D-2-Hydroxyglutarate Attenuates Sinonasal Inflammation in Murine Allergic Rhinitis
Abstract
1. Introduction
2. Methods
2.1. Mice
2.2. Flow Cytometry
2.3. Staining for IgE B Cells
2.4. Nasal Lavage and Cytology
2.5. ELISA
2.6. Statistical Analysis
3. Results
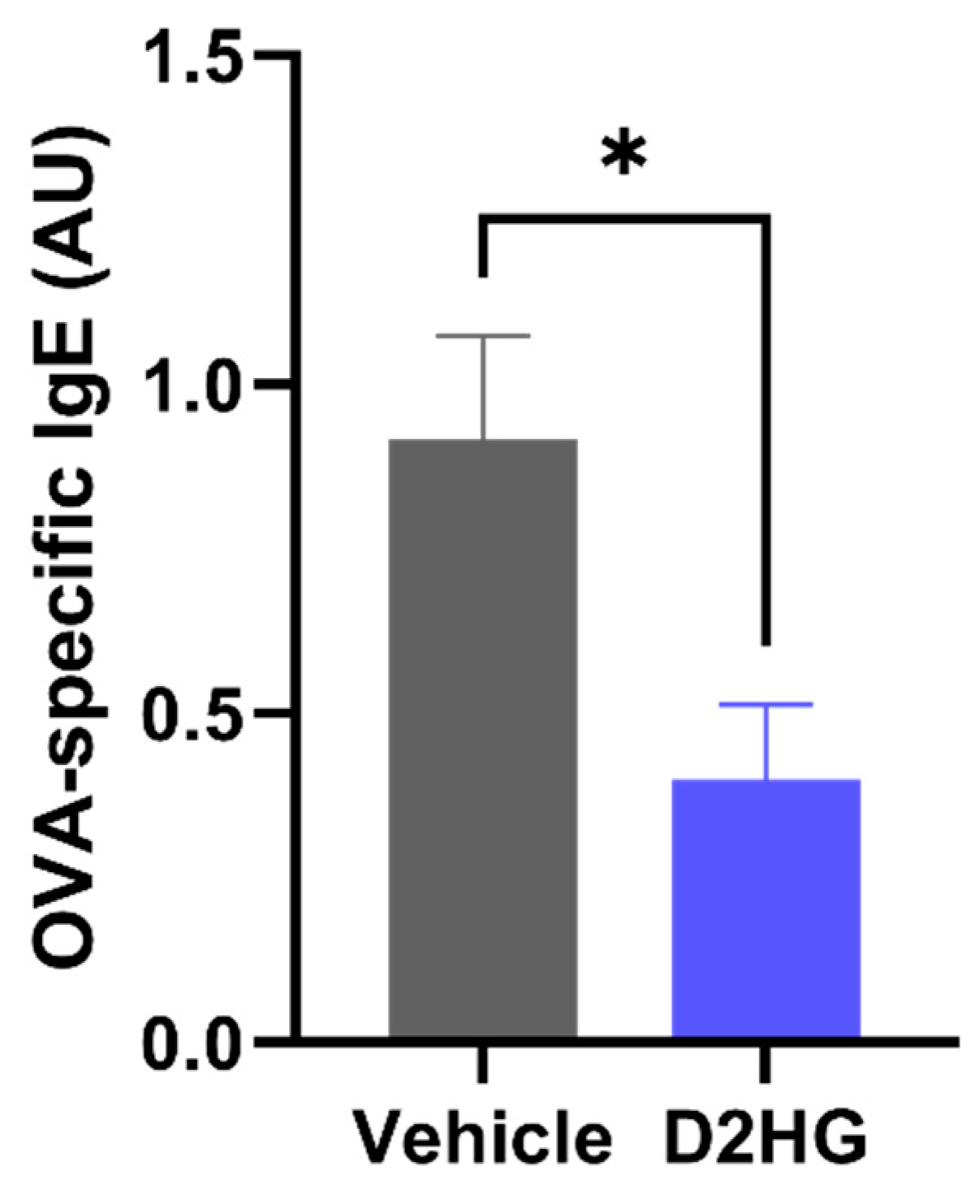
3.1. D2HG Suppresses Allergic Sensitization In Vivo
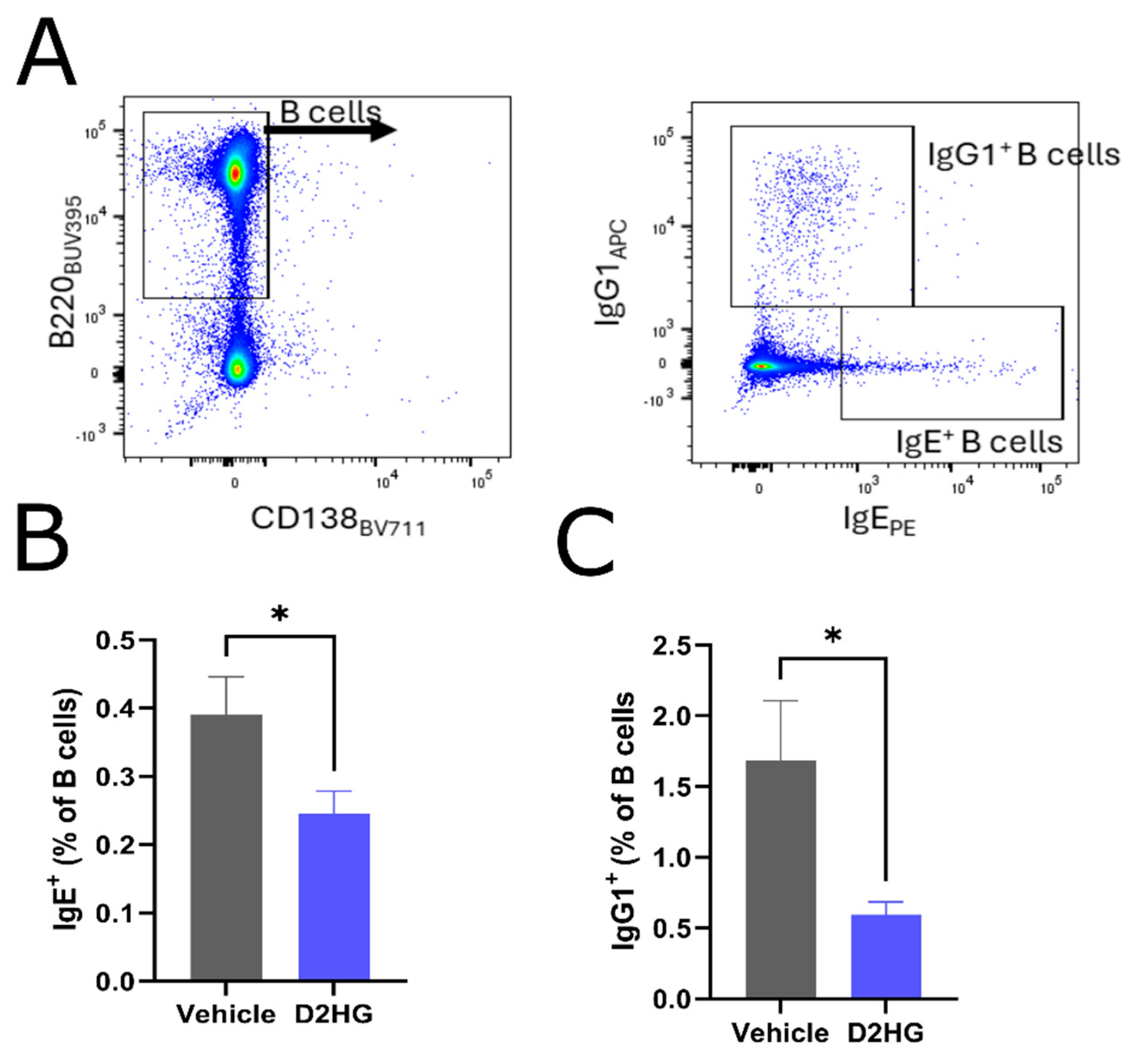
3.2. IgE Synthesis in Draining Lymph Nodes Is Impaired by D2HG Administration
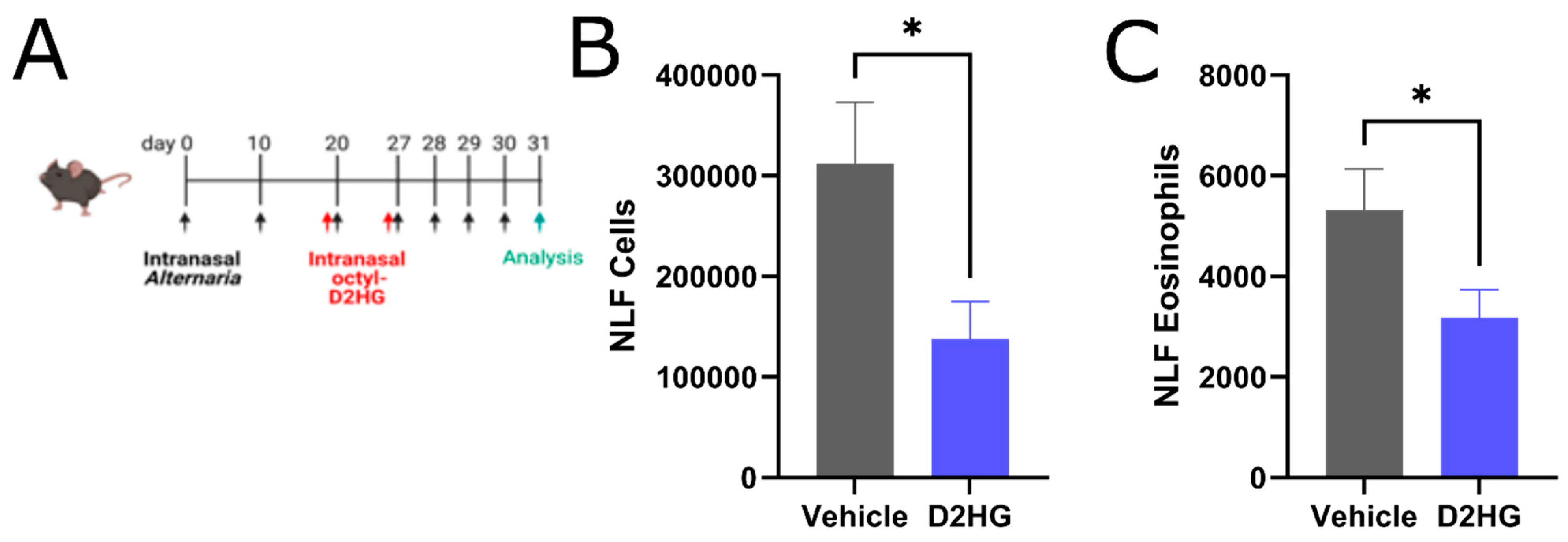
3.3. D2HG Ameliorates Sinonasal Allergic Inflammation in Murine AR
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Savoure, M.; Bousquet, J.; Jaakkola, J.J.K.; Jaakkola, M.S.; Jacquemin, B.; Nadif, R. Worldwide prevalence of rhinitis in adults: A review of definitions and temporal evolution. Clin. Transl. Allergy 2022, 12, e12130. [Google Scholar] [CrossRef] [PubMed]
- International Rhinitis Management Working Group. International Consensus Report on the diagnosis and management of rhinitis. Allergy 1994, 49, 5–34. [Google Scholar]
- Skoner, D.P. Allergic rhinitis: Definition, epidemiology, pathophysiology, detection, and diagnosis. J. Allergy Clin. Immunol. 2001, 108, S2–S8. [Google Scholar] [CrossRef]
- Thwe, P.M.; Pelgrom, L.R.; Cooper, R.; Beauchamp, S.; Reisz, J.A.; D’Alessandro, A.; Everts, B.; Amiel, E. Cell-Intrinsic Glycogen Metabolism Supports Early Glycolytic Reprogramming Required for Dendritic Cell Immune Responses. Cell Metab. 2017, 26, 558–567.e555. [Google Scholar] [CrossRef] [PubMed]
- Everts, B.; Amiel, E.; Huang, S.C.; Smith, A.M.; Chang, C.H.; Lam, W.Y.; Redmann, V.; Freitas, T.C.; Blagih, J.; van der Windt, G.J.; et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nat. Immunol. 2014, 15, 323–332. [Google Scholar] [CrossRef]
- Wculek, S.K.; Khouili, S.C.; Priego, E.; Heras-Murillo, I.; Sancho, D. Metabolic Control of Dendritic Cell Functions: Digesting Information. Front. Immunol. 2019, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- Guak, H.; Al Habyan, S.; Ma, E.H.; Aldossary, H.; Al-Masri, M.; Won, S.Y.; Ying, T.; Fixman, E.D.; Jones, R.G.; McCaffrey, L.M.; et al. Glycolytic metabolism is essential for CCR7 oligomerization and dendritic cell migration. Nat. Commun. 2018, 9, 2463. [Google Scholar] [CrossRef]
- Tharakan, A.; Kumar, A.; Allegood, J.; Cowart, L.A.; Conrad, D.H.; Martin, R.K. D-2-hydroxyglutarate suppresses allergic sensitization in a murine model of experimental asthma. Allergy 2023, 78, 3014–3016. [Google Scholar] [CrossRef]
- Zhu, Z.; Lee, P.H.; Chaffin, M.D.; Chung, W.; Loh, P.R.; Lu, Q.; Christiani, D.C.; Liang, L. A genome-wide cross-trait analysis from UK Biobank highlights the shared genetic architecture of asthma and allergic diseases. Nat. Genet. 2018, 50, 857–864. [Google Scholar] [CrossRef]
- Ferreira, M.A.R.; Mathur, R.; Vonk, J.M.; Szwajda, A.; Brumpton, B.; Granell, R.; Brew, B.K.; Ullemar, V.; Lu, Y.; Jiang, Y.; et al. Genetic Architectures of Childhood- and Adult-Onset Asthma Are Partly Distinct. Am. J. Hum. Genet. 2019, 104, 665–684. [Google Scholar] [CrossRef]
- Du, X.; Hu, H. The Roles of 2-Hydroxyglutarate. Front. Cell Dev. Biol. 2021, 9, 651317. [Google Scholar] [CrossRef]
- Lin, A.P.; Abbas, S.; Kim, S.W.; Ortega, M.; Bouamar, H.; Escobedo, Y.; Varadarajan, P.; Qin, Y.; Sudderth, J.; Schulz, E.; et al. D2HGDH regulates alpha-ketoglutarate levels and dioxygenase function by modulating IDH2. Nat. Commun. 2015, 6, 7768. [Google Scholar] [CrossRef]
- Yang, Z.; Sullivan, B.M.; Allen, C.D. Fluorescent in vivo detection reveals that IgE(+) B cells are restrained by an intrinsic cell fate predisposition. Immunity 2012, 36, 857–872. [Google Scholar] [CrossRef]
- Cho, S.H.; Oh, S.Y.; Zhu, Z.; Lee, J.; Lane, A.P. Spontaneous eosinophilic nasal inflammation in a genetically-mutant mouse: Comparative study with an allergic inflammation model. PLoS ONE 2012, 7, e35114. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Iijima, K.; Radhakrishnan, S.; Mehta, V.; Vassallo, R.; Lawrence, C.B.; Cyong, J.C.; Pease, L.R.; Oguchi, K.; Kita, H. Asthma-related environmental fungus, Alternaria, activates dendritic cells and produces potent Th2 adjuvant activity. J. Immunol. 2009, 182, 2502–2510. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Choi, S.Y.; Oh, T.I.; Kan, S.Y.; Kang, H.; Lee, S.; Oh, T.; Ko, H.M.; Lim, J.H. IDH1(R132H) Causes Resistance to HDAC Inhibitors by Increasing NANOG in Glioblastoma Cells. Int. J. Mol. Sci. 2019, 20, 2679. [Google Scholar] [CrossRef]
- Choi, B.Y.; Han, M.; Kwak, J.W.; Kim, T.H. Genetics and Epigenetics in Allergic Rhinitis. Genes 2021, 12, 2004. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y. Risk factors of allergic rhinitis: Genetic or environmental? Ther. Clin. Risk Manag. 2005, 1, 115–123. [Google Scholar] [CrossRef]
- Liu, P.S.; Wang, H.; Li, X.; Chao, T.; Teav, T.; Christen, S.; Di Conza, G.; Cheng, W.C.; Chou, C.H.; Vavakova, M.; et al. alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017, 18, 985–994. [Google Scholar] [CrossRef]
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.T.; et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef]
- Crake, R.L.I.; Burgess, E.R.; Royds, J.A.; Phillips, E.; Vissers, M.C.M.; Dachs, G.U. The Role of 2-Oxoglutarate Dependent Dioxygenases in Gliomas and Glioblastomas: A Review of Epigenetic Reprogramming and Hypoxic Response. Front. Oncol. 2021, 11, 619300. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tharakan, A.; Kumar, A.; Camarena, C.; Conrad, D.H.; Martin, R.K. D-2-Hydroxyglutarate Attenuates Sinonasal Inflammation in Murine Allergic Rhinitis. Allergies 2025, 5, 13. https://doi.org/10.3390/allergies5020013
Tharakan A, Kumar A, Camarena C, Conrad DH, Martin RK. D-2-Hydroxyglutarate Attenuates Sinonasal Inflammation in Murine Allergic Rhinitis. Allergies. 2025; 5(2):13. https://doi.org/10.3390/allergies5020013
Chicago/Turabian StyleTharakan, Anuj, Ankit Kumar, Carmen Camarena, Daniel H. Conrad, and Rebecca K. Martin. 2025. "D-2-Hydroxyglutarate Attenuates Sinonasal Inflammation in Murine Allergic Rhinitis" Allergies 5, no. 2: 13. https://doi.org/10.3390/allergies5020013
APA StyleTharakan, A., Kumar, A., Camarena, C., Conrad, D. H., & Martin, R. K. (2025). D-2-Hydroxyglutarate Attenuates Sinonasal Inflammation in Murine Allergic Rhinitis. Allergies, 5(2), 13. https://doi.org/10.3390/allergies5020013





