Structural Insights on Cross-Reactivity of Mite Allergens with Helminth Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of Most Relevant Groups of Mite Allergens
2.2. Protein Sequences Retrieval and Functional Analysis
2.3. Search for Orthologs in other Organisms
2.4. Sequence Alignment and Similarity Levels
2.5. Post Translational Modifications and Analysis of Evolutionary Conservation
2.6. Prediction, Refinement, Validation, Structural Alignment, and Visualizationof Protein Tertiary Structure
2.7. B Cell Epitope Prediction and Representation
2.8. Computational Docking of Mite and Helminth Proteins with Antibodies
3. Results
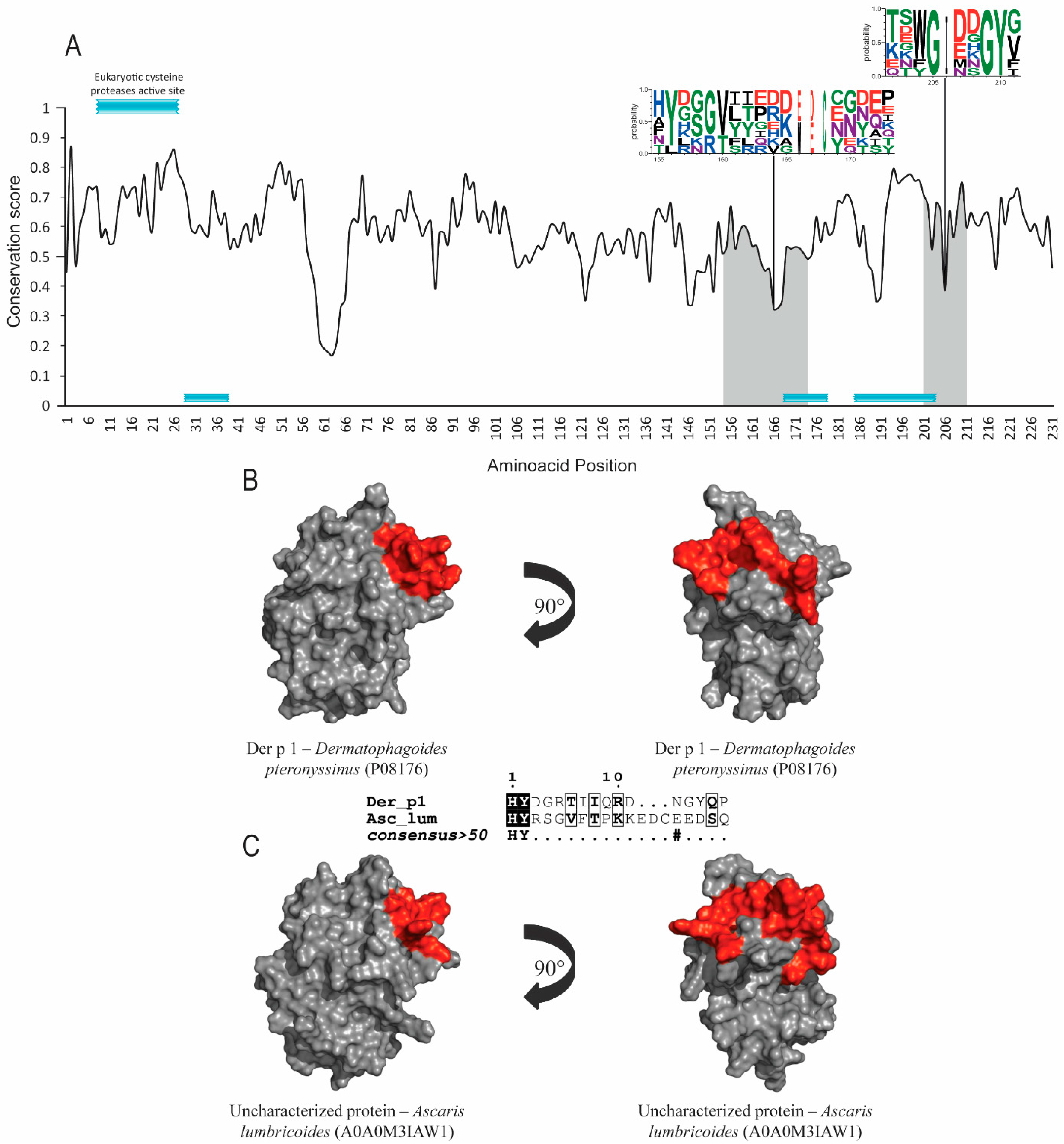
3.1. Allergens with Protease Activity (Groups 1 and 9)
3.2. Lipid-Binding Proteins (Group 2)
3.3. Unknown-Function Proteins (Groups 5 and 21)
3.4. Tropomyosins (Group 10)
3.5. Chitin-Binding Domains (Groups 18 and 23)
3.6. Confirmation of Predicted Regions by Docking Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheurer, S.; Toda, M.; Vieths, S. What makes an allergen? Clin. Exp. Allergy 2015, 45, 1150–1161. [Google Scholar] [CrossRef]
- Bajoriuniene, I.; Malakauskas, K.; Lavinskiene, S.; Jeroch, J.; Sakalauskas, R. Th17 response to Dermatophagoides pteronyssinus is related to late-phase airway and systemic inflammation in allergic asthma. Int. Immunopharmacol. 2013, 17, 1020–1027. [Google Scholar] [CrossRef]
- Kubo, M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol. Rev. 2017, 278, 162–172. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Zhang, H.; Hu, L.; Liu, J.; Wang, L.; Wang, T.; Zhang, H.; Cong, L.; Wang, Q. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Vrtala, S. Allergens from house dust and storage mites. Allergo J. Int. 2022, 31, 267–271. [Google Scholar] [CrossRef]
- Calderon, M.A.; Casale, T.B.; Nelson, H.S.; Demoly, P. An evidence-based analysis of house dust mite allergen immunotherapy: A call for more rigorous clinical studies. J. Allergy Clin. Immunol. 2013, 132, 1322–1336. [Google Scholar] [CrossRef]
- Aggarwal, P.; Senthilkumaran, S. Dust Mite Allergy. In StatPearls; Ineligible Companies: Treasure Island, FL, USA, 2024. [Google Scholar]
- Poulsen, L.K. What makes an allergen more than an allergen? Clin. Exp. Allergy 2009, 39, 623–625. [Google Scholar] [CrossRef]
- Aglas, L.; Gilles, S.; Bauer, R.; Huber, S.; Araujo, G.R.; Mueller, G.; Scheiblhofer, S.; Amisi, M.; Dang, H.H.; Briza, P.; et al. Context matters: T(H)2 polarization resulting from pollen composition and not from protein-intrinsic allergenicity. J. Allergy Clin. Immunol. 2018, 142, 984–987.e986. [Google Scholar] [CrossRef]
- Daschner, A.; Gonzalez Fernandez, J. Allergy in an Evolutionary Framework. J. Mol. Evol. 2020, 88, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Bannon, G.A. What makes a food protein an allergen? Curr. Allergy Asthma Rep. 2004, 4, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Radauer, C.; Breiteneder, H. Evolutionary biology of plant food allergens. J. Allergy Clin. Immunol. 2007, 120, 518–525. [Google Scholar] [CrossRef]
- Poulsen, L.K.; Hummelshoj, L. Triggers of IgE class switching and allergy development. Ann. Med. 2007, 39, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Scudellari, M. News Feature: Cleaning up the hygiene hypothesis. Proc. Natl. Acad. Sci. USA 2017, 114, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Gaitan, M.I. Multiple sclerosis and environmental factors: The role of vitamin D, parasites, and Epstein-Barr virus infection. Acta Neurol. Scand. 2015, 132, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Miftahussurur, M.; Nusi, I.A.; Graham, D.Y.; Yamaoka, Y. Helicobacter, Hygiene, Atopy, and Asthma. Front. Microbiol. 2017, 8, 1034. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Endo, A.; Isolauri, E.; Scalabrin, D. Early Gut Colonization With Lactobacilli and Staphylococcus in Infants: The Hygiene Hypothesis Extended. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.M.C.; Carneiro, V.L.; Galvao, A.A.; Fonseca, T.R.; Vitor, R.W.A.; Alcantara-Neves, N.M.; Cruz, A.A.; Figueiredo, C.A. Toxoplasma gondii protects from IgE sensitization and induces Th1/Th2 immune profile. Parasite Immunol. 2020, 42, e12694. [Google Scholar] [CrossRef] [PubMed]
- Versini, M.; Jeandel, P.Y.; Bashi, T.; Bizzaro, G.; Blank, M.; Shoenfeld, Y. Unraveling the Hygiene Hypothesis of helminthes and autoimmunity: Origins, pathophysiology, and clinical applications. BMC Med. 2015, 13, 81. [Google Scholar] [CrossRef]
- Yazdanbakhsh, M.; Kremsner, P.G.; van Ree, R. Allergy, parasites, and the hygiene hypothesis. Science 2002, 296, 490–494. [Google Scholar] [CrossRef]
- Cruz, A.A.; Cooper, P.J.; Figueiredo, C.A.; Alcantara-Neves, N.M.; Rodrigues, L.C.; Barreto, M.L. Global issues in allergy and immunology: Parasitic infections and allergy. J. Allergy Clin. Immunol. 2017, 140, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M. Parasitic helminth infections and the control of human allergic and autoimmune disorders. Clin. Microbiol. Infect. 2016, 22, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.; Huang, C.H.; Lee, B.W. Shellfish and House Dust Mite Allergies: Is the Link Tropomyosin? Allergy Asthma Immunol. Res. 2016, 8, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, L.; Acevedo, N. Allergy in the tropics: The impact of cross-reactivity between mites and ascaris. Front Biosci. 2011, 3, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, N.; Sanchez, J.; Erler, A.; Mercado, D.; Briza, P.; Kennedy, M.; Fernandez, A.; Gutierrez, M.; Chua, K.Y.; Cheong, N.; et al. IgE cross-reactivity between Ascaris and domestic mite allergens: The role of tropomyosin and the nematode polyprotein ABA-1. Allergy 2009, 64, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.B.; Amor, A.L.M.; Santos, L.N.; Galvao, A.A.; Oviedo Vera, A.V.; Silva, E.S.; Barbosa, C.G.; Goncalves, M.S.; Cooper, P.J.; Figueiredo, C.A.; et al. Risk factors for Toxocara spp. seroprevalence and its association with atopy and asthma phenotypes in school-age children in a small town and semi-rural areas of Northeast Brazil. Acta Trop. 2017, 174, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Santiago, H.C.; Bennuru, S.; Boyd, A.; Eberhard, M.; Nutman, T.B. Structural and immunologic cross-reactivity among filarial and mite tropomyosin: Implications for the hygiene hypothesis. J. Allergy Clin. Immunol. 2011, 127, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, L.; Coronado, S. Parasite allergens. Mol. Immunol. 2018, 100, 113–119. [Google Scholar] [CrossRef]
- Shen, C.Y.; Tsai, J.J.; Liao, E.C. Cross-reactivity of sIgE to mite and shrimp induced allergies in different age groups and clinical profiles of shrimp sIgE in vegetarians. Sci. Rep. 2019, 9, 12548. [Google Scholar] [CrossRef]
- Aalberse, R.C. Allergens from mites: Implications of cross-reactivity between invertebrate antigens. Allergy 1998, 53, 47–48. [Google Scholar] [CrossRef]
- Sidenius, K.E.; Hallas, T.E.; Poulsen, L.K.; Mosbech, H. Allergen cross-reactivity between house-dust mites and other invertebrates. Allergy 2001, 56, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Amoah, A.S.; Boakye, D.A.; Yazdanbakhsh, M.; van Ree, R. Influence of Parasitic Worm Infections on Allergy Diagnosis in Sub-Saharan Africa. Curr. Allergy Asthma Rep. 2017, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Santiago Hda, C.; Ribeiro-Gomes, F.L.; Bennuru, S.; Nutman, T.B. Helminth infection alters IgE responses to allergens structurally related to parasite proteins. J. Immunol. 2015, 194, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.A.; Glesner, J.; Daniel, J.L.; Zhang, J.; Hyduke, N.; Richardson, C.M.; DeRose, E.F.; Chapman, M.D.; Peebles, R.S., Jr.; Smith, A.S.; et al. Mapping Human Monoclonal IgE Epitopes on the Major Dust Mite Allergen Der p 2. J. Immunol. 2020, 205, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Khatri, K.; Richardson, C.M.; Glesner, J.; Kapingidza, A.B.; Mueller, G.A.; Zhang, J.; Dolamore, C.; Vailes, L.D.; Wunschmann, S.; Peebles, R.S., Jr.; et al. Human IgE monoclonal antibody recognition of mite allergen Der p 2 defines structural basis of an epitope for IgE cross-linking and anaphylaxis in vivo. PNAS Nexus 2022, 1, pgac054. [Google Scholar] [CrossRef] [PubMed]
- Glesner, J.; Vailes, L.D.; Schlachter, C.; Mank, N.; Minor, W.; Osinski, T.; Chruszcz, M.; Chapman, M.D.; Pomes, A. Antigenic Determinants of Der p 1: Specificity and Cross-Reactivity Associated with IgE Antibody Recognition. J. Immunol. 2017, 198, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Glesner, J.; Kapingidza, A.B.; Godzwon, M.; Offermann, L.R.; Mueller, G.A.; DeRose, E.F.; Wright, P.; Richardson, C.M.; Woodfolk, J.A.; Vailes, L.D.; et al. A Human IgE Antibody Binding Site on Der p 2 for the Design of a Recombinant Allergen for Immunotherapy. J. Immunol. 2019, 203, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Chruszcz, M.; Pomes, A.; Glesner, J.; Vailes, L.D.; Osinski, T.; Porebski, P.J.; Majorek, K.A.; Heymann, P.W.; Platts-Mills, T.A.; Minor, W.; et al. Molecular determinants for antibody binding on group 1 house dust mite allergens. J. Biol. Chem. 2012, 287, 7388–7398. [Google Scholar] [CrossRef]
- Garcia Alonso, M.; Caballero, M.L.; Umpierrez, A.; Lluch-Bernal, M.; Knaute, T.; Rodriguez-Perez, R. Relationships between T cell and IgE/IgG4 epitopes of the Anisakis simplex major allergen Ani s 1. Clin. Exp. Allergy 2015, 45, 994–1005. [Google Scholar] [CrossRef]
- McClain, S. Bioinformatic screening and detection of allergen cross-reactive IgE-binding epitopes. Mol. Nutr. Food Res. 2017, 61, 1600676. [Google Scholar] [CrossRef]
- Hayes, M.; Rougé, P.; Barre, A.; Herouet-Guicheney, C.; Roggen, E.L. In silico tools for exploring potential human allergy to proteins. Drug Discov. Today Dis. Models 2015, 17–18, 3–11. [Google Scholar] [CrossRef]
- Mari, A.; Rasi, C.; Palazzo, P.; Scala, E. Allergen databases: Current status and perspectives. Curr. Allergy Asthma Rep. 2009, 9, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Wasmuth, E.V.; Lima, C.D. The Rrp6 C-terminal domain binds RNA and activates the nuclear RNA exosome. Nucleic Acids Res. 2017, 45, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Attwood, T.K.; Babbitt, P.C.; Bateman, A.; Bork, P.; Bridge, A.J.; Chang, H.Y.; Dosztanyi, Z.; El-Gebali, S.; Fraser, M.; et al. InterPro in 2017-beyond protein family and domain annotations. Nucleic Acids Res. 2017, 45, D190–D199. [Google Scholar] [CrossRef] [PubMed]
- Walton, S.F.; Slender, A.; Pizutto, S.; Mounsey, K.E.; Oprescu, F.; Thomas, W.R.; Hales, B.J.; Currie, B.J. Analysis of IgE binding patterns to house dust mite allergens in scabies-endemic communities: Insights for both diseases. Clin. Exp. Allergy 2016, 46, 508. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Caldas, E.; Puerta, L.; Caraballo, L.; Lockey, R.F. Mite allergens. Clin. Allergy Immunol. 2008, 21, 161–182. [Google Scholar] [PubMed]
- Bessot, J.C.; Pauli, G. Mite allergens: An overview. Eur. Ann. Allergy Clin. Immunol. 2011, 43, 141–156. [Google Scholar]
- Cui, Y. Structural biology of mite allergens. Mol. Biol. Rep. 2013, 40, 681–686. [Google Scholar] [CrossRef] [PubMed]
- UniProt, C. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Attwood, T.K.; Babbitt, P.C.; Blum, M.; Bork, P.; Bridge, A.; Brown, S.D.; Chang, H.Y.; El-Gebali, S.; Fraser, M.I.; et al. InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 2019, 47, D351–D360. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.Q.; Junqueira, A.C.; Abellana, R.; Barrio, P.C.; Terrazas, W.C.; Sodre, F.C.; Boia, M.N.; Ascaso, C. Prevalence of intestinal parasites and risk factors forspecific and multiple helminth infections in a remote city of the Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2016, 49, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Chammartin, F.; Guimaraes, L.H.; Scholte, R.G.; Bavia, M.E.; Utzinger, J.; Vounatsou, P. Spatio-temporal distribution of soil-transmitted helminth infections in Brazil. Parasit. Vectors 2014, 7, 440. [Google Scholar] [CrossRef] [PubMed]
- Casavechia, M.T.; Lonardoni, M.V.; Venazzi, E.A.; Campanerut-Sa, P.A.; da Costa Benalia, H.R.; Mattiello, M.F.; Menechini, P.V.; Dos Santos, C.A.; Teixeira, J.J. Prevalence and predictors associated with intestinal infections by protozoa and helminths in southern Brazil. Parasitol. Res. 2016, 115, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Combet, C.; Blanchet, C.; Geourjon, C.; Deleage, G. NPS@: Network protein sequence analysis. Trends Biochem. Sci. 2000, 25, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
- Lang-Yona, N.; Shuster-Meiseles, T.; Mazar, Y.; Yarden, O.; Rudich, Y. Impact of urban air pollution on the allergenicity of Aspergillus fumigatus conidia: Outdoor exposure study supported by laboratory experiments. Sci. Total Environ. 2016, 541, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Carlsson, M.C.; Madsen, C.B.; Brand, S.; Moller, S.R.; Olsen, C.E.; Vakhrushev, S.Y.; Brimnes, J.; Wurtzen, P.A.; Ipsen, H.; et al. Glycoproteomic analysis of seven major allergenic proteins reveals novel post-translational modifications. Mol. Cell Proteom. 2015, 14, 191–204. [Google Scholar] [CrossRef]
- de Castro, E.; Sigrist, C.J.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. ScanProsite: Detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006, 34, W362–W365. [Google Scholar] [CrossRef]
- Capra, J.A.; Singh, M. Predicting functionally important residues from sequence conservation. Bioinformatics 2007, 23, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; DiMaio, F.; Wang, R.Y.; Kim, D.; Miles, C.; Brunette, T.; Thompson, J.; Baker, D. High-resolution comparative modeling with RosettaCM. Structure 2013, 21, 1735–1742. [Google Scholar] [CrossRef]
- Kleffner, R.; Flatten, J.; Leaver-Fay, A.; Baker, D.; Siegel, J.B.; Khatib, F.; Cooper, S. Foldit Standalone: A video game-derived protein structure manipulation interface using Rosetta. Bioinformatics 2017, 33, 2765–2767. [Google Scholar] [CrossRef]
- Benkert, P.; Tosatto, S.C.; Schomburg, D. QMEAN: A comprehensive scoring function for model quality assessment. Proteins 2008, 71, 261–277. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Peng, J.; Xu, J. Protein structure alignment beyond spatial proximity. Sci. Rep. 2013, 3, 1448. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, J.; Bui, H.H.; Li, W.; Fusseder, N.; Bourne, P.E.; Sette, A.; Peters, B. ElliPro: A new structure-based tool for the prediction of antibody epitopes. BMC Bioinform. 2008, 9, 514. [Google Scholar] [CrossRef]
- Ansari, H.R.; Raghava, G.P. Identification of conformational B-cell Epitopes in an antigen from its primary sequence. Immunome Res. 2010, 6, 6. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.H.; Vreven, T.; Weng, Z. ZDOCK server: Interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef]
- Papia, F.; Bellia, C.; Uasuf, C.G. Tropomyosin: A panallergen that causes a worldwide allergic problem. Allergy Asthma Proc. 2021, 42, e145–e151. [Google Scholar] [CrossRef]
- McKenna, O.E.; Asam, C.; Araujo, G.R.; Roulias, A.; Goulart, L.R.; Ferreira, F. How relevant is panallergen sensitization in the development of allergies? Pediatr. Allergy Immunol. 2016, 27, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.F.; Mendonca, T.N.; Melo, J.M.; Moreno, A.S.; Januario, Y.C.; DaSilva, L.L.; Dias, M.M.; Meireles, P.R.; Santos, K.S.; Yang, A.C.; et al. Reactions to Shrimp Including Severe Anaphylaxis in Mite- and Cockroach-Allergic Patients Who Have Never Eaten Shrimp: Clinical Significance of IgE Cross-Reactivity to Tropomyosins From Different Sources. J. Investig. Allergol. Clin. Immunol. 2019, 29, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Figo, D.D.; Cordeiro Macedo, P.R.; Gadermaier, G.; Remuzgo, C.; Castro, F.F.M.; Kalil, J.; Galvao, C.E.S.; Santos, K.S. IgE and IgG4 Epitopes of Dermatophagoides and Blomia Allergens before and after Sublingual Immunotherapy. Int. J. Mol. Sci. 2023, 24, 4173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.M.; Xiao, H.; Nowak-Wegrzyn, A.; Zhou, P. IgE-binding epitope mapping of tropomyosin allergen (Exo m 1) from Exopalaemon modestus, the freshwater Siberian prawn. Food Chem. 2020, 309, 125603. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, X.M.; Wen, Y.Q.; Zhao, J.L.; Xu, T.C.; Yong, L.; Lin, H.; Zhang, H.W.; Li, Z.X. Comparison of tropomyosin released peptide and epitope mapping after in vitro digestion from fish (Larimichthys crocea), shrimp (Litopenaeus vannamei) and clam (Ruditapes philippinarum) through SWATH-MS based proteomics. Food Chem. 2023, 403, 134314. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.Y.; Leung, N.Y.; Ho, M.H.; Gershwin, L.J.; Shu, S.A.; Leung, P.S.; Chu, K.H. Immunization with Hypoallergens of shrimp allergen tropomyosin inhibits shrimp tropomyosin specific IgE reactivity. PLoS ONE 2014, 9, e111649. [Google Scholar] [CrossRef] [PubMed]
- Takai, T.; Kato, T.; Yasueda, H.; Okumura, K.; Ogawa, H. Analysis of the structure and allergenicity of recombinant pro- and mature Der p 1 and Der f 1: Major conformational IgE epitopes blocked by prodomains. J. Allergy Clin. Immunol. 2005, 115, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; McKerrow, J. Erratum to “Cysteine proteases of parasitic organisms”: [Mol. Biochem. Parasitol. 120 (2002) 1–21]. Mol. Biochem. Parasitol. 2002, 121, 159. [Google Scholar] [CrossRef]
- Mueller, G.A.; Pedersen, L.C.; Glesner, J.; Edwards, L.L.; Zakzuk, J.; London, R.E.; Arruda, L.K.; Chapman, M.D.; Caraballo, L.; Pomes, A. Analysis of glutathione S-transferase allergen cross-reactivity in a North American population: Relevance for molecular diagnosis. J. Allergy Clin. Immunol. 2015, 136, 1369–1377. [Google Scholar] [CrossRef]
- Santiago, H.C.; LeeVan, E.; Bennuru, S.; Ribeiro-Gomes, F.; Mueller, E.; Wilson, M.; Wynn, T.; Garboczi, D.; Urban, J.; Mitre, E.; et al. Molecular mimicry between cockroach and helminth glutathione S-transferases promotes cross-reactivity and cross-sensitization. J. Allergy Clin. Immunol. 2012, 130, 248–256.e249. [Google Scholar] [CrossRef]




| Allergen 1/ Accession Number 2 | Orthologs | Accession Numbers 2 | Annotated Domain 2 | Similarity to Der p Allergen 3 | RMSD Value between all PDB and Number of Residues (Cα) Aligned 4 |
|---|---|---|---|---|---|
| Der p 1 P08176 | Der f 1 Dermatophagoides farinae | A1YW13 | Cysteine Protease | 85.84% | 1.40Å 194 residues |
| Blo t 1 Blomia tropicalis | A1KXI0 | 42.92% | |||
| Peptidase C1 Trichuris trichiura | A0A077Z108 | 39.15% | |||
| Fibroinase Loa loa | A0A1S0TN97 | 37.93% | |||
| Peptidase C1 family Schistosoma mansoni | G4LUT7 | 39.52% | |||
| Putative cysteine proteinase Toxocara canis | A0A0B2V1K6 | 41.03% | |||
| Uncharacterized protein Ascaris lumbricoides | A0A0M3IAW1 | 41.50% | |||
| Der p 2 A6XEP9 | Der f 2 Dermatophagoides farinae | Q5TIW1 | Lipid binding domain—NPC2 | 91.52% | 3.01Å 100 residues |
| Blo t 2 Blomia tropicalis | A6XEN9 | 54.23% | |||
| E1 DerP2 DerF2 domain containing protein Trichuris trichiura | A0A077ZES0 | 29.66% | |||
| Uncharacterized protein Loa loa | A0A1I7VNT1 | 34.74% | |||
| Uncharacterized protein Toxocara canis | A0A0B2USJ2 | 33.89% | |||
| Der p 9 Q7Z163 | Der f 9 Dermatophagoides farinae | A0A088SCQ8 | Serine protease | 90.41% | 1.29Å 212 residues |
| Blo t 9 Blomia tropicalis | A1KXI5 | 69.40% | |||
| Transmembrane protease serine 9 Trichuris trichiura | A0A077Z3H4 | 40.63% | |||
| Uncharacterized protein Loa loa | A0A1I7V6N8 | 38.35% | |||
| Serine protease 3 Schistosoma mansoni | G4M1A6 | 41.09% | |||
| Transmembrane protease serine 9 Toxocara canis | A0A0B2VCY3 | 41.09% | |||
| Uncharacterized protein Ascaris lumbricoides | A0A0M3HRF9 | 41.55% | |||
| Der p 10 Q304Y3 | Der f 10 Dermatophagoides farinae | A7XZI8 | Tropomyosin | 98.22% | 1.9Å 174 residues |
| Blo t 10 Blomia tropicalis | A7XZI4 | 95.37% | |||
| Tropomyosin Trichuris trichiura | A0A077ZIM1 | 80.78% | |||
| Tropomyosin Loa loa | A0A1S0UJV8 | 80.42% | |||
| Tropomyosin Schistosoma mansoni | G4VN74 | 69.75% | |||
| Tropomyosin Toxocara canis | A0A0B2VDB8 | 56.66% | |||
| Tropomyosin Ascaris lumbricoides | C0L3K2 | 81.13% | |||
| Der p 18 Q4JK71 | Der f 18 Dermatophagoides farinae | Q86R84 | Chitin metabolic process (Chitinase) | 94.27% | 1.67Å 293 residues |
| Blo t 18 Blomia tropicalis | A1KXI8 | 71.85% | |||
| Acidic mammalian chitinase Trichuris trichiura | A0A077Z8H9 | 37.29% | |||
| Uncharacterized protein Loa loa | A0A1I7W393 | 33.24% | |||
| Putative endochitinase Toxocara canis | A0A0B2V5U4 | 30.66% | |||
| Uncharacterized protein Ascaris lumbricoides | A0A0M3I4H8 | 32.72% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisboa, A.B.P.; Alcantara-Neves, N.M.; Aguiar, E.R.G.R.; Pinheiro, C.S.; Pacheco, L.G.C.; da Silva, E.S. Structural Insights on Cross-Reactivity of Mite Allergens with Helminth Proteins. Allergies 2024, 4, 64-79. https://doi.org/10.3390/allergies4020006
Lisboa ABP, Alcantara-Neves NM, Aguiar ERGR, Pinheiro CS, Pacheco LGC, da Silva ES. Structural Insights on Cross-Reactivity of Mite Allergens with Helminth Proteins. Allergies. 2024; 4(2):64-79. https://doi.org/10.3390/allergies4020006
Chicago/Turabian StyleLisboa, Ayrton B. P., Neuza M. Alcantara-Neves, Eric R. G. R. Aguiar, Carina S. Pinheiro, Luis G. C. Pacheco, and Eduardo S. da Silva. 2024. "Structural Insights on Cross-Reactivity of Mite Allergens with Helminth Proteins" Allergies 4, no. 2: 64-79. https://doi.org/10.3390/allergies4020006







