, Rac1, ROS, and MAPK to exert anti-allergic effect. Mol. Immunol. 2008, 45, 2537–2547. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Goomer, R.S.; Harwood, F.; Kubo, T.; Hirasawa, Y.; Amiel, D. The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1beta (IL-1beta), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthr. Cartil. 1999, 7, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Campo, G.M.; Avenoso, A.; Nastasi, G.; Micali, A.; Prestipino, V.; Vaccaro, M.; D’Ascola, A.; Calatroni, A.; Campo, S. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim. Biophys. Acta 2011, 1812, 1170–1181. [Google Scholar] [CrossRef] [Green Version]
- Mangano, K.; Vergalito, F.; Mammana, S.; Mariano, A.; De Pasquale, R.; Meloscia, A.; Bartollino, S.; Guerra, G.; Nicoletti, F.; Di Marco, R. Evaluation of hyaluronic acid-P40 conjugated cream in a mouse model of dermatitis induced by oxazolone. Exp. Med. 2017, 14, 2439–2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T.; et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786.e1–7. [Google Scholar] [CrossRef] [Green Version]
- Nadeau, P.; Henehan, M.; De Benedetto, A. Activation of protease-activated receptor 2 leads to impairment of keratinocyte tight junction integrity. J. Allergy Clin. Immunol. 2018, 142, 281–284.e7. [Google Scholar] [CrossRef] [Green Version]
- Sugita, K.; Kabashima, K. Tight junctions in the development of asthma, chronic rhinosinusitis, atopic dermatitis, eosinophilic esophagitis, and inflammatory bowel diseases. J. Leukoc. Biol. 2020, 107, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Wang, S.S.; Lau, Z.; Lee, H.C.; Lee, K.K.C.; Leung, T.F.; Luk, N.M. Pseudoceramide for childhood eczema: Does it work? Hong Kong Med. J. 2011, 17, 132–136. [Google Scholar] [PubMed]
- Hon, K.L.E.; Ching, G.K.; Leung, T.F.; Choi, C.Y.; Lee, K.K.C.; Ng, P.C. Estimating emollient usage in patients with eczema. Clin. Exp. Dermatol. 2010, 35, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Pong, N.H.; Wang, S.S.; Lee, V.W.; Luk, N.M.; Leung, T.F. Acceptability and efficacy of an emollient containing ceramide-precursor lipids and moisturizing factors for atopic dermatitis in pediatric patients. Drugs RD 2013, 13, 37–42. [Google Scholar]
- Lodén, M.; Andersson, A.C.; Andersson, C.; Frödin, T.; Oman, H.; Lindberg, M. Instrumental and dermatologist evaluation of the effect of glycerine and urea on dry skin in atopic dermatitis. Ski. Res. Technol. 2001, 7, 209–213. [Google Scholar]
- Son, S.W.; Park, S.Y.; Ha, S.H.; Park, G.M.; Kim, M.G.; Moon, J.S.; Yoo, D.S.; Oh, C.H. Objective evaluation for severity of atopic dermatitis by morphologic study of skin surface contours. Ski. Res. Technol. 2005, 11, 272–280. [Google Scholar] [CrossRef]
- Hon, K.L.; Kung, J.S.C.; Ng, W.G.G.; Leung, T.F. Emollient treatment of atopic dermatitis: Latest evidence and clinical considerations. Drugs Context 2018, 7, 212530. [Google Scholar] [CrossRef] [Green Version]
| CHEMICAL COMPOSITION |
|---|
| DISODIUM EDTA |
| XYLITYLGLUCOSIDE + ANHYDROXYLITOL + XYLITOL |
| POLYMETHYL METHACRYLATE |
| C14-22 ALCOHOLS AND C12-20 ALKYL GLUCOSIDE |
| ISONONYL ISONONANOATE |
| COCO CAPRYLATE/CAPRATE |
| HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER + SQUALANE + POLYSORBATE 60 |
| SODIUM HYALURONATE HMW (800.000 Da) (0.100%) |
| SODIUM HYALURONATE LMW (300.000 Da) (0.100%) |
| L-ARGININE |
| PHENOXYETHANOL, BENZOIC ACID, DEHYDROACETIC ACID, ETHYLHEXYLGLYCERIN |
| SALVIA HAENKEI EXTRACT (0.250%) |
| PURIFIED WATER |
| Control Group (Vehicle) | Active Group (IALUSET VITAL®) | |
|---|---|---|
| Subjects enrolled (n) | 20 | 20 |
| Ethnicity | ||
| Caucasian (n) | 20 | 20 |
| Gender | ||
| Female n (%) | 15 (75) | 16 (80) |
| Male n (%) | 5 (25) | 4 (20) |
| Age (years) | ||
| median (range) | 49 (20−65) | 45 (19−63) |
| SCORAD Median (IQR) | 30.10 (25.80−32.25) | 27.80 (25.37−31.07) |
| POEM Median (IQR) | 10.50 (8.00−12.00) | 9.00 (6.25−12.00) |
| Itching sensation Median (IQR) | 5.25 (4.02−6.32) | 4.95 (3.87−6.00) |
| Skin moisturization Median (IQR) | 25.30 (20.75−31.13) | 25.05 (20.70−30.48) |
| TEWL Median (IQR) | 15.95 (12.10−20.03) | 15.80 (12.68−20.28) |
| Baseline (T0) | T0’ | T30min | T60min | T7 | T14 | T28 | |||
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | Median (IQR) | Scorad | 30.10 (25.80–32.25) | – | – | – | 25.55 (23.00–31.38) | 25.05 (18.68–30.50) | 21.50 (16.48–28.86) |
| Poem | 10.50 (8.00–12.00) | – | – | – | 8.50 (4.00–11.76) | 8.50 (6.00–11.00) | 8.00 (5.25–11.00) | ||
| Itching | 5.25 (4.03–6.33) | 3.85 (3.23–5.76) | 3.50 (2.05–4.55) | 2.10 (0.95–3.10) | – | – | – | ||
| Skin Moisturization | 25.30 (20.75–31.13) | – | – | 28.35 (23.60–34.05) | 27.75 (19.70–32.90) | 28.65 (21.55–33.08) | 27.20 (21.35–32.50) | ||
| TEWL | 15.95 (12.10–20.03) | – | – | 14.75 (10.38–19.80) | 15.10 (9.65–19.15) | 15.00 (9.05–20.65) | 15.20 (9.73–21.08) | ||
| Mean | Scorad | 30.09 | – | – | – | 27.41 (p < 0.05) | 24.92 (p < 0.01) | 22.67 (p < 0.001) | |
| Poem | 10.00 | – | – | – | 8.05 (p < 0.01) | 8.35 (p < 0.01) | 8.10 (p < 0.01) | ||
| Itching | 5.26 | 4.27 (p < 0.01) | 3.69 (p < 0.01) | 2.49 (p < 0.001) | – | – | – | ||
| Skin Moisturization | 25.93 | – | – | 29.20 (p < 0.01) | 27.24 | 28.71 | 27.26 | ||
| TEWL | 18.79 | – | – | 17.48 | 17.42 | 17.51 | 18.25 | ||
| Variance | Scorad | 20.90 | – | – | – | 37.56 | 52.69 | 65.68 | |
| Poem | 13.79 | – | – | – | 19.63 | 15.61 | 15.46 | ||
| Itching | 2.11 | 2.78 | 3.71 | 3.30 | – | – | – | ||
| Skin Moisturization | 55.38 | – | – | 68.17 | 80.35 | 101.69 | 68.15 | ||
| TEWL | 146.83 | – | – | 118.91 | 118.36 | 128.57 | 157.39 | ||
| IALUSET VITAL® | Median (IQR) | Scorad | 27.80 (25.38–31.08) | – | – | – | 24.70 (21.13–26.63) | 21.00 (19.95–24.13) | 16.25 (13.45–18.98) |
| Poem | 9.00 (6.25–12.00) | – | – | – | 8.00 (5.00–9.00) | 8.00 (3.25–9.00) | 7.50 (2.50–9.00) | ||
| Itching | 4.95 (3.88–6.00) | 4.10 (2.55–4.80) | 3.35 (2.38–4.20) | 1.30 (0.93–2.28) | – | – | – | ||
| Skin Moisturization | 25.05 (20.70–30.48) | – | – | 31.70 (24.95–35.08) | 27.65 (21.48–32.00) | 30.60 (22.58–35.38) | 32.05 (26.23–34.23) | ||
| TEWL | 15.80 (12.68–20.28) | – | – | 15.20 (10.73–16.95) | 13.90 (10.90–16.63) | 14.95 (10.98–17.83) | 14.10 (11.08–15.58) | ||
| Mean | Scorad | 28.96 | – | – | – | 24.43 (p < 0.001) | 21.51 (p < 0.001) | 16.40 (p < 0.001) | |
| Poem | 9.70 | – | – | – | 7.80 (p < 0.01) | 7.35 (p < 0.01) | 6.50 (p < 0.01) | ||
| Itching | 4.93 | 3.75 (p < 0.001) | 3.15 (p < 0.001) | 1.78 (p < 0.001) | – | – | – | ||
| Skin Moisturization | 25.63 | – | – | 30.50 (p < 0.001) | 27.80 (p < 0.01) | 29.26 (p < 0.001) | 31.06 (p < 0.001) | ||
| TEWL | 17.84 | – | – | 16.34 | 15.88 | 16.36 | 15.94 | ||
| Variance | Scorad | 16.96 | – | – | – | 19.91 | 11.18 | 13.91 | |
| Poem | 17.80 | – | – | – | 11.96 | 17.92 | 17.11 | ||
| Itching | 1.51 | 2.38 | 1.26 | 1.45 | – | – | – | ||
| Skin Moisturization | 69.64 | – | – | 75.50 | 70.74 | 69.11 | 59.67 | ||
| TEWL | 91.89 | – | – | 69.61 | 65.91 | 82.09 | 78.77 | ||
| Changes from Baseline | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vehicle | IALUSET VITAL ® | ||||||||||||
| T0’ | T30min | T60min | T7 | T14 | T28 | T0’ | T30min | T60min | T7 | T14 | T28 | ||
| Median | Scorad | – | – | – | −0.50 | −4.35 | −6.35 | – | – | – | −2.05 | −6.80 | −11.55 |
| Poem | – | – | – | −1.50 | −1.50 | −1.00 | – | – | – | −1.00 | −1.50 | −2.50 | |
| Itching | −0.85 | −1.00 | −2.65 | – | – | – | −1.10 | −1.40 | −2.85 | – | – | – | |
| Skin Moisturization | – | – | 2.79 | 0.99 | 1.36 | 1.98 | – | – | 4.31 | 1.75 | 2.83 | 4.41 | |
| TEWL | – | – | −0.05 | −0.85 | −1.20 | −1.10 | – | – | −0.65 | −1.15 | −0.75 | −1.95 | |
| Mean | Scorad | – | – | – | −2.68 | −5.17 | −7.42 | – | – | – | −4.54 | −7.45 | −12.57 |
| Poem | – | – | – | −1.95 | −1.65 | −1.90 | – | – | – | −1.90 | −2.35 | −3.20 | |
| Itching | −1.00 | −1.58 | −2.77 | – | – | – | −1.18 | −1.78 | −3.15 | – | – | – | |
| Skin Moisturization | – | – | 3.27 | 1.31 | 2.78 | 1.33 | – | – | 4.87 | 2.17 | 3.62 | 5.43 | |
| TEWL | – | – | −1.31 | −1.37 | −1.28 | −0.54 | – | – | −1.50 | −1.96 | −1.48 | −1.90 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vita, F.; Ferravante, A.; Vecchi, G.; Nobile, V.; Giori, A.M. Evaluation of the Efficacy of IALUSET VITAL® Cream in Helping the Improvement of the Atopic Dermatitis Symptoms in Adults: A Randomized, Double Blind, Vehicle-Controlled Clinical Trial. Allergies 2021, 1, 195-205. https://doi.org/10.3390/allergies1040018
De Vita F, Ferravante A, Vecchi G, Nobile V, Giori AM. Evaluation of the Efficacy of IALUSET VITAL® Cream in Helping the Improvement of the Atopic Dermatitis Symptoms in Adults: A Randomized, Double Blind, Vehicle-Controlled Clinical Trial. Allergies. 2021; 1(4):195-205. https://doi.org/10.3390/allergies1040018
Chicago/Turabian StyleDe Vita, Fernanda, Angela Ferravante, Gabriele Vecchi, Vincenzo Nobile, and Andrea Maria Giori. 2021. "Evaluation of the Efficacy of IALUSET VITAL® Cream in Helping the Improvement of the Atopic Dermatitis Symptoms in Adults: A Randomized, Double Blind, Vehicle-Controlled Clinical Trial" Allergies 1, no. 4: 195-205. https://doi.org/10.3390/allergies1040018
APA StyleDe Vita, F., Ferravante, A., Vecchi, G., Nobile, V., & Giori, A. M. (2021). Evaluation of the Efficacy of IALUSET VITAL® Cream in Helping the Improvement of the Atopic Dermatitis Symptoms in Adults: A Randomized, Double Blind, Vehicle-Controlled Clinical Trial. Allergies, 1(4), 195-205. https://doi.org/10.3390/allergies1040018







