Multiplex Specific IgE Profiling in Neonatal Stool of Preterms Predicts IgE-Mediated Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Ethics
2.2. Fecal Sample Preparation
2.3. Allergen Multiplex Detection of Fecal Specific IgE
2.4. Western Blot Detection of sIgE
2.5. Eosinophil Activity Assessment
2.6. Data Expression and Analysis
3. Results
3.1. Demographic and Clinical Characterization of the Study Population
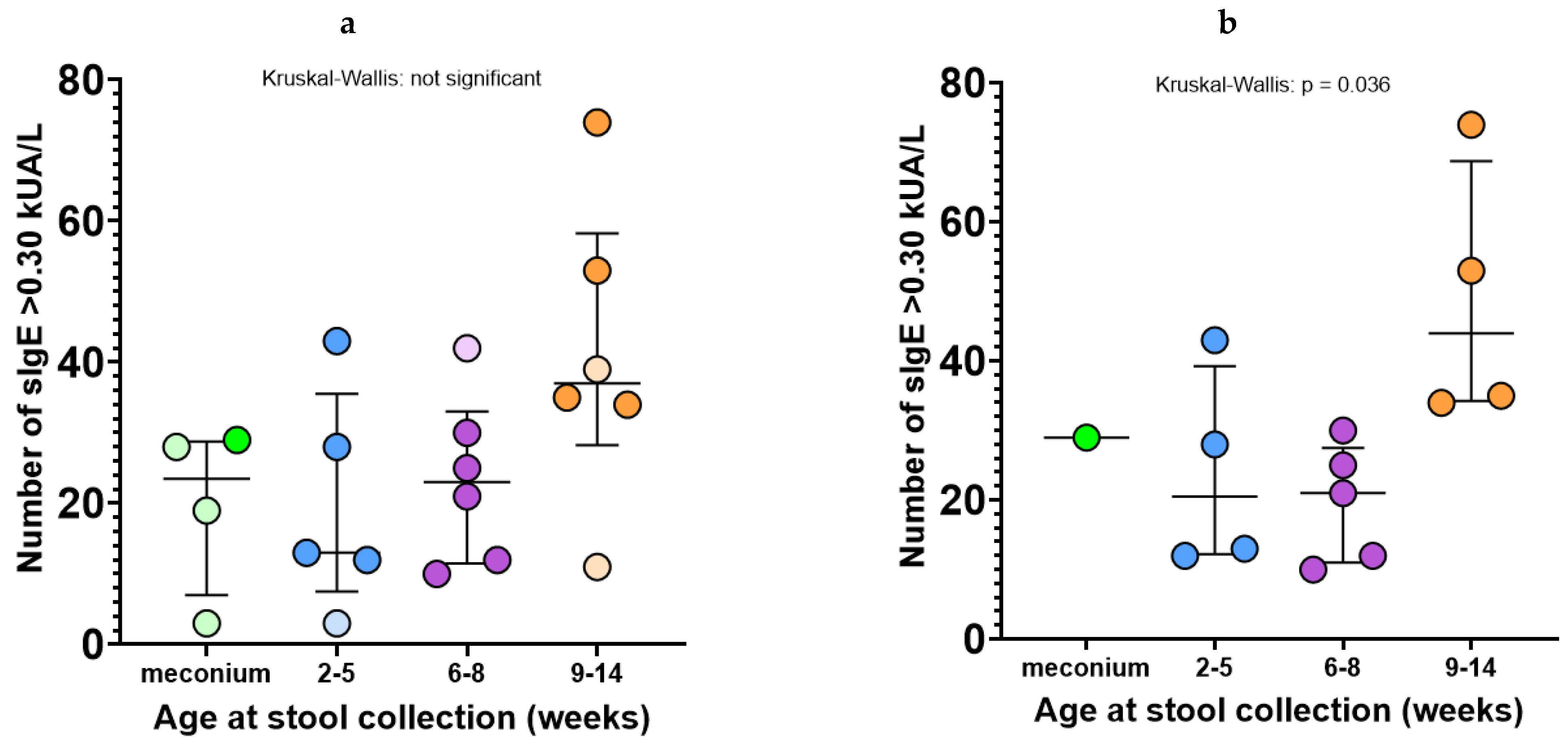
3.2. Association of Fecal sIgE Detection with Age and the Presence of Doctor-Diagnosed Atopic Conditions at the Age of 1 Year
3.3. Fecal sIgE Detection as a Function of Allergen Families and Atopic Conditions at the Age of 1 Year
3.4. Quantitative Analysis of Neonatal Fecal sIgE Reactivity
3.5. Identification of Cow’s Milk Protein sIgE by Western Blot in Stool Samples
3.6. Fecal EDN Detection and Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Platts-Mills, T.A.E. The allergy epidemics: 1870–2010. J. Allergy Clin. Immunol. 2015, 136, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Hornef, M.W.; Torow, N. ‘Layered immunity’ and the ‘neonatal window of opportunity’—Timed succession of non-redundant phases to establish mucosal host-microbial homeostasis after birth. Immunology 2020, 159, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Sereme, Y.; Michel, M.; Mezouar, S.; Guindo, C.O.; Kaba, L.; Grine, G.; Mura, T.; Mege, J.L.; Tran, T.A.; Corbeau, P.; et al. A Non-Invasive Neonatal Signature Predicts Later Development of Atopic Diseases. J. Clin. Med. 2022, 11, 2749. [Google Scholar] [CrossRef] [PubMed]
- Szépfalusi, Z.; Pichler, J.; Elsässer, S.; van Duren, K.; Ebner, C.; Bernaschek, G.; Urbanek, R. Transplacental priming of the human immune system with environmental allergens can occur early in gestation. J. Allergy Clin. Immunol. 2000, 106, 530–536. [Google Scholar] [CrossRef]
- Msallam, R.; Balla, J.; Rathore, A.P.S.; Kared, H.; Malleret, B.; Saron, W.A.A.; Liu, Z.; Hang, J.W.; Dutertre, C.A.; Larbi, A.; et al. Fetal mast cells mediate postnatal allergic responses dependent on maternal IgE. Science 2020, 370, 941–950. [Google Scholar] [CrossRef]
- Sereme, Y.; Guindo, C.O.; Filleron, A.; Corbeau, P.; Tran, T.A.; Drancourt, M.; Vitte, J.; Grine, G. Meconial Methanobrevibacter smithii suggests intrauterine methanogen colonization in preterm neonates. Curr. Res. Microb. Sci. 2021, 2, 100034. [Google Scholar]
- Sonnenschein-van der Voort, A.M.; Arends, L.R.; de Jongste, J.C.; Annesi-Maesano, I.; Arshad, S.H.; Barros, H.; Basterrechea, M.; Bisgaard, H.; Chatzi, L.; Corpeleijn, E.; et al. Preterm birth, infant weight gain, and childhood asthma risk: A meta-analysis of 147,000 European children. J. Allergy Clin. Immunol. 2014, 133, 1317–1329. [Google Scholar] [CrossRef]
- Mitselou, N.; Hallberg, J.; Stephansson, O.; Almqvist, C.; Melén, E.; Ludvigsson, J.F. Cesarean delivery, preterm birth, and risk of food allergy: Nationwide Swedish cohort study of more than 1 million children. J. Allergy Clin. Immunol. 2018, 142, 1510–1514. [Google Scholar] [CrossRef]
- Haataja, P.; Korhonen, P.; Ojala, R.; Hirvonen, M.; Paassilta, M.; Gissler, M.; Luukkaala, T.; Tammela, O. Asthma and atopic dermatitis in children born moderately and late preterm. Eur. J. Pediatr. 2016, 175, 799–808. [Google Scholar] [CrossRef]
- Wickman, M.; Lupinek, C.; Andersson, N.; Belgrave, D.; Asarnoj, A.; Benet, M.; Pinart, M.; Wieser, S.; Garcia-Aymerich, J.; Baar, A.; et al. Detection of IgE Reactivity to a Handful of Allergen Molecules in Early Childhood Predicts Respiratory Allergy in Adolescence. EBioMedicine 2017, 26, 91–99. [Google Scholar] [CrossRef]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gomez, R.M.; et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ J. 2020, 13, 100080. [Google Scholar] [CrossRef]
- Nunes, M.P.O.; van Tilburg, M.F.; Tramontina Florean, E.O.P.; Guedes, M.I.F. Detection of serum and salivary IgE and IgG1 immunoglobulins specific for diagnosis of food allergy. PLoS ONE 2019, 14, e0214745. [Google Scholar] [CrossRef]
- Leonardi, A.; Borghesan, F.; Faggian, D.; Plebani, M. Microarray-based IgE detection in tears of patients with vernal keratoconjunctivitis. Pediatr. Allergy Immunol. 2015, 26, 641–645. [Google Scholar] [CrossRef]
- Aghayan-Ugurluoglu, R.; Ball, T.; Vrtala, S.; Schweiger, C.; Kraft, D.; Valenta, R. Dissociation of allergen-specific IgE and IgA responses in sera and tears of pollen-allergic patients: A study performed with purified recombinant pollen allergens. J. Allergy Clin. Immunol. 2000, 105, 803–813. [Google Scholar] [CrossRef]
- Chawes, B.L.; Wolsk, H.M.; Carlsson, C.J.; Rasmussen, M.A.; Følsgaard, N.; Stokholm, J.; Bonnelykke, K.; Brix, S.; Schoos, M.A.M.; Bisgaard, H. Neonatal airway immune profiles and asthma and allergy endpoints in childhood. Allergy 2021, 76, 3713–3722. [Google Scholar] [CrossRef]
- Kolmannskog, S.; Haneberg, B.; Marhaug, G.; Bolle, R. Immunoglobulin E in Extracts of Feces from Children. Int. Arch. Allergy Immunol. 1984, 74, 50–54. [Google Scholar] [CrossRef]
- Hoh, R.A.; Joshi, S.A.; Lee, J.-Y.; Martin, B.A.; Varma, S.; Kwok, S.; Nielsen, S.C.A.; Nejad, P.; Haraguchi, E.; Dixit, P.S.; et al. Origins and clonal convergence of gastrointestinal IgE + B cells in human peanut allergy. Sci. Immunol. 2020, 5, eaay4209. [Google Scholar] [CrossRef]
- Heffler, E.; Puggioni, F.; Peveri, S.; Montagni, M.; Canonica, G.W.; Melioli, G. Extended IgE profile based on an allergen macroarray: A novel tool for precision medicine in allergy diagnosis. World Allergy Organ. J. 2018, 11, 7. [Google Scholar] [CrossRef]
- IUIS/WHO Allergen Nomenclature. Available online: http://allergen.org (accessed on 3 January 2023).
- Daffos, F.; Grangeot-Keros, L.; Lebon, P.; Forestier, F.; Pavlovsky, M.C.; Chartier, M.; Pillot, J. Prenatal diagnosis of congenital rubella. Lancet 1984, 324, 1–3. [Google Scholar] [CrossRef]
- Casas, R.; Björkstén, B. Detection of Fel d 1-immunoglobulin G immune complexes in cord blood and sera from allergic and non-allergic mothers: Fel d 1-IgG immune complexes in cord blood and sera. Pediatr. Allergy Immunol. 2001, 12, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Starkl, P.; Gaudenzio, N.; Marichal, T.; Reber, L.L.; Sibilano, R.; Watzenboeck, M.L.; Fontaine, F.; Mueller, A.C.; Tsai, M.; Knapp, S.; et al. IgE antibodies increase honeybee venom responsiveness and detoxification efficiency of mast cells. Allergy 2022, 77, 499–512. [Google Scholar] [CrossRef]
- Starkl, P.; Watzenboeck, M.L.; Popov, L.M.; Zahalka, S.; Hladik, A.; Lakovits, K.; Radhouani, M.; Haschemi, A.; Marichal, T.; Reber, L.L.; et al. IgE Effector Mechanisms, in Concert with Mast Cells, Contribute to Acquired Host Defense against Staphylococcus aureus. Immunity 2020, 53, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Roca, M.; Donat, E.; Rodriguez Varela, A.; Carvajal, E.; Cano, F.; Armisen, A.; Ekoff, H.; Cañada-Martínez, A.J.; Rydell, N.; Ribes-Koninckx, C. Fecal Calprotectin and Eosinophil-Derived Neurotoxin in Children with Non-IgE-Mediated Cow’s Milk Protein Allergy. J. Clin. Med. 2021, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Vidova, V.; Benesova, E.; Klanova, J.; Thon, V.; Spacil, Z. Simultaneous quantitative profiling of clinically relevant immune markers in neonatal stool swabs to reveal inflammation. Sci. Rep. 2021, 11, 10222. [Google Scholar] [CrossRef]






| Condition at Age 1 Year | n | sIgE+ Allergens (%) | sIgE− Allergens (%) | p | Level of Significance | RR (CI 95%) | OR (CI 95%) | |
|---|---|---|---|---|---|---|---|---|
| CMA/meconium samples | CMA+ | 1 | 29 (16%) | 157 (84%) | 0.014 | * | 1.56 (1.11–2.10) | 1.88 (1.13–3.05) |
| CMA− | 3 | 50 (9%) | 508 (91%) | |||||
| Any IgE-mediated disease | AA+ | 13 | 390 (16%) | 2028 (84%) | 0.027 | * | 1.06 (1.01–1.11) | 1.3 (1.03–1.67) |
| AA− | 4 | 95 (13%) | 649 (87%) | |||||
| CMA | CMA+ | 8 | 298 (20%) | 1190 (80%) | <0.0001 | **** | 1.38 (1.27–1.50) | 1.99 (1.64–2.43) |
| CMA− | 9 | 187 (11%) | 1487 (89%) | |||||
| Asthma | Asthma+ | 8 | 249 (17%) | 1239 (83%) | 0.043 | * | 1.11 (1.01–1.22) | 1.23 (1.01–1.49) |
| Asthma− | 9 | 236 (14%) | 1438 (86%) | |||||
| AD | AD+ | 3 | 93 (17%) | 465 (83%) | ns | 0 | 1.10 (0.90–1.34) | 1.13 (0.88–1.45) |
| AD− | 14 | 392 (15%) | 2212 (85%) |
| Condition at Age 1 Year | sIgE+ Allergens (%) | Total Group Allergen Count | p | Level of Significance | RR (CI 95%) | OR (CI 95%) | |
|---|---|---|---|---|---|---|---|
| Milk allergens (Bos d 4, Bos d 5, Bos d 8, Ovi a _milk, Cam d) | CMA+ | 10 (25%) | 40 | 0.002 | ** | 2.2 (1.4–3.1) | 14.7 (2.2–162) |
| CMA− | 1 (2%) | 45 | |||||
| Kiwifruit marker allergens (Act d 1, Act d 5) | CMA+ | 6 (38%) | 16 | 0.03 | * | 2.4 (1.2–4.3) | 10.8 (1.2–129) |
| CMA− | 1 (5.3%) | 18 | |||||
| Storage proteins | CMA+ | 29 (28%) | 104 | <0.0001 | *** | 1.9 (1.5–2.4) | 5.3 (2.2–11.4) |
| CMA− | 8 (7%) | 117 | |||||
| Indoor airborne allergens | CMA+ | 32 (19%) | 168 | 0.0007 | *** | 1.6 (1.3–2.0) | 3.2 (1.6–6.5) |
| CMA− | 13 (7%) | 189 | |||||
| Indoor and outdoor airborne allergens | CMA+ | 52 (19%) | 280 | 0.01 | * | 1.3 (1.1–1.6) | 1.8 (1.1–2.9) |
| CMA− | 35 (11%) | 315 | |||||
| Indoor and outdoor airborne allergens | Asthma+ | 51 (18%) | 280 | 0.003 | ** | 1.4 (1.1–1.7) | 2.1 (1.3–3.4) |
| Asthma− | 30 (10%) | 315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sereme, Y.; Michel, M.; Mezouar, S.; Orain, N.; Cezar, R.; Tu Anh, T.; Corbeau, P.; Filleron, A.; Vitte, J. Multiplex Specific IgE Profiling in Neonatal Stool of Preterms Predicts IgE-Mediated Disease. Allergies 2023, 3, 58-71. https://doi.org/10.3390/allergies3010005
Sereme Y, Michel M, Mezouar S, Orain N, Cezar R, Tu Anh T, Corbeau P, Filleron A, Vitte J. Multiplex Specific IgE Profiling in Neonatal Stool of Preterms Predicts IgE-Mediated Disease. Allergies. 2023; 3(1):58-71. https://doi.org/10.3390/allergies3010005
Chicago/Turabian StyleSereme, Youssouf, Moïse Michel, Soraya Mezouar, Nicolas Orain, Renaud Cezar, Tran Tu Anh, Pierre Corbeau, Anne Filleron, and Joana Vitte. 2023. "Multiplex Specific IgE Profiling in Neonatal Stool of Preterms Predicts IgE-Mediated Disease" Allergies 3, no. 1: 58-71. https://doi.org/10.3390/allergies3010005
APA StyleSereme, Y., Michel, M., Mezouar, S., Orain, N., Cezar, R., Tu Anh, T., Corbeau, P., Filleron, A., & Vitte, J. (2023). Multiplex Specific IgE Profiling in Neonatal Stool of Preterms Predicts IgE-Mediated Disease. Allergies, 3(1), 58-71. https://doi.org/10.3390/allergies3010005






