Mechanisms and Comparative Treatments of Allergic Rhinitis including Phototherapy
Abstract
:1. Introduction
2. Background to the Mechanism of Allergic Rhinitis
2.1. Symptoms of Allergic Rhinitis
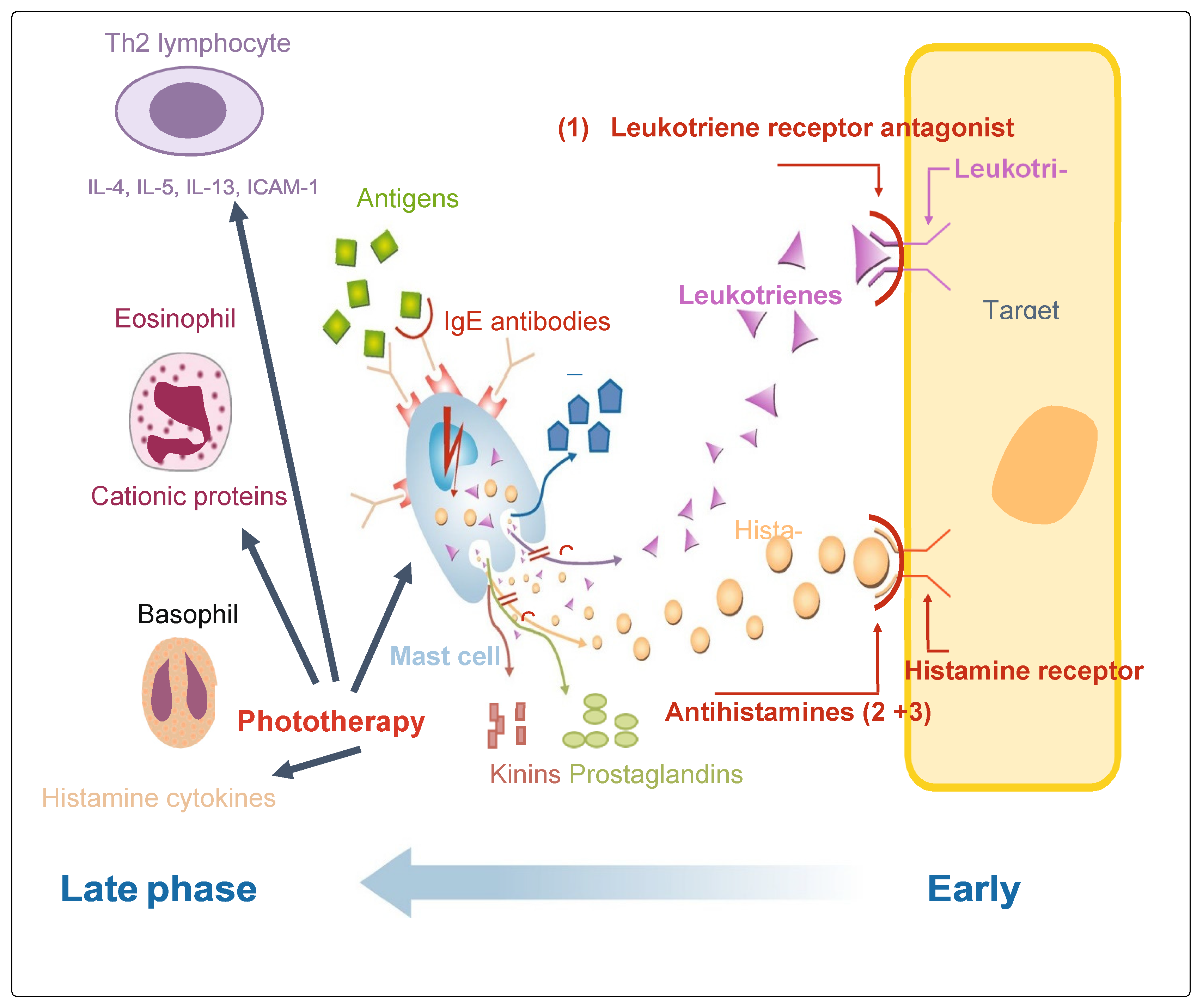
2.2. Potential for Phototherapy-Mediated Early and Late Phase Allergic Responses
2.3. Characteristics of Early Phase Responses
2.4. Characteristics of Late Phase Responses
2.5. AR Treatment According to ARIA (Allergic Rhinitis and Its Impact on Asthma) Guidelines
3. Phototherapy Treatment of AR
3.1. Phototherapy Treatment of AR Using Visible Wavelengths
3.2. Phototherapy Effects of Red and Infrared Wavelengths
3.3. Evidence for Phototherapy as a Successful Treatment for Allergic Rhinitis
3.4. Safety of Visible and NIR Wavelengths Used in Phototherapy
3.5. Use of Phototherapy as a Reliever of Symptoms in Allergic Rhinitis
4. Treatments for Allergic Rhinitis Using Pharmacological Intervention
4.1. Approaches to the Use of Allergic Rhinitis Pharmacotherapies
4.2. Leukotriene Receptors (LTRAs)
4.3. Oral Antihistamines (OAHs)
4.4. Intranasal Antihistamines (INAH)
4.5. Intranasal Corticosteroids (INSs)
5. Other Treatments for Allergic Rhinitis Using Non-Pharmacological Interventions
5.1. Avoidance
5.2. Pollen Barriers and Non-Pharmacotherapies
6. Allergen Immunotherapy (AIT)
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bousquet, J.; van Cauwenberge, P.; Khaltaev, N.; Aria Workshop Group; World Health Organization. Allergic Rhinitis and Its Impact on Asthma. J. Allergy Clin. Immunol. 2001, 108, S147–S334. [Google Scholar] [CrossRef]
- D’Amato, G.; Spieksma, F.T.M.; Liccardi, G.; Jager, S.; Russo, M.; Kontou-Fili, K.; Nikkels, H.; Wuthrich, B.; Bonini, S. Pollen related allergy in Europe. Allergy 1998, 53, 567–578. [Google Scholar] [CrossRef]
- North, M.L.; Ellis, A.K. The role of epigenetics in the developmental origins of allergic disease. Ann. Allergy Asthma Immunol. 2011, 106, 355–361. [Google Scholar] [CrossRef]
- Moodycliffe, A.M.; Kimber, I.; Norval, M. The effect of ultraviolet B irradiation and urocanic acid isomers on dendritic cell migration. Immunology 1992, 77, 394–399. [Google Scholar]
- Hollingsworth, J.W.; Maruoka, S.; Boon, K.; Garantziotis, S.; Li, Z.; Tomfohr, J.; Bailey, N.; Potts, E.N.; Whitehead, G.; Brass, D.M.; et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J. Clin. Investig. 2008, 118, 3462–3469. [Google Scholar] [CrossRef]
- Silva-Filho, J.L.; Caruso-Neves, C.; Pinheiro, A.A.S. IL-4: An important cytokine in determining the fate of T cells. Biophys. Rev. 2014, 6, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.; Candotti, F.; Smith, S.; O’Shea, J.J.; Weinberg, K.; Johnston, J.A. Interleukin-4 Signalling in B Lymphocytes from Patients with X-linked Severe Combined Immunodeficiency Open Access. Cell Biol. Metab. 1997, 272, 7314–7319. [Google Scholar] [CrossRef]
- Satitsuksanoa, P.; Daanje, M.; Akdis, M.; Boyd, S.D.; van de Veen, W. Allergy Biology and dynamics of B cells in the context of IgE-mediated food allergy. Allergy 2021, 76, 1707–1717. [Google Scholar] [CrossRef]
- Naclerio, R.M. Allergic rhinitis. N. Engl. J. Med. 1991, 325, 860–869. [Google Scholar] [PubMed]
- Bradding, P.; Feather, I.H.; Wilson, S.; Bardin, P.; Heusser, C.H.; Holgate, S.T.; Howarth, P.H. Immuno-localization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL- 4, IL-5, and IL-6 in human allergic mucosal inflammation. J. Immunol. 1993, 151, 3853–3865. [Google Scholar] [CrossRef] [PubMed]
- Pawankar, R.U.; Okuda, M.; Hasegawa, S.; Suzuki, K.; Yssel, H.; Okubo, K.; Okumura, K.; Ra, C. Interleukin-13 expression in the nasal mucosa of perennial allergic rhinitis. Am. J. Respir. Crit. Care Med. 1995, 152, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xia, Y.; Nguyen, A.; Lai, Y.H.; Feng, L.; Mosmann, T.R.; Lo, D. Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J. Immunol. 1999, 162, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Miyamasu, M.; Imanishi, M.; Yamada, H.; Nakajima, T.; Yamaguchi, M.; Fujisawa, T.; Pawankar, R.; Sano, Y.; Ohta, K.; et al. Inducible expression of a Th2- type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J. Immunol. 2000, 165, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Ozu, C.; Pawankar, R.; Takizawa, R.; Yamagishi, S.; Yagi, T. Regulation of mast cell migration into the allergic nasal epithelium by RANTES and not SCF. J. Allergy Clin. Immunol. 2004, 113, S28. [Google Scholar] [CrossRef]
- Lilly, C.M.; Nakamura, H.; Kesselman, H.; Nagler-Anderson, C.; Asano, K.; Garcia-Zepeda, E.A.; Rothenberg, M.E.; Drazen, J.M.; Luster, A.D. Expression of eotaxin by human lung epithelial cells: Induction by cytokines and inhibition by glucocorticoids. J. Clin. Investig. 1997, 99, 1767–1773. [Google Scholar] [CrossRef]
- Pawankar, R.; Ra, C. Heterogeneity of mast cells and T cells in the nasal mucosa. J. Allergy Clin. Immunol. 1996, 98, S248–S262. [Google Scholar] [CrossRef] [PubMed]
- Bjermer, L.; Westman, M.; Holmström, M.; Wickman, M.C. The complex pathophysiology of allergic rhinitis: Scientific rationale for the development of an alternative treatment option. Allergy Asthma Clin. Immunol. 2019, 15, 24. [Google Scholar] [CrossRef]
- Klimek, L.; Bachert, C.; Pfaar, O.; Becker, S.; Bieber, T.; Brehler, R.; Buhl, R.; Casper, I.; Chaker, A.; Czech, W.; et al. ARIA guideline 2019: Treatment of allergic rhinitis in the German health system. Allergo J. Int. 2019, 28, 255–276. [Google Scholar] [CrossRef]
- Sherman, R.E.; Anderson, S.A.; Dal Pan, G.J.; Gray, G.W.; Gross, T.; Hunter, N.L.; LaVange, L.; Marinac-Dabic, D.; Marks, P.W.; Robb, M.A.; et al. Real-world evidence—What is it and what can it tell us? N. Engl. J. Med. 2016, 375, 2293–2297. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Wallace, D.; Dykewicz, M.; Shneyer, L. Minimal clinically important difference (MCID) in allergic rhinitis: Agency for Healthcare Research and quality or anchor-based thresholds? J. Allergy Clin. Immunol. Pract. 2016, 4, 682–688. [Google Scholar] [CrossRef]
- Oyinlola, J.O.; Campbell, J.; Kousoulis, A.A. Isreal world evidence influencing practice? Asystematic review of CPRD research in NICE guidances. BMC Health Serv. Res. 2016, 16, 299. [Google Scholar] [CrossRef]
- Koreck, A.I.; Csoma, Z.; Bodai, L.; Ignacz, F.; Kenderessy, A.S.; Kadocsa, E.; Szabo, G.; Bor, Z.; Erdei, A.; Szony, B.; et al. Rhinophototherapy: A new therapeutic tool for the management of allergic rhinitis. Allergy Clin. Immunol. 2005, 115, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Hallen, S.; Oliveberg, M.; Brzezinski, P. Light-induced Structural Changes in Cytochrome c Oxidase. Measurements of Electrogenic Events and Absorbance Changes. FEBS Lett. 1993, 318, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Koreck, A.; Csoma, Z.; Ignácz, F.; Bodai, L.; Kadocsa, E.; Szabó, G.; Bor, Z.; Nékám, K.; Dobozy., A.; Kemény, L.; et al. Intranasal phototherapy for the treatment of allergic rhinitis. Orvosi Hetil. 2005, 146, 965–969. [Google Scholar] [CrossRef]
- Kemeny, L.; Koreck, A. Ultraviolet light phototherapy for allergic rhinitis. J. Photochem. Photobiol. B 2007, 87, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Csoma, Z.; Koreck, A.; Ignacz, F.; Bor, Z.; Szabo, G.; Bodai, L.; Dobozy, A.; Kemeny, L.J. PUVA treatment of the nasal cavity improves the clinical symptoms of allergic rhinitis and inhibits the immediate-type hypersensitivity reaction in the skin. Photochem. Photobiol. B 2006, 83, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Cingi, C.; Yaz, A.; Cakli, H.; Ozudogru, E.; Kecik, C.; Bal, C. The effects of phototherapy on quality of life in allergic rhinitis cases. Eur. Arch. Otorhinolaryngol. 2009, 266, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Norihero, I.; Katsuura, T.; Kikuchi, Y.; Miwa, E. Effect of far infrared radiation on forearm skin blood flow. Ann. Physiol. Anthropol. 1987, 6, 31–32. [Google Scholar]
- Hu, K.-H.; Li, W.-T. Clinical Effects of Far-Infrared Therapy in Patients with Allergic Rhinitis. In Proceedings of the 29th Annual International Conference of the IEEE EMBS, Lyon, France, 23–26 August 2007; pp. 1479–1482. [Google Scholar]
- Kennedy, R.; Robertson, L. Study on the effect of phototherapy for inhibition of symptoms associated with allergic rhinitis. Eur. Ann. Allergy Clin. Immunol. 2020, 52, 66–73. [Google Scholar] [CrossRef]
- Hu, K.-H.; Li, W.-T. Phototherapy for the Treatment of Allergic Rhinitis; Allergic Rhinitis; Kowalski, M., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0288-5. Available online: http://www.intechopen.com/books/allergic-rhinitis/photoherapy-for-allergic-rhinitis (accessed on 30 September 2023).
- Brehmer, D. Endonasal phototherapy with Rhinolight for the treatment of allergic rhinitis. Expert. Rev. Med. Devices 2010, 7, 21–26. [Google Scholar] [CrossRef]
- Neuman, I.; Finkelstein, Y. Narrow-band Red Light Phototherapy in Perennial Allergic Rhinitis and Nasal Polyposis. Ann. Allergy Asthma Immunol. 1997, 78, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Tatar, E.Ç.; Korkmaz, H.; Sürenoğlu, U.A.; Saylam, G.; Ozdek, A. Effects of rhinophototherapy on quality of life in persistant allergic rhinitis. Clin. Exp. Otorhinolaryngol. 2013, 6, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Emberlin, J.C.; Lewis, R.A. Pollen challenge study of a phototherapy device for reducing the symptoms of hay fever. Curr. Med. Res. Opin. 2009, 25, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Nakamura, Y.; Takagi, S.; Sakai, K. Effects of azelastine on the level of serum interleukin-4 and soluble CD23 antigen in the treatment of nasal allergy. Arzneimittelforschung 1998, 48, 1143–1147. [Google Scholar]
- Sliney, D.; Wolbarsht, M. Optical Radiation Hazards to the Skin. In Safety with Lasers and Other Optical Sources; Springer: Boston, MA, USA, 1980. [Google Scholar] [CrossRef]
- Bozkurt, A.; Onaral, B. Safety assessment of near infrared light emitting diodes for diffuse optical measurements. Biomed. Eng. Online 2004, 3, 9. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Fornari, R.; Kamimura, H. Comprehensive Semiconductor Science and Technology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 154–167, ISBN 9780444531438, eBook ISBN 9780080932286. [Google Scholar]
- Kang, J.-I.; Min, K.-J.; Lee, D.-H. Intranasal Phototherapy for Allergic Rhinitis: A systematic review. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2020, 33, 55–73. [Google Scholar] [CrossRef]
- Bousquet, J.; Van Cauwenberge, P.; Bachert, C.; Canonica, G.W.; Demoly, P.; Durham, S.R.; Fokkens, W.; Lockey, R.J.; Meltzer, E.O.; Mullol, J.; et al. Requirements for medications commonly used in the treatment of allergic rhinitis. European Academy of Allergy and Clinical Immunology (EAACI), Allergic Rhinitis and its Impact on Asthma. Allergy 2003, 58, 165–263. [Google Scholar] [CrossRef] [PubMed]
- Kurowski, M.; Kuna, P.; Górski, P. Montelukast plus cetirizine in the prophylactic treatment of seasonal allergic rhinitis: Influence on clinical symptoms and nasal allergic inflammation. Allergy 2004, 59, 280–288. [Google Scholar] [CrossRef]
- Akhouri, S.; House, S.A. Allergic Rhinitis. [Updated 16 July 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK538186/ (accessed on 30 September 2023).
- Britten, N.; Ukoumunne, O.C.; Boulton, M.G. Patients’ attitudes to medicines and expectations for prescriptions. Health Expect. 2002, 5, 256–269. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Gleason, M.M.; Togias, A. Cysteinyl leukotrienes multi-functional mediators in allergic rhinitis. Clin. Exp. Allergy. 2006, 36, 689–703. [Google Scholar] [CrossRef]
- Ramires, R.; Caiaffa, M.F.; Tursi, A.; Haeggstrom, J.Z.; Macchia, L. Novel inhibitory effect on 5-lipoxygenase activity by the anti-asthma drug montelukast. Biochem. Biophys. Res. Commun. 2004, 324, 815–821. [Google Scholar] [CrossRef]
- Anderson, R.; Theron, A.J.; Gravett, C.M.; Steel, H.C.; Tintinger, G.R.; Feldman, C. Montelukast inhibits neutrophil pro-inflammatory activity by a cyclic AMP-dependent mechanism. Br. J. Pharmacol. 2009, 156, 105–115. [Google Scholar] [CrossRef]
- Woszczek, G.; Chen, L.Y.; Alsaaty, S.; Nagineni, S.; Shelhamer, J.H. Concentration-dependent noncysteinyl leukotriene type 1 receptormediated inhibitory activity of leukotriene receptor antagonists. J. Immunol. 2010, 184, 2219–2225. [Google Scholar] [CrossRef]
- Robinson, A.J.; Kashanin, D.; O’Dowd, F.; Williams, V.; Walsh, G.M. Montelukast inhibition of resting and GM-CSF-stimulated eosinophil adhesion to VCAM-1 under flow conditions appears inde-pendent of cysLT(1)R antagonism. J. Leukoc. Biol. 2008, 83, 1522–1529. [Google Scholar] [CrossRef]
- Meliton, A.Y.; Munoz, N.M.; Leff, A.R. Blockade of avidity and focal clustering of beta 2-integrin by cysteinyl leukotriene antagonism attenuates eosinophil adhesion. J. Allergy Clin. Immunol. 2007, 120, 1316–1323. [Google Scholar] [CrossRef]
- Weimer, L.K.; Gamache, D.A.; Yanni, J.M. Histamine-stimulated cytokine secretion from human conjunctival epithelial cells: Inhibition by the histamine H1 antagonist emedastine. Int. Arch. Allergy Immunol. 1998, 115, 288–293. [Google Scholar] [CrossRef]
- Dobashi, K.; Iizuka, K.; Houjou, S.; Sakai, H.; Watanabe, K.; Mori, M.; Nakazawaet, T. Effect of cetirizine on antigen-induced tracheal contraction of passively sensitized guinea pigs. Ann. Allergy Asthma Immunol. 1996, 77, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Anthes, J.C.; Gilchrest, H.; Richard, C.; Eckel, S.; Hesk, D.; West, R.E., Jr.; Williams, S.M.; Greenfeder, S.; Billah, M.; Kreutner, W.; et al. Biochemical characterization of desloratadine, a potent antagonist of the human histamine H(1) receptor. Eur. J. Pharmacol. 2002, 449, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Kanei, A.; Asano, K.; Kanai, K.; Furuta, A.; Sasaki, K.; Suzaki, H. Inhibitory action of levocetirizine on the production of eosinophil chemoattractants RANTES and eotaxin in vitro and in vivo. In Vivo 2014, 28, 657–666. [Google Scholar] [PubMed]
- Kusters, S.; Schuligoi, R.; Huttenbrink, K.B.; Rudert, J.; Wachs, A.; Szelenyi, I.; Peskar, B.A. Effects of antihistamines on leukotriene and cytokine release from dispersed nasal polyp cells. Arzneimittelforschung 2002, 52, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Canonica, G.W.; Blaiss, M. Antihistaminic, anti-inflammatory, and antiallergic properties of the nonsedating second-generation antihistamine desloratadine: A review of the evidence. World Allergy Organ. J. 2011, 4, 47–53. [Google Scholar] [CrossRef]
- Horak, F.; Zieglmayer, U.P. Azelastine nasal spray for the treatment of allergic and nonallergic rhinitis. Expert. Rev. Clin. Immunol. 2009, 5, 659–669. [Google Scholar] [CrossRef]
- Van Hoecke, H.; Vandenbulcke, L.; Van Cauwenberge, P. Histamine and leukotriene receptor antagonism in the treatment of allergic rhinitis: An update. Drugs 2007, 67, 2717–2726. [Google Scholar] [CrossRef]
- Kempuraj, D.; Huang, M.; Kandere-Grzybowska, K.; Basu, S.; Boucher, W.; Letourneau, R.; Athanassiou, A.; Theoharides, T.C. Azelastine inhibits secretion of IL-6, TNF-alpha and IL-8 as well as NF-kappaB activation and intracellular calcium ion levels in normal human mast cells. Int. Arch. Allergy Immunol. 2003, 132, 231–239. [Google Scholar] [CrossRef]
- Matsuo, S.; Takayama, S. Influence of the anti-allergic agent, azelastine, on tumor necrosis factor-alpha (TNF-alpha) secretion from cultured mouse mast cells. In Vivo 1998, 12, 481–484. [Google Scholar] [PubMed]
- Yoneda, K.; Yamamoto, T.; Ueta, E.; Osaki, T. Suppression by azelastine hydrochloride of NF-kappa B activation involved in generation of cytokines and nitric oxide. Jpn. J. Pharmacol. 1997, 73, 145–153. [Google Scholar] [CrossRef]
- Ciprandi, G.; Pronzato, C.; Passalacqua, G.; Ricca, V.; Grogen, J.; Mela, G.S. Topical azelastine reduces eosinophil activation and intercellular adhesion molecule-1 expression on nasal epithelial cells: An antiallergic activity. J. Allergy Clin. Immunol. 1996, 98 Pt 1, 1088–1096. [Google Scholar] [CrossRef]
- Wallace, D.V.; Dykewicz, M.S.; Bernstein, D.I.; Blessing-Moore, J.; Cox, L.; Khan, D.A.; Lang, D.M.; Nicklas, R.A.; Oppenheimer, J.; Portnoy, J.M.; et al. The diagnosis and management of rhinitis: An updated practice parameter. J. Allergy Clin. Immunol. 2008, 122, S1–S84. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.; Dijkstra, M.; Kleinjan, A.; Severijnen, L.A.; Boks, S.; Mulder, P.; Fokkens, W. Fluticasone propionate aqueous nasal spray reduces inflammatory cells in unchallenged allergic nasal mucosa: Effects of single allergen challenge. J. Allergy Clin. Immunol. 2001, 107, 627–633. [Google Scholar] [CrossRef]
- Alvarado-Valdes, C.A.; Blomgren, J.; Weiler, D.; Gleich, G.J.; Reed, C.E.; Field, E.A.; Wisniewski, M.E.; Pobinert, B.F. The effect of fluticasone propionate aqueous nasal spray on eosinophils and cytokines in nasal secretions of patients with ragweed allergic rhinitis. Clin. Ther. 1997, 19, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Weido, A.J.; Reece, L.M.; Alam, R.; Cook, C.K.; Sim, T.C. Intranasal fluticasone propionate inhibits recovery of chemokines and other cytokines in nasal secretions in allergen-induced rhinitis. Ann. Allergy Asthma Immunol. 1996, 77, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Rak, S.; Jacobson, M.R.; Sudderick, R.M.; Masuyama, K.; Juliusson, S.; Kay, A.B.; Hamid, Q.; Löwhagen, O.; Durham, S.R. Influence of prolonged treatment with topical corticosteroid (fluticasone propionate) on early and late phase nasal responses and cellular infiltration in the nasal mucosa after allergen challenge. Clin. Exp. Allergy 1994, 24, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Takizawa, R.; Yokoshima, K.; Okubo, K.; Okuda, M.; Yagi, T. Generation of tumor necrosis factor alpha by human nasal epithelial cells and inhibition by fluticasone propionate. Arerugi 1995, 44, 1216–1222. [Google Scholar] [PubMed]
- Ohnishi, M.; Takizawa, R.; Ohkubo, K.; Yokosima, K.; Okuda, M.; Yagi, T. Fluticasone propionate reduced the production of GM-CSF, IL-6 and IL-8 generated from cultured nasal epithelial cells. Arerugi 1994, 43, 441–447. [Google Scholar]
- Wandalsen, G.F.; Mendes, A.I.; Sole, D. Objective improvement in nasal congestion and nasal hyperreactivity with use of nasal steroids in persistent allergic rhinitis. Am. J. Rhinol. Allergy. 2010. [CrossRef]
- Tovey, E.R.; Liu-Brennan, D.; Garden, F.L.; Oliver, B.G.; Perzanowski, M.S.; Marks, G.B. Time-Based Measurement of Personal Mite Allergen Bioaerosol Exposure over 24 Hour Periods. PLoS ONE 2016, 11, e0153414. [Google Scholar] [CrossRef]
- Van Cauwenberge, P.; Bachert, C.; Passalacqua, G.; Bousquet, J.; Canonica, G.W.; Durham, S.R.; Fokkens, W.J.; Howarth, P.H.; Lund, V.; Malling, H.J.; et al. Consensus statement on the treatment of allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy 2000, 55, 116–134. [Google Scholar] [CrossRef]
- Nicholas, C.; Wegienka, G.; Havstad, S.; Ownby, D.; Johnson, C.C. Influence of cat characteristics on Fel d 1 levels in the home. Ann. Allergy Asthma Immunol. 2008, 101, 47–50. [Google Scholar] [CrossRef]
- Nicholas, C.; Wegienka, G.; Havstad, S.; Zoratti, E.; Ownby, D.; Johnson, C.C. Dog characteristics and allergen levels in the home. Ann. Allergy Asthma Immunol. 2010, 105, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Kenney P Hilberg O Laursen AC Peel RG Sigsgaard T Preventive effect of nasal filters on allergic rhinitis: A randomized, double-blind, placebo-controlled crossover park study. J. Allergy Clin. Immunol. 2015, 136, 1566–1572. [CrossRef] [PubMed]
- Wolffsohn, J.S.; Naroo, S.A.; Bilkhu, P.S.; Kennedy, R.; Robertson, L.A. Investigating ocular allergy: Can we determine a better measurement? Clin. Exp. Allergy 2013, 43, 1441. [Google Scholar]
- Dhami, S.; Kakourou, A.; Asamoah, F.; Agache, I.; Lau, S.; Jutel, M.; Muraro, A.; Roberts, G.; Akdis, C.A.; Bonini, M.; et al. Allergen immunotherapy for allergic asthma: A systematic review and meta-analysis. Allergy 2017, 72, 1825–1848. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.K.; Schlosser, R.J. Subcutaneous and sublingual immunotherapy for allergic rhinitis: What is the evidence? Am. J. Rhinol. Allergy 2012, 26, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.; Li, T.; Nelson, H.; Lockey, R. Allergen immunotherapy: A practice parameter second update. J. Allergy Clin. Immunol. 2007, 120, S25–S85. [Google Scholar] [CrossRef]
- Kennedy, R. Phototherapy as a Treatment for Dermatological Diseases, Cancer, Aesthetic Dermatologic Conditions and Allergenic Rhinitis in Adult and Paediatric Medicine. Life 2023, 13, 196. [Google Scholar] [CrossRef]
| Uncoated Nostril | Nostril with Pollen Barrier | % Increase | |
|---|---|---|---|
| Grass pollen | 67.3 | 185.7 | 64 |
| Other pollen | 5.7 | 20.3 | 72 |
| Total pollen | 102.4 | 376.9 | 73 |
| Study Criteria | Wavelength | Main Effects | Main References |
|---|---|---|---|
| Randomized, single-blind, placebo-controlled study | UVAB (UV-A and UV-B, visible light) | Decrease in the severity of symptoms of nasal obstruction, nasal itching, nasal discharge and sneezing variables (p < 0.001). | [27] |
| Double-blind, placebo-controlled study | UV-B (5%), UV-A (25%) and visible light (70%) | Decrease in severity of allergic rhinitis. | [32] |
| Randomized, double-blind study (49 patients) | mUV/VIS irradiation | Improvement in clinical symptoms of sneezing (p < 0.016), rhinorrhea (p < 0.007), nasal itching (p < 0.014) and total nasal score (p < 0.004). | [22] |
| A blind, placebo-controlled study | Visible light (mUV/VIS) and infrared light (660 nm 940 nm) | Significantly improved nasal symptoms of allergic rhinitis arising from exposure to indoor and outdoor allergens. | [30] |
| Double-blind, randomized prospective study (50 patients) | Intranasal illumination at 660 nm for 4.4 min three times a day for 14 days | Improvement in symptoms was reported by 72% of the allergic rhinitis patients. | [33] |
| Randomized study (65 Patients) | Visible light, ultraviolet A and ultraviolet B | Improvements in all variables of the quality of life—nasal symptom scores and VAS were statistically significant. | [34] |
| Randomized, double-blind study | Low-dose UVB, low-dose UVA and visible light | Reduced symptom scores for sneezing, rhinorrhea, nasal itching and the total nasal score in ragweed allergic patients. | [25] |
| Randomized into the treatment group (phototherapy was performed once a day for 7 days) and control group (antihistamine once a day for 7 days) | Phototherapy with LEDs consisting of two wavelengths, 660 and 850 nm | Significant improvements were observed in all of the symptoms of AR patients. | [31] |
| Double-blind, placebo-controlled grass pollen challenge | Red and infrared spectra: 652 nm and 940 nm | Significant reductions in severity of symptom scores were found for sneezing, running nose, running eyes and itchy mouth/palate (p < or =0.05). | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennedy, R. Mechanisms and Comparative Treatments of Allergic Rhinitis including Phototherapy. Allergies 2024, 4, 17-29. https://doi.org/10.3390/allergies4010002
Kennedy R. Mechanisms and Comparative Treatments of Allergic Rhinitis including Phototherapy. Allergies. 2024; 4(1):17-29. https://doi.org/10.3390/allergies4010002
Chicago/Turabian StyleKennedy, Roy. 2024. "Mechanisms and Comparative Treatments of Allergic Rhinitis including Phototherapy" Allergies 4, no. 1: 17-29. https://doi.org/10.3390/allergies4010002
APA StyleKennedy, R. (2024). Mechanisms and Comparative Treatments of Allergic Rhinitis including Phototherapy. Allergies, 4(1), 17-29. https://doi.org/10.3390/allergies4010002






