Abstract
Mast cells (MC) are key effector cells in allergic diseases and are increasingly recognized for their roles in the immunopathogenesis of tuberculosis (TB). In allergic conditions, MCs are hyperactivated, driving T-helper Type 2 (Th2)-skewed immune responses that may antagonize the T-helper Type 1 (Th1)-mediated immunity essential for controlling Mycobacterium tuberculosis (Mtb) infection. This immunological imbalance may contribute to increased TB susceptibility, altered granuloma dynamics, and accelerated fibrotic remodeling. Histopathological and in vivo studies have revealed that MCs are recruited to TB lesions, where they release a spectrum of mediators, including histamine, IL-17A, TNF-α, TGF-β, tryptase, and chymase. These mediators can either support initial immune defense or promote chronic inflammation and tissue damage, depending on context and regulation. Moreover, individuals with chronic allergic diseases such as asthma and allergic rhinitis may experience worse TB outcomes due to their baseline immune dysregulation. Environmental exposures (e.g., air pollution, smoking), genetic polymorphisms (e.g., IL-4 −589C/T, IL-13 R130Q), and gut-lung axis disturbances further modulate MC activity and TB pathogenesis. This review synthesizes current findings on MC involvement in TB, particularly in allergic settings, and highlights the need for epidemiological studies and mechanistic research. It also explores the promise of host-directed therapies (HDTs) that target MCs or their mediators, such as antihistamines, MC stabilizers, leukotriene inhibitors, and cytokine modulators, as novel adjuncts to standard TB treatment. Personalized approaches that consider immune profiles, genetic risk, and comorbid allergies may improve TB outcomes and inform future clinical guidelines.
1. Introduction
Mast cells (MC) are long-lived, granule-containing immune cells that have historically been associated with allergic and anaphylactic reactions. Located predominantly in mucosal and epithelial tissues, including the skin, gastrointestinal tract, and respiratory system, MCs play a sentinel role by responding rapidly to environmental stimuli through the release of a vast array of mediators such as histamine, tryptase, chymase, cytokines, and chemokines [1,2]. Classically, MCs have been studied in the context of type I hypersensitivity reactions, where crosslinking of IgE bound to the high-affinity FcεRI receptor triggers degranulation and the subsequent allergic cascade [3]. However, growing evidence suggests that the biological role of MCs extends well beyond allergies, encompassing both innate and adaptive immune responses, including antimicrobial defense, tissue repair, and fibrosis [4].
One particularly intriguing area of recent research is the involvement of MCs in infectious diseases, especially chronic infections such as tuberculosis (TB). TB, caused by Mycobacterium tuberculosis (Mtb), remains one of the leading causes of death worldwide due to an infectious agent, claiming over 1.6 million lives annually as of the most recent World Health Organization (WHO) report [5]. TB is primarily a pulmonary disease, and since MCs are densely distributed in the lungs, especially at mucosal and perivascular sites, their potential interaction with Mtb is of significant immunopathological interest [6].
Historically, macrophages and T cells have been viewed as the central players in TB immunity, particularly via the T-helper Type 1 (Th1) response characterized by interferon-gamma (IFN-γ) production and macrophage activation [5]. However, recent studies have uncovered that MCs can also phagocytose Mtb, respond to pathogen-associated molecular patterns via toll-like receptors (TLRs), and release pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interleukin-1beta (IL-1β), and interleukin-17A (IL-17A) [4,7]. MCs have also been shown to contain intracellular Mtb antigens, especially in fibrotic regions of human TB lesions, suggesting not only their active involvement in the local immune response but also a possible role as a reservoir for the bacteria during chronic or latent infection stages [8].
In allergic individuals, the situation becomes more complex. Allergic diseases, such as asthma, allergic rhinitis, and atopic dermatitis, are characterized by a T-helper Type 2 (Th2)-biased immune environment that favors the production of interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-13 (IL-13), and immunoglobulin E (IgE). This Th2 dominance can suppress Th1 responses, which are essential for controlling intracellular pathogens like Mtb [9,10]. Several studies have shown that patients with chronic allergic conditions or Th2-skewed environments exhibit impaired macrophage activation and altered granuloma formation, which may predispose them to TB infection or exacerbate disease progression [11]. Furthermore, genetic polymorphisms in cytokine genes, including IL-4 and IL-13, which drive Th2 responses and MC activation, have been associated with increased susceptibility to TB and reduced capacity to mount an effective Th1-mediated immune response [12].
The concept of MCs acting as immunological “double agents” in TB pathogenesis capable of both promoting host defense and contributing to immune dysregulation is supported by evidence from both murine models and human post-mortem lung studies. For example, transfer of Toll-Like Receptor 2 (TLR2)-expressing MCs into TLR2-deficient mice has been shown to rescue granuloma formation, normalize myeloid cell recruitment, and restore Th1 cytokine production, ultimately improving bacterial control [7]. Conversely, MCs residing at the periphery of necrotic granulomas and within fibrotic tissue express pro-fibrotic mediators such as transforming growth factor-beta (TGF-β) and chymase, potentially contributing to lung tissue remodeling and long-term sequelae of TB [8].
Environmental exposures common in allergic individuals, such as air pollution, cigarette smoke, and airborne allergens, may further exacerbate this immunological imbalance. Pollutants like particulate matter ≤ 2.5 µm (PM2.5) have been shown to activate mast cells via ROS/JNK signaling, promoting IgE-mediated degranulation and cytokine release [13]. Additionally, diesel exhaust particles impair macrophage function—reducing phagocytosis and cytokine production (e.g., TNF-α, IL-8, IL-1β) and compromising antimycobacterial defense in vitro and in vivo [14]. Emerging studies on the gut–lung axis highlight the impact of intestinal dysbiosis on systemic immunity and possibly mast cell behavior, further complicating TB susceptibility in allergic populations [15].
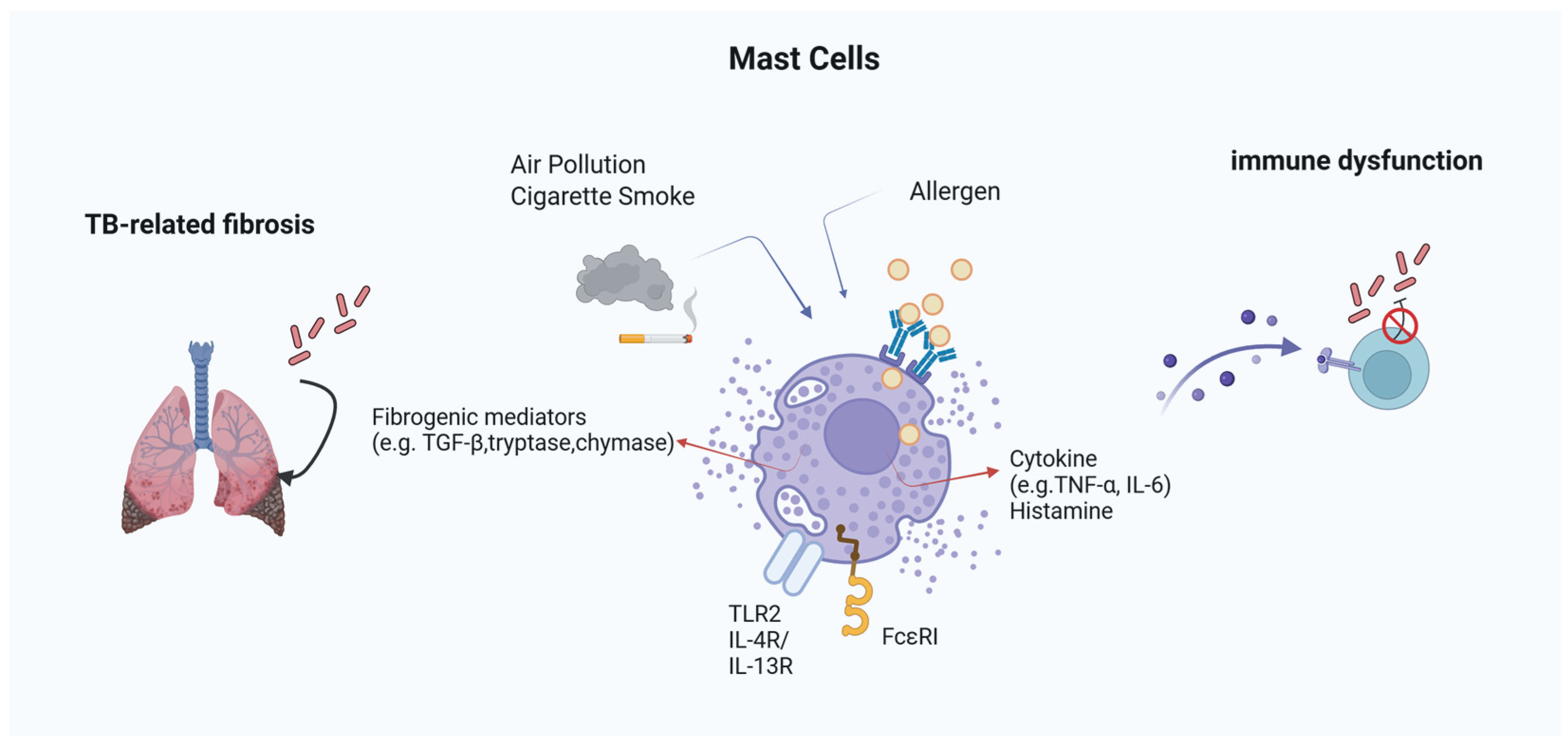
To provide an integrative overview of these mechanisms, Figure 1 summarizes the proposed pathways by which MCs influence TB pathogenesis in the context of allergic inflammation. This conceptual framework highlights the dualistic nature of MC activity, illustrating how MC-derived mediators can either support protective immunity or drive pathological inflammation and fibrosis depending on the immunological and environmental context.
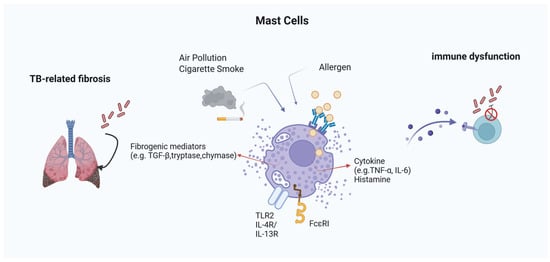
Figure 1.
Mast Cell-Mediated Mechanisms in TB within Allergic Environments. MCs integrate signals from allergens and environmental pollutants to modulate TB pathogenesis. Through FcεRI and TLR2, they release histamine, cytokines (TNF-α, IL-6), and fibrogenic mediators (e.g., tryptase, chymase, TGF-β). These mediators influence both granuloma dynamics and fibrosis, while histamine can suppress IFN-γ and impair macrophage function. External factors like cigarette smoke and PM2.5 further enhance MC activation, contributing to immune dysfunction and tissue remodeling in TB under allergic conditions.
From a clinical perspective, these findings underscore the urgent need to investigate the epidemiological and mechanistic links between allergic diseases and TB. Understanding the extent to which allergic inflammation, MC activation, and Th2-skewed immunity contribute to TB susceptibility and progression could open new avenues for both diagnosis and treatment [2,16]. Particularly, the field of host-directed therapies (HDTs) targeting MCs and related pathways, such as MC stabilizers, antihistamines, leukotriene antagonists, and IL-4/IL-13 inhibitors, holds promise for improving TB treatment outcomes, especially in patients with pre-existing allergic conditions [17,18,19].
In this review, we aim to synthesize the current understanding of the role of MCs in TB immunopathogenesis, with a particular emphasis on the intersection of allergy and infection. We explore how genetic, environmental, and immunological factors converge to modulate MC behavior and how this impacts TB pathogenesis, granuloma architecture, fibrosis, and treatment responses. Furthermore, we discuss the potential of MC-targeted HDTs as novel adjunctive strategies in TB management and the importance of personalized medicine approaches in addressing the needs of this unique and vulnerable patient population.
2. Prevalence and Epidemiology of Allergic Diseases and Tuberculosis
Allergic diseases and TB are two major global health burdens whose intersection has important implications for public health, clinical management, and immunological research. Over the past few decades, the global prevalence of allergic diseases, including asthma, allergic rhinitis, and atopic dermatitis, has markedly increased, particularly in urbanized regions with westernized lifestyles. According to the World Allergy Organization (WAO), approximately 10–30% of the global population is affected by allergic rhinitis and up to 10% by asthma, with incidence rates continuing to rise due to environmental and lifestyle changes [1]. In parallel, TB remains one of the leading causes of death from infectious disease worldwide, with 10.6 million new cases and 1.3 million deaths reported by the WHO in 2022 [2]. This epidemiological overlap raises pressing questions regarding whether and how allergic immune profiles, particularly those mediated by MCs, influence susceptibility to and outcomes of TB.
2.1. Th2 Immune Polarization and Impaired TB Control
Several studies have reported that individuals with allergic diseases, particularly those with allergic rhinitis or asthma, have an increased risk of developing TB. This increased susceptibility is likely due to the suppression of Th1 immunity by Th2 cytokines. Moreover, genetic polymorphisms in IL-4 and IL-13 genes, which promote a Th2-dominant immune profile, have been linked to increased IgE production and excessive secretion of Th2 cytokines, further undermining Th1 responses [4,5]. These immune alterations weaken the activation of macrophages, a key defense mechanism against Mtb infection. Allergic diseases are primarily characterized by a Th2-skewed immune response, marked by elevated levels of IL-4, IL-5, and IL-13, leading to eosinophilia, IgE production, and MC hyperreactivity. In contrast, effective control of Mtb infection requires a robust Th1-type response, in which IFN-γ activates macrophages to eliminate intracellular bacteria [5,6]. A growing body of evidence suggests that chronic Th2 polarization often present in allergic individuals can suppress Th1 responses, thereby compromising host defense against Mtb [4]. This is particularly concerning given that IFN-γ plays a central role in granuloma formation and bacterial containment during TB infection.
Several cohort studies have supported the notion of immunological antagonism between allergic diseases and TB. In a recent nationwide cohort study conducted in Korea, individuals with allergic conditions such as asthma and allergic rhinitis demonstrated a significantly increased risk of developing mycobacterial diseases, including TB, particularly among those with elevated total IgE levels and eosinophilic inflammation (adjusted HRs up to 1.33) [20]. In another large-scale cohort study, patients with asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS) exhibited a more than twofold increased risk of incident TB compared to non-ACOS individuals (adjusted HR: 2.41) [21]. These findings collectively suggest that allergic inflammation may not only predispose individuals to TB infection but also impair immune control mechanisms, potentially contributing to poor recovery and increased risk of latent tuberculosis infection (LTBI) reactivation.
2.2. Genetic Polymorphisms and Population-Specific Risk
Genetic predisposition plays a critical role in shaping individual immune responses. Polymorphisms in cytokine genes such as IL-4 (−589C/T), IL-13 (R130Q), and IL-10 (−1082A/G) have been shown to promote Th2 bias and are frequently associated with both allergic diseases and increased TB susceptibility [10,12]. For example, IL-4 and IL-13 polymorphisms not only enhance IgE production but also downregulate IFN-γ, which is essential for macrophage activation. Furthermore, the IFN-γ +874T/A polymorphism has shown a variable association with TB across populations: the AA genotype was linked to higher TB risk in Asians and Africans, while the TT genotype appeared protective in Caucasians [14]. This illustrates that gene–environment interactions may modulate TB risk differently across ethnicities and should be considered in population-specific screening and intervention strategies.
2.3. Environmental Influences: Air Pollution, Tobacco Smoke, and Allergen Exposure
Environmental factors significantly modulate immune responses and may further influence the relationship between allergy and TB. Air pollutants such as PM2.5 and diesel exhaust particles can directly activate MCs through oxidative stress and Toll-like receptor (TLR) pathways, leading to increased secretion of pro-inflammatory cytokines (e.g., TNF-α, IL-6) and suppression of antimicrobial immunity. Diesel exhaust particles have also been shown to impair phagocytic activity of alveolar macrophages, further weakening the first-line defense against Mtb [13,14].
Cigarette smoke, another potent environmental risk factor, promotes MC degranulation and enhances vascular permeability in the lungs. It not only aggravates allergic inflammation but also impairs mucociliary clearance and antigen presentation, creating a permissive environment for Mtb colonization and replication. Notably, both smoking and allergic diseases such as asthma are independently associated with an increased risk of TB, as demonstrated in large-scale cohort and meta-analytic studies [20,22,23]. Smoking has been shown to elevate TB risk by up to 2.7-fold [22,23], while asthma and allergic rhinitis are also linked to significantly higher incidence of mycobacterial diseases [20]. Although no study to date has directly quantified the synergistic effect of smoking and allergic conditions combined, the co-existence of these risk factors may plausibly lead to even greater TB susceptibility. The immunosuppressive effects of chronic allergic inflammation, compounded by smoking-induced impairment of pulmonary immunity, suggest a potential additive or even multiplicative risk that warrants further investigation.
2.4. Gut–Lung Axis and Microbiota-Immune Crosstalk
Recent studies have highlighted the gut–lung axis as a critical modulator of systemic immunity. Dysbiosis in the gut microbiota, often observed in individuals with allergic diseases, can influence immune cell differentiation and cytokine profiles at distal mucosal sites, including the lungs [24,25,26]. Germ-free or antibiotic-treated mice display impaired pulmonary IFN-γ responses and increased susceptibility to Mtb, suggesting that a balanced gut microbiota is essential for maintaining protective immunity [25]. In allergic individuals, dietary patterns high in processed foods and low in fiber can exacerbate this dysbiosis, skewing immune responses further toward Th2 dominance [24,26]. Interventions targeting the microbiota through probiotics, prebiotics, or dietary changes may therefore represent novel strategies for enhancing host resistance to TB, particularly in allergic populations [15,25,26].
2.5. Clinical and Public Health Implications of Mast Cell-Driven Immune Dysregulation in Tuberculosis
Understanding the complex interplay between allergic diseases and TB holds profound implications for both clinical care and public health policy. Individuals with chronic allergic conditions such as asthma, allergic rhinitis, or atopic dermatitis often exhibit a Th2-biased immune profile, which can undermine Th1-mediated defenses critical for controlling Mtb. This immunological imbalance may contribute to increased susceptibility to TB infection and complicate disease progression (Table 1).

Table 1.
Comparative Overview of Mast Cell Activity in Allergic Diseases and Tuberculosis.
From a public health perspective, this necessitates more tailored approaches to TB surveillance and prevention. Targeted screening for LTBI among patients with allergic diseases, particularly in TB-endemic regions, could enable early detection and reduce the risk of reactivation. Moreover, mitigating environmental risk factors such as exposure to air pollution, cigarette smoke, and indoor allergens could simultaneously benefit both allergic and TB-related health outcomes. Urban environmental policies aimed at reducing fine particulate matter (PM2.5) may thus serve dual roles in infectious disease and allergy prevention [2,16].
At the clinical level, a growing interest in HDTs has opened the door to novel treatment strategies that address the underlying immune dysregulation present in allergic individuals. Antihistamines such as cetirizine have been shown in randomized controlled trials to enhance IFN-γ production and improve macrophage activation in atopic individuals [27,28]. MC stabilizers, with cromolyn sodium demonstrating in a Phase II clinical trial the ability to attenuate TGFβ levels and slow progression of pulmonary fibrosis in patients with chronic asthma, may help limit MC degranulation and pro-fibrotic cytokine release [29]. Likewise, leukotriene receptor antagonists (e.g., montelukast) may attenuate inflammation by inhibiting leukotriene-mediated immune recruitment, which plays a dual role in allergy exacerbation and TB lesion formation [30].
In individuals with marked Th2 dominance, cytokine-modulating therapies targeting IL-4, IL-5, or IL-13 (e.g., dupilumab) may also rebalance immune responses toward a Th1-predominant state more conducive to bacterial clearance. Although such interventions are in earlier stages of exploration for TB, they are already in clinical use for allergic disorders and could be repurposed within well-designed translational studies [31].
Furthermore, interventions involving the gut–lung axis, such as probiotic supplementation or high-fiber dietary modifications, may support systemic immune modulation and reduce allergic inflammation, potentially enhancing pulmonary defenses against Mtb [24,25,26]. Future clinical trials are warranted to investigate the synergistic benefits of these adjunctive therapies in TB patients with comorbid allergic conditions.
Finally, personalized medicine approaches incorporating cytokine profiling, MC activity assays, and genotyping of immune-related polymorphisms (e.g., IL-4 −589C/T, IL-13 R130Q, IFN-γ +874T/A) could help stratify patients by risk and optimize treatment selection [12,32]. A precision immunology framework tailored to the allergic-TB spectrum would not only improve individual outcomes but also inform more cost-effective public health interventions.
3. Gene–Environment Interactions and Molecular Mechanisms in Mast Cell-Mediated TB Pathogenesis
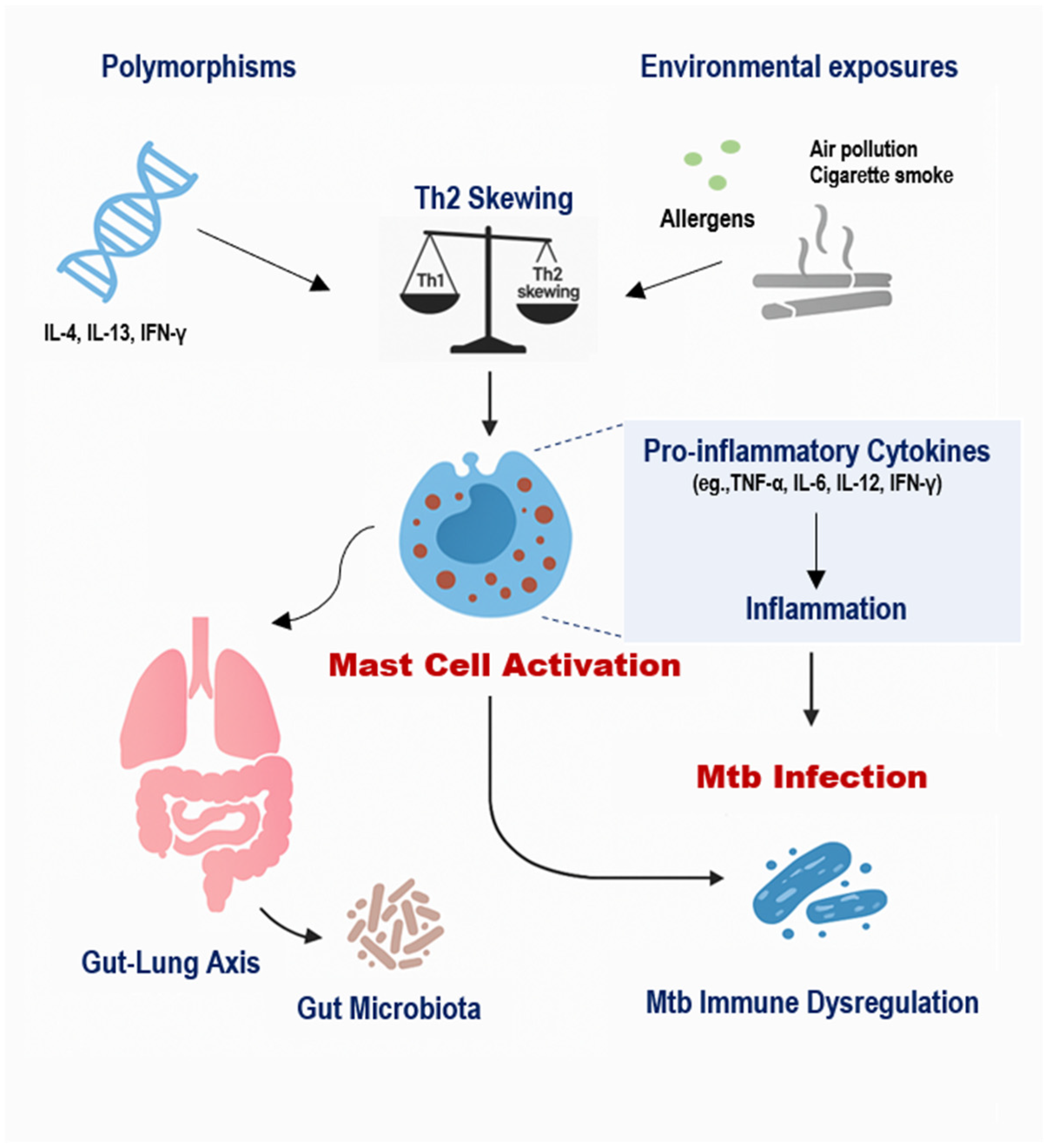
The immunopathogenesis of TB in individuals with allergic diseases is influenced by a multifaceted interplay between host genetics, environmental exposures, and MC-mediated immune responses (Figure 2) [1,32]. MCs, long recognized for their role in allergic inflammation, are increasingly appreciated as active participants in the host response to Mtb [2,4]. Recent studies have elucidated both protective and pathogenic roles of MCs in TB, suggesting that their function is highly context-dependent, influenced by genetic background, receptor expression, local cytokine milieu, and environmental stimuli [6,17,19].
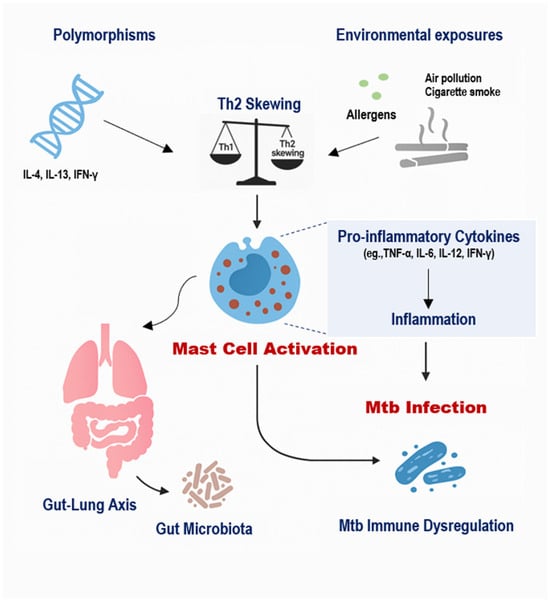
Figure 2.
Integrated Model of Genetic, Environmental, and Microbial Regulation of Mast Cell Activity in TB. Genetic polymorphisms such as IL-4 −589C/T, IL-13 R130Q, and IFN-γ +874T/A influence cytokine profiles and predispose individuals to Th2-skewed immunity, which enhances MC activation. Environmental exposures, including air pollution, cigarette smoke, and allergens, further stimulate MCs, leading to the release of histamine, TGF-β, and pro-inflammatory cytokines. Additionally, gut dysbiosis impairs regulatory immune pathways and promotes systemic inflammation, indirectly amplifying MC responses. Together, these factors modulate MC behavior in the lung, contributing to impaired macrophage activation, altered granuloma formation, and increased fibrosis in TB, especially among individuals with allergic backgrounds.
3.1. Genetic Factors Regulating Mast Cell Responses in TB
Genetic polymorphisms in immune-regulatory genes significantly influence the susceptibility and progression of TB, particularly in individuals with Th2-skewed responses. Allelic variations in cytokine genes such as IL-4 (−589C/T), IL-13 (R130Q), and IFN-γ (+874T/A) are well-documented in the literature and have been associated with altered transcriptional activity and cytokine production profiles [12,32,33]. These polymorphisms often promote a Th2-dominant phenotype, characterized by elevated IgE, IL-4, IL-5, and IL-13 levels, which in turn enhance MC proliferation and sensitivity to activation [19,33,34,35]. Such a shift may impair Th1-driven macrophage activation critical for the intracellular killing of Mtb and thereby increase host susceptibility [7,8,9,24,35].
In the context of TB, IL-12 is a key cytokine for initiating Th1 responses and stimulating IFN-γ production by T cells and NK cells. Polymorphisms in IL-12B (such as the 1188A/C variant) have been shown to diminish IL-12 production and have been associated with higher TB susceptibility [7,8]. Since MCs are capable of producing IL-12 upon microbial stimulation, these polymorphisms may affect not only systemic T cell responses but also local MC-derived IL-12 signaling, impairing the early containment of Mtb.
3.2. TLR2-Mediated Mast Cell Activation: A Crucial Defense Axis
Recent murine studies have demonstrated that MCs can directly sense Mtb components via pattern recognition receptors, particularly TLR2. TLR2-deficient (TLR2−/−) mice infected with Mtb exhibit defective immune responses characterized by impaired recruitment of myeloid cells, poorly organized granulomas, and reduced levels of key cytokines (e.g., IL-1β, TNF-α, IL-6). Notably, restoration of mast cell function via adoptive transfer of TLR2-competent bone marrow-derived mast cells (BMMCs) reestablishes protective immunity, including improved granuloma structure and CD8+ T cell localization in the lungs [6]. These findings suggest that TLR2-dependent MC activation is essential for orchestrating both innate and adaptive responses during TB infection.
Furthermore, Mtb engages additional MC surface molecules, such as CD48, a GPI-anchored receptor involved in microbial recognition. Studies have shown that CD48 ligation by Mtb leads to intracellular calcium flux and MC degranulation, accompanied by the release of TNF-α, IL-6, and leukotriene B4, which are critical for neutrophil recruitment and early granuloma formation [2]. However, excessive release of these mediators may also amplify inflammation and tissue injury, especially in sensitized individuals with allergic backgrounds [4,9].
3.3. Mast Cell Distribution and Phenotype in Human TB Lesions
Human autopsy studies of TB-infected lungs have provided compelling evidence for dynamic MC redistribution and phenotypic shifts during disease progression. In a study of 44 TB necropsies, MCs were found to accumulate at the periphery of granulomas, within pneumonic infiltrates, and particularly in fibrotic regions of the lung. These cells exhibited immunoreactivity for both tryptase and chymase (MCTC phenotype), indicating a transition from mucosal-type MCs (MCT) to connective-tissue-type MCs, known to promote fibrosis via release of TGF-β and matrix remodeling enzymes [1,4].
Importantly, these MCs were shown to contain intracellular Mtb antigens, suggesting their role as potential phagocytic reservoirs of Mtb, although the extent to which they can restrict or harbor replicating bacilli remains unclear [1,2,7]. Co-staining for IL-17A and TGF-β further demonstrated the dual functionality of MCs in early pro-inflammatory signaling and late-stage fibrosis development [1,4]. This phenotypic plasticity underscores the complexity of MC involvement in TB pathogenesis.
In line with this, population-based studies have also identified structural lung diseases such as COPD as significant risk factors for TB, suggesting that chronic pulmonary remodeling and inflammation—processes involving MCs—may render the lung more susceptible to Mtb infection and tissue injury [36].
3.4. Environmental Triggers of Mast Cell Activation and TB Susceptibility
Environmental exposures such as particulate matter (PM2.5), cigarette smoke, and microbial products are known to activate MCs and exacerbate allergic inflammation. Diesel exhaust particles, for example, induce MC degranulation and elevate TNF-α and IL-6 release, which can impair alveolar macrophage function and reduce phagocytic clearance of Mtb [14]. Cigarette smoke similarly promotes MC activation via oxidative stress pathways, increases histamine release, and disrupts epithelial barrier integrity factors that collectively enhance TB susceptibility [13,22,23].
Moreover, allergen exposure in sensitized individuals (e.g., house dust mites, pollen) primes MCs through IgE cross-linking, which may further amplify Th2 polarization and inhibit IFN-γ-mediated macrophage responses to Mtb [19,27]. These environmental cofactors, in combination with genetic predisposition, create a permissive immunological environment for Mtb persistence and disease progression [9,12,32].
3.5. Gut-Lung Axis, Microbiota, and Mast Cell Crosstalk
Recent studies suggest that the gut microbiota can influence pulmonary immunity through systemic modulation of MC activity. Dysbiosis, induced by Western diets or antibiotic overuse, is associated with increased gut permeability, systemic endotoxemia, and enhanced Th2 responses. This altered gut-lung axis may contribute to MC hyperreactivity and impaired containment of Mtb in the lungs [24,25,26]. Certain probiotics, such as Lactobacillus rhamnosus, have been shown to reduce MC degranulation, restore epithelial barrier integrity, and downregulate IL-4 and IL-13 expression [24,26]. These effects offer potential adjunctive benefits for TB management in allergic individuals, although direct clinical evidence in TB cohorts remains limited and requires further investigation [2,15].
3.6. Dual Roles of Mast Cell-Derived Mediators in TB Immunopathology
MC-derived mediators exhibit dual roles in TB, often exerting protective or pathological effects depending on the context (Table 2). Histamine, released from activated MCs, suppresses IFN-γ synthesis by T cells and NK cells, thereby hindering macrophage activation and intracellular killing of Mtb [28,37]. Elevated histamine levels also promote vasodilation and vascular leakage, potentially facilitating bacterial dissemination [27].

Table 2.
Dual Roles of Mast Cell-Derived Mediators in TB Immunopathology.
Leukotriene B4 (LTB4), another MC product, contributes to neutrophil recruitment required for granuloma formation, but excessive levels may drive tissue damage and lung pathology [6]. Similarly, tryptase and chymase promote extracellular matrix (ECM) turnover during inflammation, yet their sustained release has been linked to pulmonary fibrosis and structural remodeling in TB [1,4].
4. The Role of Bacterial Contamination in TB Pathogenesis and Allergy Sensitization
Environmental exposure to bacterial components can exert both protective and pathogenic effects on immune system development and disease susceptibility. According to the hygiene hypothesis, reduced microbial exposure during early childhood may impair the maturation of the immune system, leading to an increased prevalence of allergic disorders [38]. This is primarily attributed to a skewed Th2 response in the absence of sufficient microbial stimuli that would otherwise promote Th1 or regulatory immune pathways [26,37].
In contrast, regular exposure to certain environmental mycobacteria, including non-tuberculous species, may induce cross-protective immune responses that help contain Mtb [39]. Experimental models have shown that innate immune priming through microbial ligands, particularly those engaging pattern recognition receptors such as toll-like receptors (TLRs), can enhance resistance to TB [6,17]. For example, activation of TLR2 by bacterial lipoproteins has been shown to stimulate MCs to release TNF-α and IL-6, which are important for macrophage recruitment and early granuloma formation [6]. Mice lacking TLR2 exhibit impaired granuloma architecture and reduced myeloid cell infiltration, a phenotype that is reversed upon adoptive transfer of TLR2-positive MCs, highlighting the role of bacterial recognition pathways in both innate activation and tissue remodeling during TB infection [17].
Beyond protective priming, environmental bacterial contamination, including microbial residues in food or polluted air, may act as adjuvants that potentiate allergic sensitization. These bacterial fragments can activate MCs and other antigen-presenting cells through PRRs, leading to excessive cytokine production and inappropriate immune skewing [26,38]. MCs, in particular, respond differentially to bacterial genera, releasing unique profiles of cytokines depending on the microbial stimulus [6]. In the context of food contamination, repeated low-dose exposure to bacterial endotoxins may chronically activate mucosal MCs, increasing the risk of both allergic sensitization and immune dysregulation [38]. On the other hand, bacterial residues present in food, dust, or polluted air can act as immunological adjuvants that enhance allergic sensitization. For example, repeated exposure to low concentrations of endotoxins such as lipopolysaccharide can prime mucosal mast cells and skew the immune response toward Th2, increasing susceptibility to allergic diseases [13,26,38].
Moreover, environmental endotoxins such as lipopolysaccharide (LPS) can enhance MC degranulation and induce IL-1β, TNF-α, and IL-6 production, contributing to an inflammatory milieu that may facilitate Mtb pathogenesis [14]. This is especially relevant in urban environments with high levels of airborne bacterial and fungal fragments. Epidemiological studies suggest that early-life exposure to farm environments rich in microbial diversity is associated with reduced incidence of allergic asthma and potentially lower TB susceptibility, likely due to balanced immune maturation [37,38].
The interplay between bacterial contamination and MC activity underscores the importance of context in determining disease trajectory. While controlled microbial exposure can support immune competence and resistance to TB, dysregulated or chronic exposure, particularly in allergen-sensitized individuals, may instead exacerbate inflammation, impair Th1 immunity, and disrupt pulmonary barrier function [26,38,39].
5. The Role of Gut Microbiota in TB and Allergy Pathogenesis
The human gut microbiota plays a critical role in shaping both systemic and mucosal immunity, with far-reaching implications for the pathogenesis of allergic diseases and infections such as TB. Increasing evidence supports the existence of a bidirectional gut–lung axis, whereby alterations in intestinal microbial composition can influence pulmonary immune responses and disease outcomes [15,25,26]. This connection is particularly relevant in the context of MC biology, as MC activation and recruitment can be modulated by microbial-derived signals originating in the gut [37,38].
In allergic diseases, gut dysbiosis is often characterized by reduced microbial diversity and a lower abundance of immunoregulatory bacteria such as Bifidobacterium and Faecalibacterium prausnitzii. This microbial imbalance fosters a pro-inflammatory environment and favors the development of Th2-dominant responses, leading to heightened IgE production, eosinophilic infiltration, and MC hyperactivation [24,38]. Similarly, in TB, perturbations in gut microbiota have been associated with weakened Th1 immunity and impaired host defense mechanisms. Individuals with active TB often exhibit a significant depletion of short-chain fatty acid (SCFA)-producing bacteria, which are essential for maintaining regulatory T cell (Treg) homeostasis and limiting excessive inflammation [2,15].
Recent studies have demonstrated that specific gut microbiota-derived metabolites, such as butyrate and propionate, can directly inhibit MC degranulation and cytokine release. These SCFAs act through G protein-coupled receptors (e.g., GPR41, GPR43) expressed on MCs and other immune cells, ultimately modulating the production of key mediators such as IL-6, TNF-α, and IL-13 [37,38]. In the context of TB, this regulatory axis may be essential to prevent immunopathology while preserving effective bacterial clearance. Conversely, dysbiosis-induced loss of SCFA signaling can heighten MC reactivity, enhancing inflammation and tissue remodeling in the lungs [15,26].
The influence of gut microbiota on TB progression is further underscored by experimental evidence showing that antibiotic-induced dysbiosis exacerbates Mtb infection in murine models, while fecal microbiota transplantation from healthy donors restores bacterial control and reduces lung pathology. Moreover, co-housing TB-susceptible and TB-resistant mice leads to partial microbiota transfer and a corresponding shift in disease outcome, implicating the gut microbiome as a modifiable risk factor in TB [25].
In allergic individuals, the convergence of gut dysbiosis and Mtb infection may create a synergistic vulnerability. For example, dysbiosis-induced enhancement of IL-4 and IL-13 secretion can suppress IL-12 production and IFN-γ responses, thereby impairing macrophage activation [24,33,35]. Additionally, MCs in the gastrointestinal tract interact closely with the gut microbiota and contribute to mucosal immune surveillance [38]. When primed by dysbiotic signals, these MCs may undergo phenotypic changes that affect their behavior systemically, including in the lung microenvironment, where they can exacerbate granulomatous inflammation and promote fibrosis [15,22].
Dietary interventions offer a promising route to restoring gut microbial balance and modulating systemic immunity. High-fiber diets, rich in fermentable polysaccharides, enhance the production of SCFAs and support Treg expansion [37]. Probiotic supplementation with Lactobacillus or Bifidobacterium strains has been shown to downregulate MC activation and shift the Th2/Th1 balance in favor of antimicrobial resistance [37,38]. In TB, these strategies may improve host responses to infection, particularly in individuals with concurrent allergic disease [25].
Future clinical studies should prioritize evaluating the gut microbiota composition of TB patients with and without allergic comorbidities. Longitudinal analyses would help determine whether microbial signatures correlate with disease severity, MC phenotype, or response to therapy [15,25]. Additionally, integrative approaches combining microbiome profiling, cytokine analysis, and MC functional assays could uncover novel therapeutic targets along the gut–lung axis [26,38]. Understanding how gut-derived signals intersect with pulmonary immunity via MC modulation will be crucial for developing host-directed interventions that can reduce pathology and improve treatment outcomes in TB, particularly in the growing population of patients affected by allergic conditions.
6. The Impact of Immune Mechanisms on TB Pathogenesis and Mast Cell Activity
The immune response against Mtb is orchestrated through a dynamic interplay between innate and adaptive immune mechanisms. MCs, traditionally associated with allergic responses, have emerged as important players in the innate immune landscape of TB, influencing both early host defense and long-term tissue remodeling. However, the dual role of MCs—protective in some contexts and pathogenic in others—underscores the need to dissect the immune mechanisms that modulate their activity during TB infection [1,4,6].
6.1. Innate Immune Recognition and MC Activation
MCs express multiple pattern recognition receptors (PRRs), including TLR2, which recognize Mtb-derived ligands such as 19 kDa lipoproteins, lipomannan, and phosphatidylinositol mannosides. Activation via TLR2 promotes the release of key pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, which are essential for recruiting neutrophils and monocytes to infection sites. Studies using TLR2-deficient mice have underscored the receptor’s critical role in host defense against TB, revealing heightened bacterial burden and disrupted granulomatous responses. Complementation with TLR2-expressing mast cells partially reverses these defects, enhancing cytokine output and lymphocyte trafficking to infected lung tissue [6]. These findings highlight the essential role of TLR2-dependent MC activation in establishing a protective immune milieu capable of controlling Mtb infection [2,6].
6.2. Adaptive Immune Cross-Talk and Cytokine Modulation
MCs influence adaptive immunity both directly and indirectly. Upon activation by Mtb antigens, MCs can release IL-12, promoting a Th1-skewed response that enhances macrophage activation [8]. However, in allergic individuals with a Th2-biased cytokine profile, MCs may instead contribute to the secretion of IL-4, IL-5, and IL-13, dampening IFN-γ production and compromising TB control [9,11,19]. The net effect of MC activity thus depends heavily on the surrounding cytokine environment. For example, MC-derived histamine has been shown to inhibit IFN-γ synthesis in both T cells and NK cells, thereby weakening cell-mediated immunity against intracellular pathogens like Mtb [3,27].
6.3. Localization and Phenotypic Shifts in Mcs in Human TB Lesions
Immunohistological studies of post-mortem lung tissues from TB patients have provided critical insights into MC distribution and function. MCs are enriched in inflammatory regions such as the periphery of granulomas, alveolar walls, and fibrotic zones. Notably, MCs in fibrotic areas exhibit an MCTC phenotype (co-expressing tryptase and chymase), which is strongly associated with tissue remodeling and fibrosis [4]. These cells also express TGF-β and IL-17A, implicating MCs in both the fibrogenic and immunomodulatory processes during chronic TB infection. Their absence in the necrotic core but presence in surrounding regions suggests a strategic positioning that allows them to shape granuloma dynamics and subsequent lesion evolution [1].
6.4. Context-Dependent Mediator Release
The dual role of MC activation is especially relevant in the therapeutic context. While MC-derived TNF-α and IL-6 promote granuloma stability and immune cell recruitment, overproduction of proteases such as tryptase and chymase, or cytokines like TGF-β, can lead to fibrosis and structural lung damage [1,3,4,5]. Likewise, excessive leukotrienes, including LTB4, may aggravate inflammation and compromise granuloma integrity [4,39]. These observations highlight the importance of context-aware modulation of MC activity. Rather than indiscriminate suppression, tailored interventions that balance their beneficial and harmful outputs could preserve host defense while minimizing immunopathology in TB.
6.5. Implications for Immunotherapeutic Targeting
Given their immunoregulatory plasticity, MCs represent a potential therapeutic target in TB, especially in individuals with predisposing allergic inflammation. Strategies that promote beneficial MC functions such as IL-12 secretion and controlled TNF-α release while minimizing pathogenic outputs like TGF-β or chymase may optimize host defenses [4,7,18]. Moreover, combining immunomodulators with current TB therapies could help reestablish immune balance in patients with a Th2-skewed background, preventing excessive fibrosis without compromising bacterial control [9,35].
7. Conclusions
Mast cells (MCs), long recognized as central effectors in allergic responses, have now emerged as multifaceted players in the immunopathogenesis of TB. This review has examined the complex and context-dependent role of MCs at the intersection of allergic inflammation and Mtb infection, highlighting their potential to act both as immune sentinels and as contributors to pathological outcomes such as chronic inflammation and pulmonary fibrosis [1,4].
Evidence from both murine models and human histopathological studies shows that MCs participate actively in the immune response to TB by releasing pro-inflammatory cytokines such as TNF-α and IL-6 and by contributing to early cellular recruitment and granuloma formation. In particular, TLR2-mediated MC activation has been shown to restore immune competence in TLR2-deficient mice infected with Mtb, reinforcing the significance of MCs in host defense [6]. Concurrently, excessive or dysregulated MC activity—characterized by chronic release of TGFβ, histamine, tryptase, and chymase—can exacerbate tissue damage, promote fibrosis, and impair Th1-mediated bacterial control [4,39].
The duality of MC function becomes critical in individuals with pre-existing allergic diseases. In these hosts, Th2-skewed environments dominate, favoring MC-mediated release of IL-4, IL-5, and IL-13, suppressing IFNγ production, and undermining macrophage activation necessary for controlling Mtb [9,20]. Genetic polymorphisms in IL-4, IL-13, and IFNγ genes further compound this vulnerability, contributing to altered MC behavior and increased TB susceptibility [12,33].
Environmental factors such as air pollution, cigarette smoke, and gut dysbiosis further modulate MC activity, with demonstrated effects on both allergic sensitization and TB outcomes [13,14,15,40]. Hence, integrating environmental health strategies alongside immunological and genetic screening is essential for future TB control frameworks.
Clinically, the growing recognition of MCs as immunological hubs suggests a new frontier for HDTs. Therapeutic strategies stabilizing MCs, inhibiting IL-4/IL-13 pathways, or reorienting immune responses toward Th1 dominance may benefit TB patients with allergic comorbidities [18,35]. Additionally, probiotic and dietary interventions targeting the gut–lung axis show promise for systemic immune modulation [26,37].
Looking ahead, future research should prioritize the following:
- -
- Comprehensive profiling of MC phenotypes and functions across TB stages;
- -
- Longitudinal epidemiological studies on TB incidence/severity in allergic populations;
- -
- Development and validation of MC-directed HDTs in preclinical/clinical TB models;
- -
- Exploration of personalized medicine approaches incorporating cytokine profiling, genetic risk markers, and environmental exposure data for patient stratification and tailored interventions.
Recent findings also suggest that alarmins such as IL-33 and TSLP—epithelial-derived cytokines involved in type 2 and innate inflammation—may influence MC activation in the context of TB. Although direct evidence is limited, IL-33 has been shown to prime human mast cells for enhanced responsiveness to mycobacterial stimuli, and co-administration of IL-33 with TB DNA vaccines augments Th1-type responses in murine models [41,42]. These observations highlight the need for further re-search into how alarmin–MC interactions may shape the immune landscape of TB, particularly in allergic individuals.
By integrating immunology, genetics, and environmental health perspectives, this review repositions MCs from peripheral allergy mediators to central regulators of TB immunity. A nuanced understanding of their dual roles may lead to optimized clinical outcomes at this intersection of major global health challenges [1,4,6,26].
Author Contributions
Conceptualization, H.S.; investigation, G.P., H.-S.L. and S.H.L.; resources, Y.H. and H.S.; writing—original draft preparation, S.H.L.; writing—review and editing, H.S.; supervision, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank their lab members for helpful discussions during the preparation of this manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| MC | Mast Cells |
| Mtb | Mycobacterium tuberculosis |
| TB | Tuberculosis |
| HDT | Host-Directed Therapy |
| LTBI | Latent Tuberculosis Infection |
| Th1 | T-Helper Type 1 |
| Th2 | T-Gelper Type 2 |
| Treg | Regulatory T Cell |
| TLR2 | Toll-Like Receptor 2 |
| TNF-α | Tumor Necrosis Factor-Alpha |
| TGF-β | Transforming Growth Factor-Beta |
| interleukin | G Protein-Coupled Receptor |
| FcεRI | High-Affinity IgE Receptor |
| CD48 | Cluster of Differentiation 48 |
| IFN-γ | Interferon-Gamma |
| PM2.5 | Particulate Matter ≤ 2.5 µm |
References
- Garcia-Rodriguez, K.M.; Bini, E.I.; Gamboa-Domínguez, A.; Espitia-Pinzón, C.I.; Huerta-Yepez, S.; Bulfone-Paus, S.; Hernández-Pando, R. Differential mast cell numbers and characteristics in human tuberculosis pulmonary lesions. Sci. Rep. 2021, 11, 10687. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, S.; Hernández-Pando, R.; Abraham, S.N.; Enciso, J.A. Mast cell activation by Mycobacterium tuberculosis: Mediator release and role of CD48. J. Immunol. 2003, 170, 5590–5596. [Google Scholar] [CrossRef]
- Etna, M.P.; Giacomini, E.; Severa, M.; Coccia, E.M. Pro- and anti-inflammatory cytokines in tuberculosis: A two-edged sword in TB pathogenesis. Semin. Immunol. 2014, 26, 543–551. [Google Scholar] [CrossRef]
- Gupta, A.; Nayak, S.; Dey, R.; Das, P.; Basu, S. Mast cells promote pathology and susceptibility in tuberculosis. Elife 2024, 13, e102634. [Google Scholar] [CrossRef]
- Smith, I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. Rev. 2003, 16, 463–496. [Google Scholar] [CrossRef] [PubMed]
- Carlos, D.; Frantz, F.G.; Souza-Júnior, D.A.; Jamur, M.C.; Oliver, C.; Ramos, S.G.; Quesniaux, V.F.; Ryffel, B.; Silva, C.L.; Bozza, M.T.; et al. TLR2-dependent mast cell activation contributes to the control of Mycobacterium tuberculosis infection. Microbes Infect. 2009, 11, 770–778. [Google Scholar] [CrossRef]
- Tait Wojno, E.D.; Hunter, C.A.; Stumhofer, J.S. The immunobiology of the interleukin-12 family: Room for discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef]
- Wang, H.; Ruan, G.; Li, Y.; Liu, X. The role and potential application of IL-12 in the immune regulation of tuberculosis. Int. J. Mol. Sci. 2025, 26, 3106. [Google Scholar] [CrossRef]
- Ashenafi, S.; Aderaye, G.; Bekele, A.; Zewdie, M.; Aseffa, G.; Hoang, A.T.N.; Carow, B.; Habtamu, M.; Wijkander, M.; Rottenberg, M.; et al. Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOCS3. Clin. Immunol. 2014, 151, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Teles, R.M.B.; Graeber, T.G.; Krutzik, S.R.; Montoya, D.; Schenk, M.; Lee, D.J.; Komisopoulou, E.; Kelly-Scumpia, K.; Chun, R.; Iyer, S.S.; et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science 2013, 339, 1448–1453. [Google Scholar] [CrossRef]
- Parasa, V.R.; Rahman, M.J.; Hoang, A.T.N.; Svensson, M.; Brighenti, S.; Lerm, M. Modeling Mycobacterium tuberculosis early granuloma formation in experimental human lung tissue. Dis. Model. Mech. 2014, 7, 281–288. [Google Scholar] [CrossRef]
- Zhen, L.; Sun, Y.; Gao, J. Interleukin-4 gene polymorphisms and the risk of tuberculosis: A meta-analysis. Cytokine 2023, 169, 156282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, N.; Mao, M.; Zhou, Y.; Wu, Y.; Li, J.; Zhang, W.; Peng, C.; Chen, X.; Li, J. Fine particulate matter (PM2.5) promotes IgE-mediated mast cell activation through ROS/Gadd45b/JNK axis. J. Dermatol. Sci. 2021, 102, 47–57. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, P.; Romero-Andrada, I.; Molina-Moya, B.; Latorre, I.; Lacoma, A.; Prat-Aymerich, C.; Tabernero, L.; Domínguez, J. Impact of diesel exhaust particles on infections with Mycobacterium bovis BCG in in vitro human macrophages and an in vivo Galleria mellonella model. Environ. Pollut. 2024, 341, 122597. [Google Scholar] [CrossRef]
- Shi, W.; Hu, Y.; Ning, Z.; Xia, F.; Wu, M.; Hu, Y.O.O.; Chen, C.; Prast-Nielsen, S.; Xu, B. Alterations of gut microbiota in patients with active pulmonary tuberculosis in China: A pilot study. Int. J. Infect. Dis. 2021, 111, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, S.; Rivas-Santiago, B.; Enciso, J.A. Mycobacterium tuberculosis entry into mast cells through cholesterol-rich membrane microdomains. Scand. J. Immunol. 2009, 70, 256–263. [Google Scholar] [CrossRef]
- Mahajan, P.; Gor, H.R.; Jadhav, S.; Joshi, M.; Nema, V. Host-Directed Therapeutic for the treatment of Mycobacterium tuberculosis. Microbiol. Res. 2025, 299, 128253. [Google Scholar] [CrossRef]
- Caslin, H.L.; Kiwanuka, K.N.; Haque, T.T.; Taruselli, M.T.; MacKnight, H.P.; Paranjape, A.; Ryan, J.J. Controlling mast cell activation and homeostasis: Work influenced by Bill Paul that continues today. Front. Immunol. 2018, 9, 868. [Google Scholar] [CrossRef]
- McLeod, J.J.A.; Baker, B.; Ryan, J.J. Mast cell production and response to IL4 and IL13. Cytokine 2015, 75, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Choi, H.; Lee, H.; Han, K.; Park, D.W.; Park, T.S.; Moon, J.-Y.; Kim, T.-H.; Sohn, J.W.; Yoon, H.J.; et al. Impact of allergic disease on the risk of mycobacterial disease. J. Allergy Clin. Immunol. Pract. 2023, 11, 2830–2838.e4. [Google Scholar] [CrossRef]
- Yeh, J.J.; Wang, Y.C.; Kao, C.H. Asthma-Chronic Obstructive Pulmonary Diseases Overlap Syndrome Increases the Risk of Incident Tuberculosis: A National Cohort Study. PLoS ONE 2016, 11, e0159012. [Google Scholar] [CrossRef]
- Lin, H.-H.; Ezzati, M.; Murray, M. Tobacco smoke, indoor air pollution and tuberculosis: A systematic review and meta-analysis. PLoS Med. 2007, 4, e20. [Google Scholar] [CrossRef] [PubMed]
- Obore, N.; Kawuki, J.; Guan, J.; Papabathini, S.; Wang, L. Association between indoor air pollution, tobacco smoke and tuberculosis: An updated systematic review and meta-analysis. Public Health 2020, 187, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, L.L.; Lu, R.Q.; Ma, W.W.; Xiao, R. Alteration of Intestinal Microbiota Composition in Oral Sensitized C3H/HeJ Mice Is Associated With Changes in Dendritic Cells and T Cells in Mesenteric Lymph Nodes. Front. Immunol. 2021, 12, 631494. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Vidyarthi, A.; Nadeem, S.; Negi, S.; Nair, G.; Agrewala, J.N. Alteration in the gut microbiota provokes susceptibility to tuberculosis. Front. Immunol. 2016, 7, 529. [Google Scholar] [CrossRef]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.A.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef]
- Mo, S.; Guo, J.; Ye, T.; Zhang, X.; Zeng, J.; Xu, Y.; Peng, B.; Dai, Y.; Xiao, W.; Zhang, P.; et al. Mycobac-terium tuberculosis utilizes host histamine receptor H1 to modulate reactive oxygen species production and phagosome maturation via the p38MAPK-NOX2 axis. mBio. 2022, 13, e02004-22. [Google Scholar] [CrossRef]
- Uğuz, A.; Naziroğlu, M.; Şahin, M.; Yüksel, M.; Aykur, M. The effect of cetirizine on IFN-gamma and IL-10 production in children with allergic rhinitis. Turk. J. Pediatr. 2005, 47, 111–115. [Google Scholar] [PubMed]
- Birring, S.S.; Wijsenbeek, M.; Agrawal, S.; van den Berg, J.; Stone, H.; Maher, T.M.; Morice, A.H. A novel formulation of inhaled sodium cromoglicate (PA101) in idiopathic pulmonary fibrosis and chronic cough: A randomised, double-blind, proof-of-concept, phase 2 trial. Lancet Respir. Med. 2017, 5, 806–815. [Google Scholar] [CrossRef]
- Hardwick, C.; White, D.; Morris, E.; Monteiro, E.F.; A Breen, R.; Lipman, M. Montelukast as a potential therapy for TB-associated immune reconstitution inflammatory syndrome. Sex. Transm. Infect. 2006, 82, 513–514. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suárez-Fariñas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, F.; Wang, Y.; Chen, J.; Yuan, X. Association between IFNGR1 gene polymorphisms and tuberculosis susceptibility: A meta-analysis. Front. Public Health 2022, 10, 976221. [Google Scholar] [CrossRef]
- Ober, C.; Yao, T.C. The genetics of asthma and allergic disease: A 21st century perspective. Immunol. Rev. 2011, 242, 10–30. [Google Scholar] [CrossRef]
- Roy, E.; Brennan, J.; Jolles, S.; Lowrie, D.B. Beneficial effect of anti-interleukin-4 antibody when administered in a murine model of tuberculosis infection. Tuberculosis 2008, 88, 197–202. [Google Scholar] [CrossRef]
- Heitmann, L.; Abad Dar, M.; Schreiber, T.; Erdmann, H.; Behrends, J.; McKenzie, A.N.J.; Brombacher, F.; Ehlers, S.; Hölscher, C. The IL-13/IL-4Rα axis is involved in tuberculosis-associated pathology. J. Pathol. 2014, 234, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Inghammar, M.; Ekbom, A.; Engström, G.; Ljungberg, B.; Romanus, V.; Löfdahl, C.-G.; Egesten, A.; Dheda, K. COPD and the Risk of Tuberculosis—A Population-Based Cohort Study. PLoS ONE 2010, 5, e10138. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.Y.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef] [PubMed]
- Ege, M.J.; Mayer, M.; Normand, A.-C.; Genuneit, J.; Cookson, W.O.; Braun-Fahrländer, C.; Heederik, D.; Piarroux, R.; von Mutius, E. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 2011, 364, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, K.; Singh, S.; Phillips, R.O. Non-tuberculous mycobacteria in TB-endemic countries: Are we neglecting the danger? PLoS Negl. Trop. Dis. 2010, 4, e615. [Google Scholar] [CrossRef]
- Li, H.; Yang, T.; Ning, Q.; Li, F.; Chen, T.; Yao, Y.; Sun, Z. Exposure to cigarette smoke alters mast cell phenotype and function in the lung. Inhal. Toxicol. 2015, 27, 822–831. [Google Scholar] [CrossRef]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.-C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.J.; et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, D.O.; Siefert, R.J.; Weiner, D.B. Alarmin IL-33 elicits potent TB-specific cell-mediated responses. Hum. Vaccin. Immunother. 2015, 11, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).