Proposal for Structured Histopathology of Nasal Secretions for Endotyping Chronic Rhinosinusitis: An Exploratory Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Inclusion
2.2. Sinonasal Sampling
2.3. Laboratory Analysis
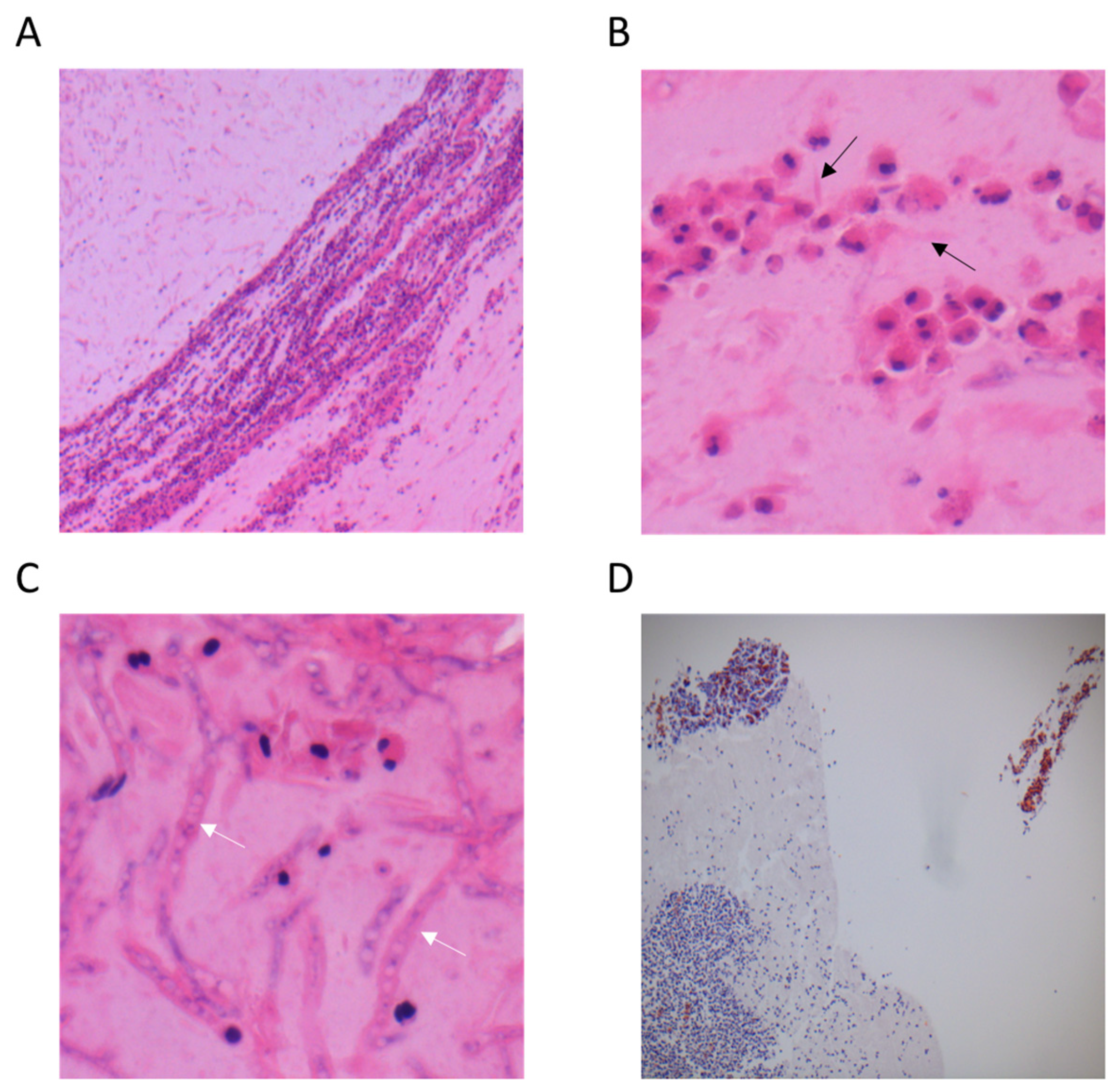
3. Results
3.1. Validity of Secretions Sampling
3.2. Reproducibility of Secretions Sampling
3.3. Laboratory Staining Techniques
3.3.1. Haematoxylin and Eosin Staining (H&E)
3.3.2. Congo Red Staining
3.3.3. Gomori Methenamine Silver Staining (GMS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Bachert, C.; Cingi, C.; Dykewicz, M.S.; Hellings, P.W.; Naclerio, R.M.; Schleimer, R.P.; Ledford, D. Endotypes and phenotypes of chronic rhinosinusitis: A PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2013, 131, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, T.; Sakashita, M.; Haruna, T.; Asaka, D.; Takeno, S.; Ikeda, H.; Nakayama, T.; Seki, N.; Ito, S.; Murata, J.; et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: The JESREC Study. Allergy 2015, 70, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Snidvongs, K.; Lam, M.; Sacks, R.; Earls, P.; Kalish, L.; Phillips, P.S.; Pratt, E.; Harvey, R.J. Structured histopathology profiling of chronic rhinosinusitis in routine practice. Int. Forum Allergy Rhinol. 2012, 2, 376–385. [Google Scholar] [CrossRef]
- Ba, N.J.D.; Marino, M.J.; Zarka, M.A.; Lal, D. Histopathological characteristics of surgical tissue from primary vs recurrent chronic rhinosinusitis with nasal polyposis patients. Laryngoscope 2020, 5, 5–10. [Google Scholar] [CrossRef]
- Kuhar, H.N.; Tajudeen, B.A.; Mahdavinia, M.; Gattuso, P.; Ghai, R.; Batra, P.S. Inflammatory infiltrate and mucosal remodeling in chronic rhinosinusitis with and without polyps: Structured histopathologic analysis. Int. Forum Allergy Rhinol. 2017, 7, 679–689. [Google Scholar] [CrossRef]
- Tajudeen, B.A.; Ganti, A.; Ba, H.N.K.; Mahdavinia, M.; Heilingoetter, A.; Gattuso, P.; Ghai, R.; Batra, P.S. The presence of eosinophil aggregates correlates with increased postoperative prednisone requirement. Laryngoscope 2019, 129, 794–799. [Google Scholar] [CrossRef]
- Unsal, A.A.; Reyes, C.; Biddinger, P.; Kountakis, S.E. Eosinophilic Mucin: A Predictor for Disease Severity in Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2021, 35, 187–194. [Google Scholar] [CrossRef]
- Vlaminck, S.; Vauterin, T.; Hellings, P.W.; Jorissen, M.; Acke, F.; Van Cauwenberge, P.; Bachert, C.; Gevaert, P. The Importance of Local Eosinophilia in the Surgical Outcome of Chronic Rhinosinusitis: A 3-Year Prospective Observational Study. Am. J. Rhinol. Allergy 2014, 28, 260–264. [Google Scholar] [CrossRef]
- Vlaminck, S.; Acke, F.; Prokopakis, E.; Speleman, K.; Kawauchi, H.; van Cutsem, J.-C.; Hellings, P.W.; Jorissen, M.; Seys, S.; Bachert, C.; et al. Surgery in Nasal Polyp Patients: Outcome After a Minimum Observation of 10 Years. Am. J. Rhinol. Allergy 2021, 35, 449–457. [Google Scholar] [CrossRef]
- Nair, P.; Goodwin, S.; Hargreave, F.E. Reproducibility, Validity, and Responsiveness of Cell Counts in Blown Nasal Secretions. Allergy Rhinol. 2011, 2, 3–5. [Google Scholar] [CrossRef]
- Green, R.H.; Brightling, C.E.; McKenna, S.; Hargadon, B.; Parker, D.; Bradding, P.; Wardlaw, A.J.; Pavord, I.D. Asthma exacerbations and sputum eosinophil counts: A randomised controlled trial. Lancet 2002, 360, 1715–1721. [Google Scholar] [CrossRef]
- Koenderman, L.; Hassani, M.; Mukherjee, M.; Nair, P. Monitoring eosinophils to guide therapy with biologics in asthma: Does the compartment matter? Allergy 2021, 76, 1294–1297. [Google Scholar] [CrossRef]
- Miyabe, Y.; Kobayashi, Y.; Fukuchi, M.; Saga, A.; Moritoki, Y.; Saga, T.; Akuthota, P.; Ueki, S. Eosinophil-mediated inflammation in the absence of eosinophilia. Asia Pac. Allergy 2021, 11, e30. [Google Scholar] [CrossRef]
- Gelardi, M.; Iannuzzi, L.; Quaranta, N.; Landi, M.; Passalacqua, G. NASAL cytology: Practical aspects and clinical relevance. Clin. Exp. Allergy 2016, 46, 785–792. [Google Scholar] [CrossRef]
- Massey, C.J.; Del Valle, F.D.; Abuzeid, W.M.; Levy, J.M.; Mueller, S.; Levine, C.G.; Smith, S.S.; Bleier, B.S.; Ramakrishnan, V.R. Sample collection for laboratory-based study of the nasal airway and sinuses: A research compendium. Int. Forum Allergy Rhinol. 2020, 10, 303–313. [Google Scholar] [CrossRef]
- Ueki, S.; Konno, Y.; Takeda, M.; Moritoki, Y.; Hirokawa, M.; Matsuwaki, Y.; Honda, K.; Ohta, N.; Yamamoto, S.; Takagi, Y.; et al. Eosinophil extracellular trap cell death–derived DNA traps: Their presence in secretions and functional attributes. J. Allergy Clin. Immunol. 2016, 137, 258–267. [Google Scholar] [CrossRef]
- Riva, G.; Tavassoli, M.; Cravero, E.; Moresco, M.; Albera, A.; Canale, A.; Pecorari, G. Long-term evaluation of nasal polyposis recurrence: A focus on multiple relapses and nasal cytology. Am. J. Otolaryngol. 2022, 43, 103325. [Google Scholar] [CrossRef]
- Brescia, G.; Alessandrini, L.; Marioni, G. Structured histopathology for endotyping and planning rational treatment in chronic rhinosinusitis. Am. J. Otolaryngol. 2021, 42, 102795. [Google Scholar] [CrossRef]
- Fettrelet, T.; Gigon, L.; Karaulov, A.; Yousefi, S.; Simon, H.-U. The Enigma of Eosinophil Degranulation. Int. J. Mol. Sci. 2021, 22, 7091. [Google Scholar] [CrossRef]
- Kjarsgaard, M.; Adatia, A.; Bhalla, A.; LaVigne, N.; Radford, K.; Huang, C.; Mukherjee, M.; Nair, P. Underestimation of airway luminal eosinophilia by quantitative sputum cytometry. Allergy Asthma Clin. Immunol. 2021, 17, 63. [Google Scholar] [CrossRef]
- Persson, C.G.; Erjefalt, J.S. “Ultimate activation” of eosinophils in vivo: Lysis and release of clusters of free eosinophil granules (Cfegs). Thorax 1997, 52, 569–574. [Google Scholar] [CrossRef][Green Version]
- Erjefalt, J.S.; Greiff, L.; Andersson, M.; Ädelroth, E.; Jeffery, P.K.; Persson, C.G.A. Degranulation patterns of eosinophil granulocytes as determinants of eosinophil driven disease. Thorax 2001, 56, 341–344. [Google Scholar] [CrossRef]
- Persson, C.; Uller, L. Theirs But to Die and Do: Primary Lysis of Eosinophils and Free Eosinophil Granules in Asthma. Am. J. Respir. Crit. Care Med. 2014, 189, 628–633. [Google Scholar] [CrossRef]
- Ponikau, J.U.; Winter, L.A.; Kephart, G.M.; Squillace, D.L.; Hershcovitch, M.D.; Moon, S.; Sherris, D.A.; Kern, E.B.; Gleich, G.J.; Kita, H. An immunologic test for chronic rhinosinusitis based on free intranasal eosinophilic major basic protein. Int. Forum Allergy Rhinol. 2015, 5, 28–35. [Google Scholar] [CrossRef]
- Plager, U.A.; Loegering, D.A.; Checkel, J.L.; Tang, J.; Kephart, G.M.; Caffes, P.L.; Adolphson, C.R.; Ohnuki, L.E.; Gleich, G.J. Major basic protein homolog (MBP2): A specific human eosinophil marker. J. Immunol. 2006, 177, 7340–7345. [Google Scholar] [CrossRef]
- Aegerter, H.; Smole, U.; Heyndrickx, I.; Verstraete, K.; Savvides, S.N.; Hammad, H.; Lambrecht, B.N. Charcot–Leyden crystals and other protein crystals driving type 2 immunity and allergy. Curr. Opin. Immunol. 2021, 72, 72–78. [Google Scholar] [CrossRef]
- Ueki, S.; Miyabe, Y.; Yamamoto, Y.; Fukuchi, M.; Hirokawa, M.; Spencer, L.A.; Weller, P.F. Charcot-Leyden Crystals in Eosinophilic Inflammation: Active Cytolysis Leads to Crystal Formation. Curr. Allergy Asthma Rep. 2019, 19, 35. [Google Scholar] [CrossRef]
- Delemarre, T.; Holtappels, G.; De Ruyck, N.; Zhang, N.; Nauwynck, H.; Bachert, C.; Gevaert, E. A substantial neutrophilic inflammation as regular part of severe type 2 chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2021, 147, 179–188.e2. [Google Scholar] [CrossRef]
- Hopkins, C.; Andrews, P.; Holy, C. Does time to endoscopic sinus surgery impact outcomes in chronic rhinosinusitis? Retrospective analysis using the UK clinical practice research data. Rhinol. J. 2015, 53, 18–24. [Google Scholar] [CrossRef]
- Alsharif, S.; Jonstam, K.; Van Zele, T.; Gevaert, P.; Mlt, G.H.; Bachert, C. Endoscopic Sinus Surgery for Type-2 CRS wNP: An Endotype-Based Retrospective Study. Laryngoscope 2019, 129, 1286–1292. [Google Scholar] [CrossRef] [PubMed]



| T1 Inflammation | T2 Inflammation | |
|---|---|---|
| Number of patients | 12 | 41 |
| Age (mean, range) | 51.6 y (18–66 y) | 55.6 y (33–86 y) |
| Gender | 4 F–8 M | 15 F–26 M |
| Phenotype | 12 CRSsNP | 1 CRPsNP–40 CRSwNP |
| Allergy | 2/12 (16.7%) | 21/41 (51.2%) |
| Asthma | 1/12 (8.3%) | 26/41 (63.4%) |
| Preoperative Nasal Blown Secretions | Preoperative Aspiration of Nasal Aspiration | Surgical Tissue | |
|---|---|---|---|
| <10 eos/HPF | 28 (68.3%) | 6 (14.6%) | 0 (0.0%) |
| 10–49 eos/HPF | 6 (14.6%) | 15 (36.6%) | 5 (12.2%) |
| 50–99 eos/HPF | 6 (14.6%) | 13 (31.7%) | 19 (46.3%) |
| >99 eos/HPF | 1 (2.4%) | 7 (7.1%) | 17 (41.5%) |
| NBS Day 0 | NBS Day 3–4 | ANS Day 0 | ANS Day 3–4 | ||
|---|---|---|---|---|---|
| T2 EFRS patients | Eosinophils | 4 (40%) | 4 (40%) | 4 (40%) | 4 (40%) |
| Neutrophils | 3 (30%) | 4 (40%) | 4 (40%) | 4 (40%) | |
| Controls | Eosinophils | 2 (20%) | 0 (0%) | 3 (30%) | 1 (10%) |
| Neutrophils | 7 (70%) | 8 (80%) | 4 (40%) | 4 (40%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlaminck, S.; Prokopakis, E.; Kawauchi, H.; Haspeslagh, M.; Van Huysse, J.; Simões, J.; Acke, F.; Gevaert, P. Proposal for Structured Histopathology of Nasal Secretions for Endotyping Chronic Rhinosinusitis: An Exploratory Study. Allergies 2022, 2, 128-137. https://doi.org/10.3390/allergies2040012
Vlaminck S, Prokopakis E, Kawauchi H, Haspeslagh M, Van Huysse J, Simões J, Acke F, Gevaert P. Proposal for Structured Histopathology of Nasal Secretions for Endotyping Chronic Rhinosinusitis: An Exploratory Study. Allergies. 2022; 2(4):128-137. https://doi.org/10.3390/allergies2040012
Chicago/Turabian StyleVlaminck, Stephan, Emmanuel Prokopakis, Hideyuki Kawauchi, Marc Haspeslagh, Jacques Van Huysse, João Simões, Frederic Acke, and Philippe Gevaert. 2022. "Proposal for Structured Histopathology of Nasal Secretions for Endotyping Chronic Rhinosinusitis: An Exploratory Study" Allergies 2, no. 4: 128-137. https://doi.org/10.3390/allergies2040012
APA StyleVlaminck, S., Prokopakis, E., Kawauchi, H., Haspeslagh, M., Van Huysse, J., Simões, J., Acke, F., & Gevaert, P. (2022). Proposal for Structured Histopathology of Nasal Secretions for Endotyping Chronic Rhinosinusitis: An Exploratory Study. Allergies, 2(4), 128-137. https://doi.org/10.3390/allergies2040012








