Bone Marrow Stromal Cells Generate a Pro-Healing Inflammasome When Cultured on Titanium–Aluminum–Vanadium Surfaces with Microscale/Nanoscale Structural Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Ti6Al4V Disk Fabrication
2.2. Surface Characterization
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. Contact Angle Analysis
2.2.3. Roughness Analysis
2.2.4. Chemical Analysis
X-Ray Photoelectron Spectroscopy (XPS)
Energy-Dispersive X-Ray (EDX) Analysis
X-Ray Diffraction (XRD)
2.3. Response to Surface Topography
2.3.1. Cell Culture
2.3.2. RNA Expression Analysis
2.4. Growth on Ti6Al4V in GM vs. Growth on TCPS in OM
2.4.1. Cell Culture
2.4.2. Gene Expression Analysis
2.4.3. Bioinformatics Analysis
2.4.4. Functional Enrichment Analysis
3. Results
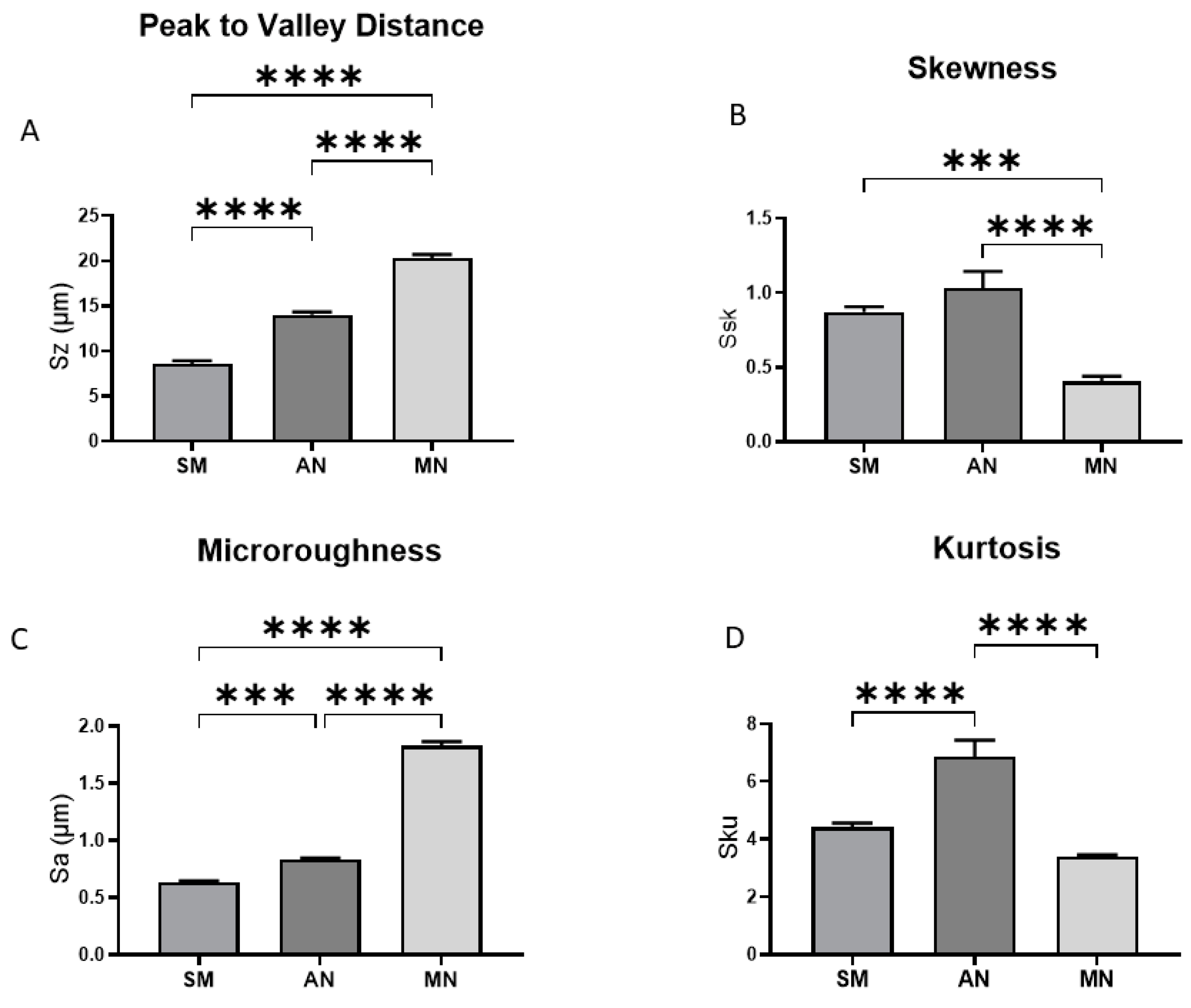
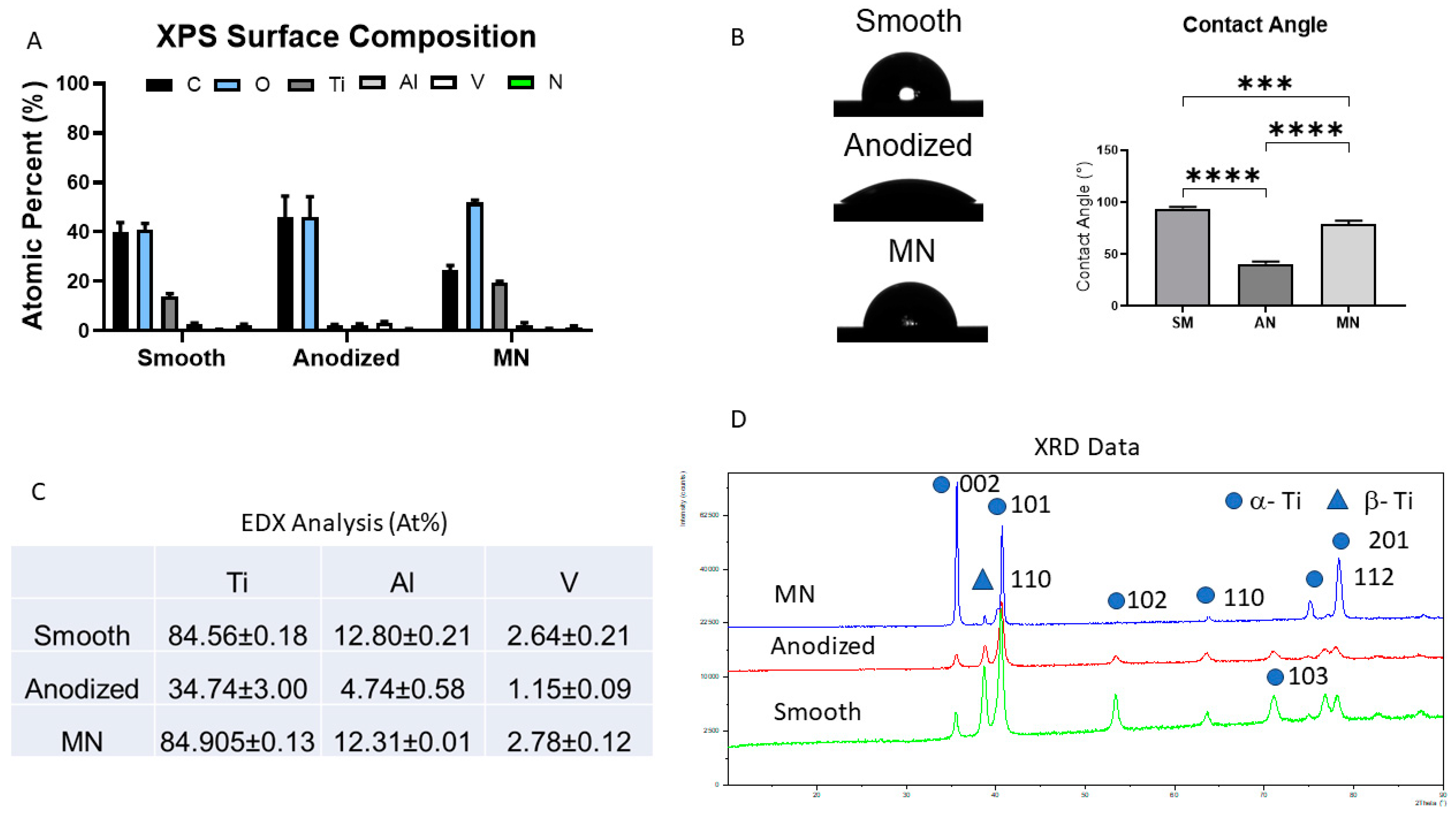
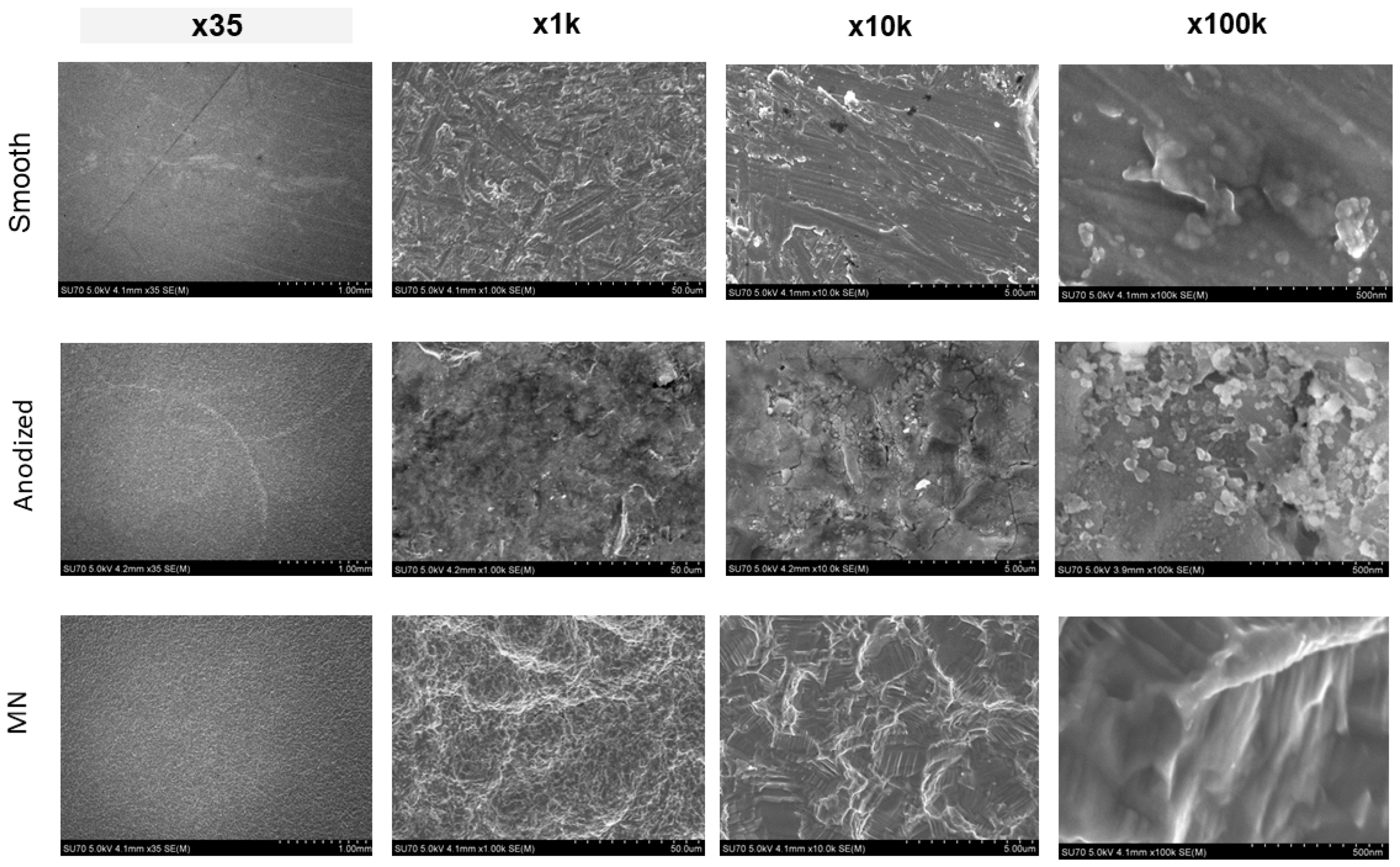
3.1. Surface Characterization and Analysis
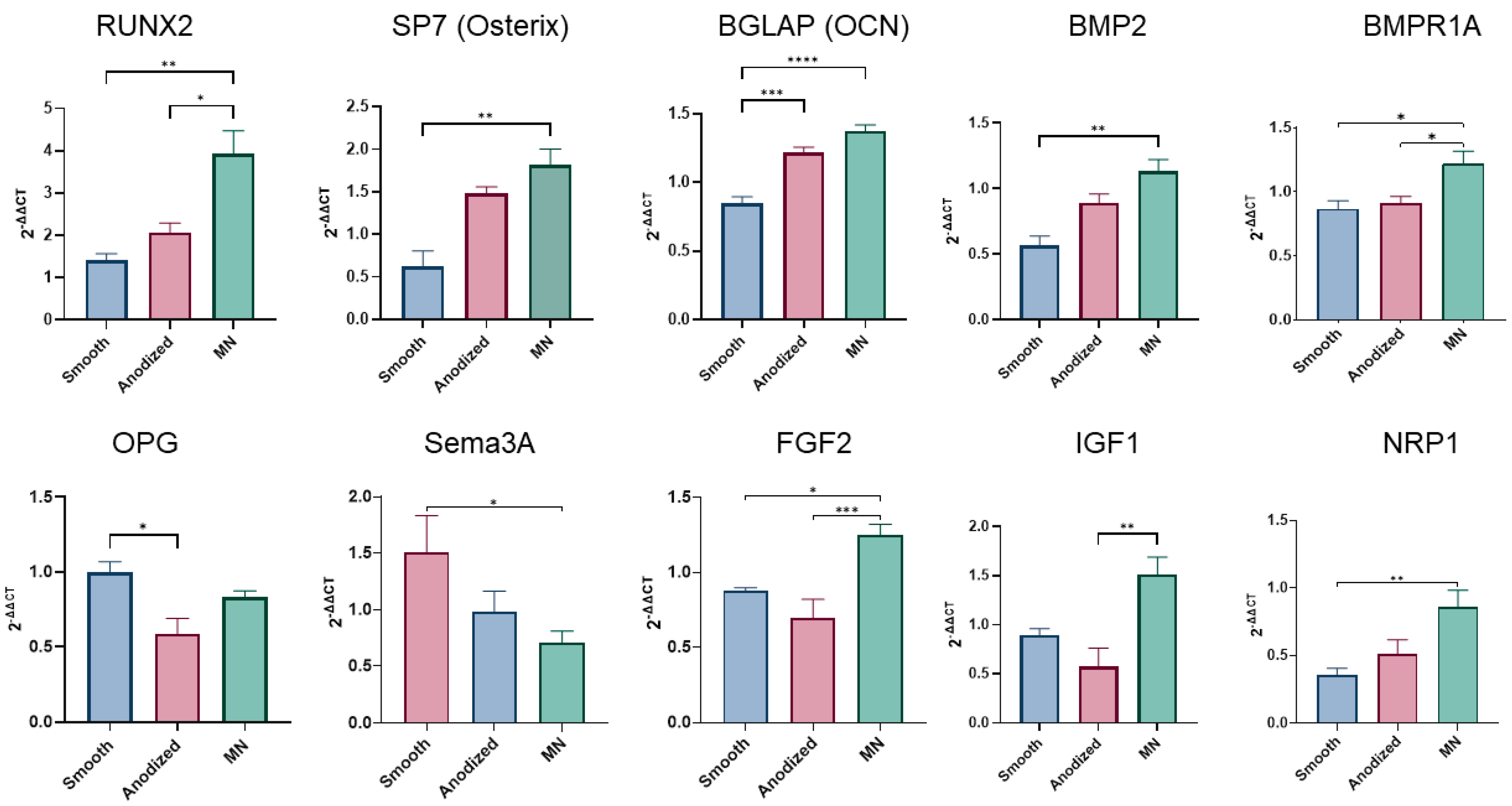
3.2. MSC Response to Surface Topography
3.2.1. Osteoblast Phenotypic Expression
3.2.2. Regulation of Apoptosis
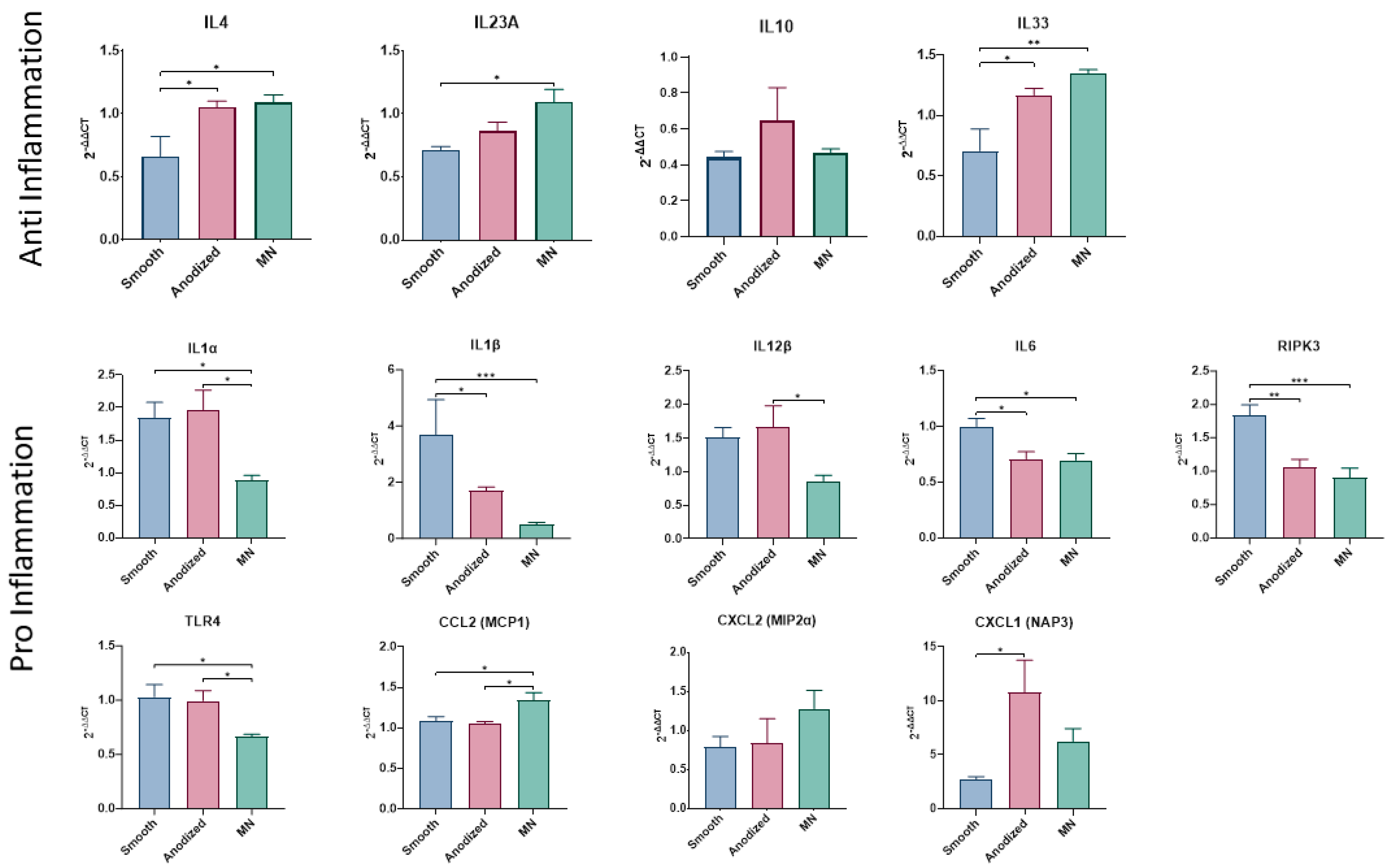
3.2.3. Inflammasome Expression
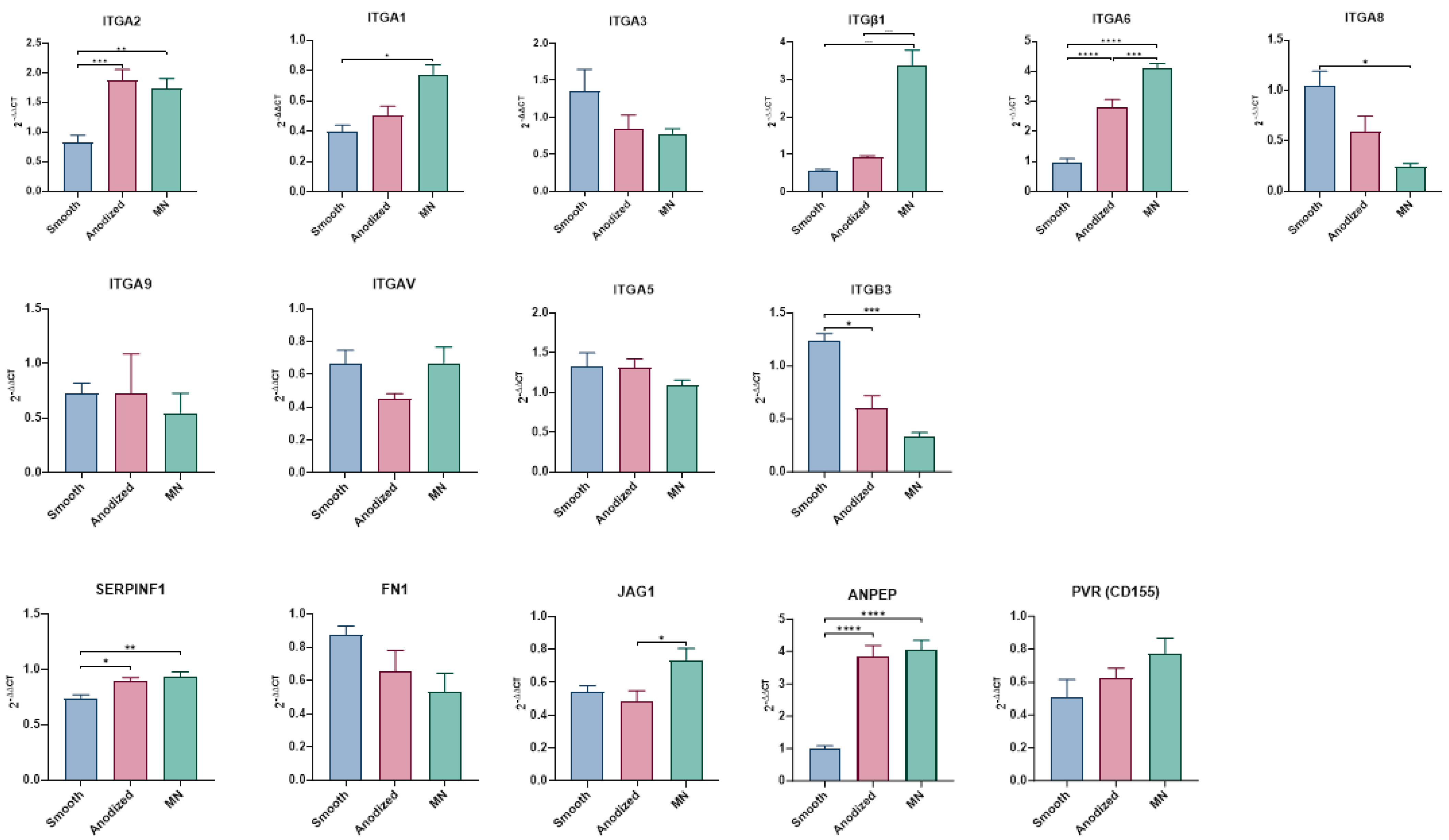
3.2.4. Integrin Expression
3.3. Differential Regulation of Gene Expression and Signaling Pathways in MSCs Cultured on MN-Modified Ti6Al4V Versus TCPS in Osteogenic Media
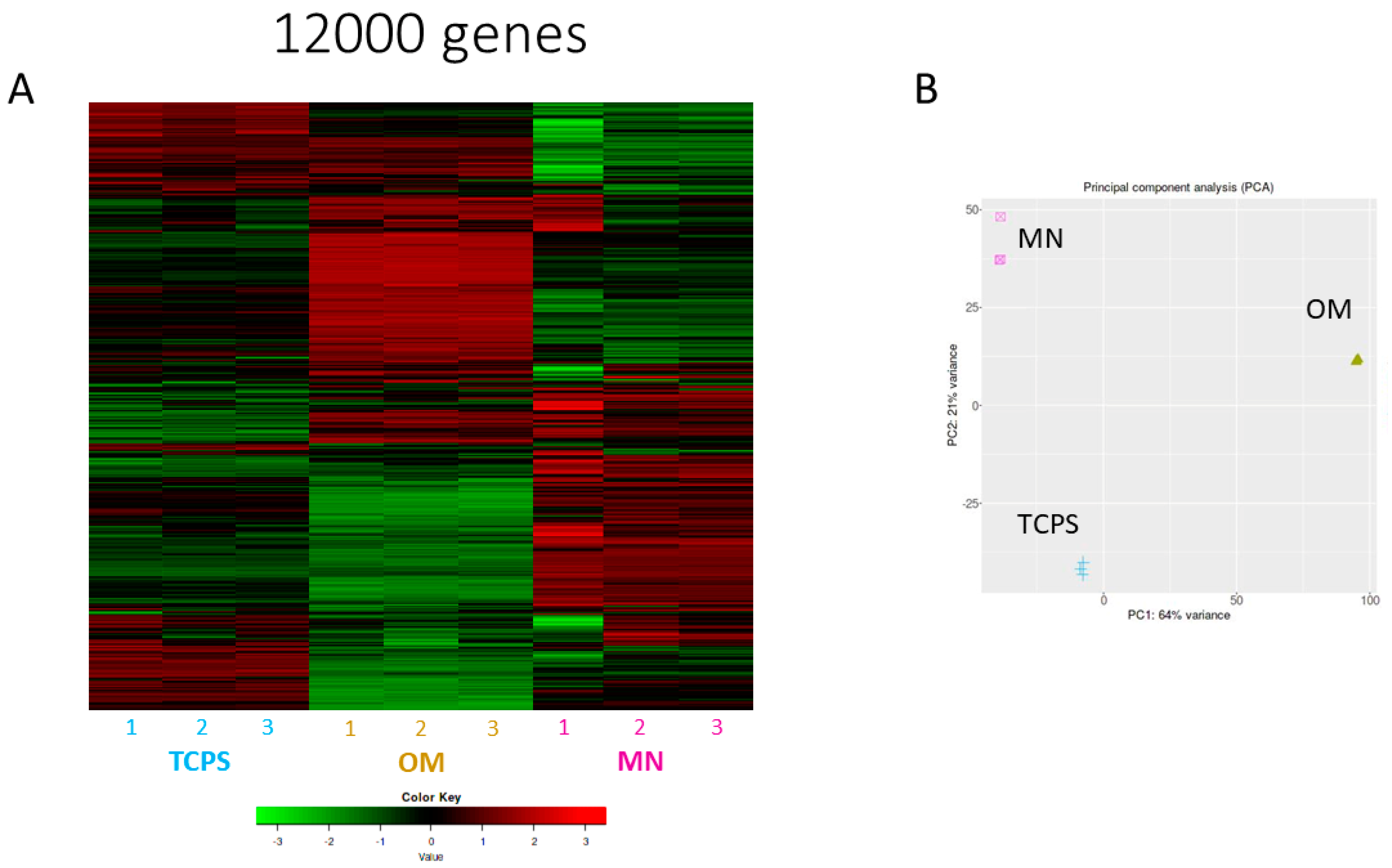
3.3.1. RNA-seq and Principal Component Analysis
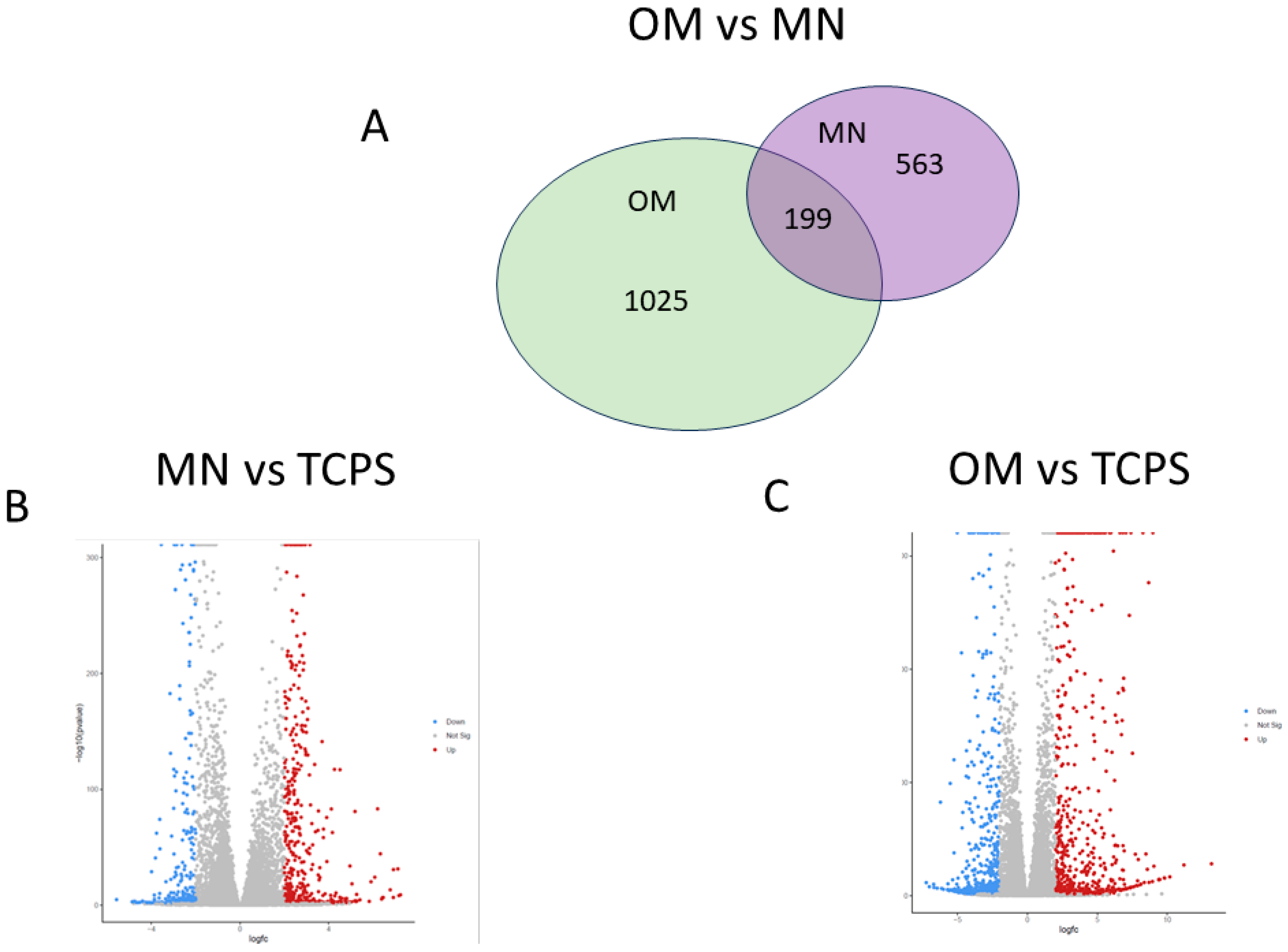
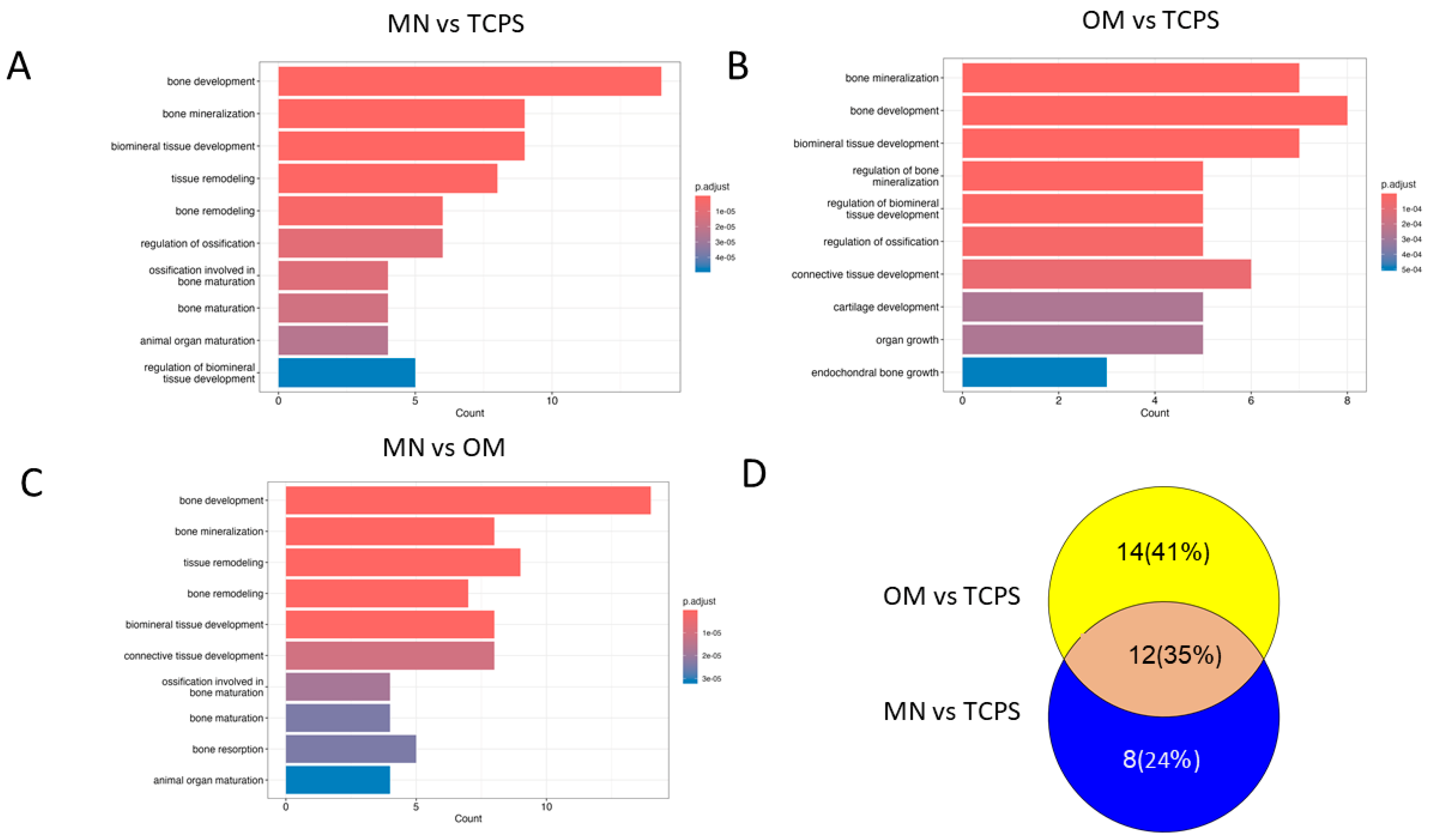
3.3.2. Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, M.B.; Slosar, P.; Schwartz, Z.; Cohen, D.J.; Goodman, S.B.; Anderson, P.A.; Boyan, B.D. A Review of Biomimetic Topographies and Their Role in Promoting Bone Formation and Osseointegration: Implications for Clinical Use. Biomimetics 2022, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Pang, Y.; Zhou, Z.; Yao, R.; Sun, W. An Integrated Cell Printing System for the Construction of Heterogeneous Tissue Models. Acta Biomater. 2019, 95, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.B.; Cohen, D.J.; Bosh, K.B.; Kapitanov, M.; Slosar, P.J.; Levit, M.M.; Gallagher, M.; Rawlinson, J.J.; Schwartz, Z.; Boyan, B.D. Bone Marrow Stromal Cells Generate an Osteoinductive Microenvironment When Cultured on Titanium–Aluminum–Vanadium Substrates with Biomimetic Multiscale Surface Roughness. Biomed. Mater. 2023, 18, 035001. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.B.; Bosh, K.B.; Jacobs, T.W.; Joshua Cohen, D.; Schwartz, Z.; Boyan, B.D. Growth Factors Produced by Bone Marrow Stromal Cells on Nanoroughened Titanium–Aluminum–Vanadium Surfaces Program Distal MSCs into Osteoblasts via BMP2 Signaling. J. Orthop. Res. 2021, 39, 1908–1920. [Google Scholar] [CrossRef] [PubMed]
- Lotz, E.M.; Berger, M.B.; Boyan, B.D.; Schwartz, Z. Regulation of Mesenchymal Stem Cell Differentiation on Microstructured Titanium Surfaces by Semaphorin 3A. Bone 2020, 134, 115260. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Cohen, D.J.; Berger, M.B.; Sabalewski, E.L.; McClure, M.J.; Boyan, B.D.; Schwartz, Z. Osseointegration of Titanium Implants in a Botox-Induced Muscle Paralysis Rat Model Is Sensitive to Surface Topography and Semaphorin 3A Treatment. Biomimetics 2023, 8, 93. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Tunger, A.; Wobus, M.; von Bonin, M.; Towers, R.; Bornhäuser, M.; Dazzi, F.; Wehner, R.; Schmitz, M. Immunomodulatory Properties of Mesenchymal Stromal Cells: An Update. Front. Cell Dev. Biol. 2021, 9, 637725. [Google Scholar] [CrossRef] [PubMed]
- Smolinská, V.; Boháč, M.; Danišovič, Ľ. Current Status of the Applications of Conditioned Media Derived from Mesenchymal Stem Cells for Regenerative Medicine. Physiol. Res. 2023, 72, S233–S245. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Hyzy, S.L.; Berg, M.E.; Schneider, J.M.; Hotchkiss, K.; Schwartz, Z.; Boyan, B.D. Osteoblast Lineage Cells Can Discriminate Microscale Topographic Features on Titanium–Aluminum–Vanadium Surfaces. Ann. Biomed. Eng. 2014, 42, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Navarrete, R.; Hyzy, S.L.; Slosar, P.J.; Schneider, J.M.; Schwartz, Z.; Boyan, B.D. Implant Materials Generate Different Peri-Implant Inflammatory Factors. Spine 2015, 40, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Berger, M.B.; Nelson, F.R.; Donahue, H.J.; Schwartz, Z. The Biological Basis for Surface-Dependent Regulation of Osteogenesis and Implant Osseointegration. J. Am. Acad. Orthop. Surg. 2022, 30, e894–e898. [Google Scholar] [CrossRef] [PubMed]
- Boland, G.M.; Perkins, G.; Hall, D.J.; Tuan, R.S. Wnt 3a Promotes Proliferation and Suppresses Osteogenic Differentiation of Adult Human Mesenchymal Stem Cells. J. Cell Biochem. 2004, 93, 1210–1230. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Olivares-Navarrete, R.; Berger, M.B.; Hyzy, S.L.; Schwartz, Z. Role of Wnt11 during Osteogenic Differentiation of Human Mesenchymal Stem Cells on Microstructured Titanium Surfaces. Sci. Rep. 2018, 8, 8588. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Navarrete, R.; Hyzy, S.L.; Park, J.H.; Dunn, G.R.; Haithcock, D.A.; Wasilewski, C.E.; Boyan, B.D.; Schwartz, Z. Mediation of Osteogenic Differentiation of Human Mesenchymal Stem Cells on Titanium Surfaces by a Wnt-Integrin Feedback Loop. Biomaterials 2011, 32, 6399–6411. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Hermann, C.D.; Cheng, A.; Olivares-Navarrete, R.; Gittens, R.A.; Bird, M.M.; Walker, M.; Cai, Y.; Cai, K.; Sandhage, K.H.; et al. Role of A2β1 Integrins in Mediating Cell Shape on Microtextured Titanium Surfaces. J. Biomed. Mater. Res. A 2015, 103, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Jullien, N.; Maudinet, A.; Leloutre, B.; Ringe, J.; Häupl, T.; Marie, P.J. Downregulation of ErbB3 by Wnt3a Contributes to Wnt-induced Osteoblast Differentiation in Mesenchymal Cells. J. Cell Biochem. 2012, 113, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Cayon, R.; Castillo-Dali, G.; Corcuera-Flores, J.; Serrera-Figallo, M.; Castillo-Oyague, R.; Gonzalez-Martin, M.; Gutierrez-Perez, J.; Torres-Lagares, D. Production of Bone Mineral Material and BMP-2 in Osteoblasts Cultured on Double Acid-Etched Titanium. Med. Oral. Patol. Oral. Cir. Bucal 2017, 22, e651–e659. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.; Kasten, P.; Stiehler, M. Culture Media for the Differentiation of Mesenchymal Stromal Cells. Acta Biomater. 2011, 7, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Bonewald, L.F.; Paschalis, E.P.; Lohmann, C.H.; Rosser, J.; Cochran, D.L.; Dean, D.D.; Schwartz, Z.; Boskey, A.L. Osteoblast-Mediated Mineral Deposition in Culture Is Dependent on Surface Microtopography. Calcif. Tissue Int. 2002, 71, 519–529. [Google Scholar] [CrossRef]
- Bonewald, L.F.; Harris, S.E.; Rosser, J.; Dallas, M.R.; Dallas, S.L.; Camacho, N.P.; Boyan, B.; Boskey, A. Von Kossa Staining Alone Is Not Sufficient to Confirm That Mineralization In Vitro Represents Bone Formation. Calcif. Tissue Int. 2003, 72, 537–547. [Google Scholar] [CrossRef]
- Leboy, P.S.; Beresford, J.N.; Devlin, C.; Owen, M.E. Dexamethasone Induction of Osteoblast MRNAs in Rat Marrow Stromal Cell Cultures. J. Cell. Physiol. 1991, 146, 370–378. [Google Scholar] [CrossRef]
- Volk, S.W.; Diefenderfer, D.L.; Christopher, S.A.; Haskins, M.E.; Leboy, P.S. Effects of Osteogenic Inducers on Cultures of Canine Mesenchymal Stem Cells. Am. J. Vet. Res. 2005, 66, 1729–1737. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Valverde, P.; Murray, D.; Dard, M.M.; Van Dyke, T.; Xu, Q.; Xu, X.; Karimbux, N.; Tu, Q.; et al. Osteogenic Effects of MicroRNA-335-5p/Lipidoid Nanoparticles Coated on Titanium Surface. Arch. Oral. Biol. 2021, 129, 105207. [Google Scholar] [CrossRef] [PubMed]
- Nahum, E.Z.; Lugovskoy, A.; Lugovskoy, S.; Sobolev, A. Synthesis of Titanium Oxide Nanotubes Loaded with Hydroxyapatite. Nanomaterials 2023, 13, 2743. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zinger, O.; Schwartz, Z.; Wieland, M.; Landolt, D.; Boyan, B.D. Osteoblast-like Cells Are Sensitive to Submicron-scale Surface Structure. Clin. Oral. Implants Res. 2006, 17, 258–264. [Google Scholar] [CrossRef]
- Berthelot, R.; Variola, F. Investigating the Interplay between Environmental Conditioning and Nanotopographical Cueing on the Response of Human MG63 Osteoblastic Cells to Titanium Nanotubes. Biomater. Sci. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Andrés-León, E.; Núñez-Torres, R.; Rojas, A.M. MiARma-Seq: A Comprehensive Tool for MiRNA, MRNA and CircRNA Analysis. Sci. Rep. 2016, 6, 25749. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 1 January 2020).
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Boutros, P.C. VennDiagram: A Package for the Generation of Highly-Customizable Venn and Euler Diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Abueg, L.A.L.; Afgan, E.; Allart, O.; Awan, A.H.; Bacon, W.A.; Baker, D.; Bassetti, M.; Batut, B.; Bernt, M.; Blankenberg, D.; et al. The Galaxy Platform for Accessible, Reproducible, and Collaborative Data Analyses: 2024 Update. Nucleic Acids Res. 2024, 52, W83–W94. [Google Scholar] [CrossRef]
- Ge, S.X.; Son, E.W.; Yao, R. IDEP: An Integrated Web Application for Differential Expression and Pathway Analysis of RNA-Seq Data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef] [PubMed]
- Vedi, M.; Nalabolu, H.S.; Lin, C.-W.; Hoffman, M.J.; Smith, J.R.; Brodie, K.; De Pons, J.L.; Demos, W.M.; Gibson, A.C.; Hayman, G.T.; et al. MOET: A Web-Based Gene Set Enrichment Tool at the Rat Genome Database for Multiontology and Multispecies Analyses. Genetics 2022, 220, iyac005. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. ClusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.-H.; Yu, G.; Cai, P. GgVennDiagram: An Intuitive, Easy-to-Use, and Highly Customizable R Package to Generate Venn Diagram. Front. Genet. 2021, 12, 706907. [Google Scholar] [CrossRef] [PubMed]
- Barberi, J.; Spriano, S. Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef]
- Park, J.H.; Schwartz, Z.; Olivares-Navarrete, R.; Boyan, B.D.; Tannenbaum, R. Enhancement of Surface Wettability via the Modification of Microtextured Titanium Implant Surfaces with Polyelectrolytes. Langmuir 2011, 27, 5976–5985. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; Cheng, A.; Anderson, D.M.; McLachlan, T.; Stephan, I.; Geis-Gerstorfer, J.; Sandhage, K.H.; Fedorov, A.G.; Rupp, F.; et al. The Roles of Titanium Surface Micro/Nanotopography and Wettability on the Differential Response of Human Osteoblast Lineage Cells. Acta Biomater. 2013, 9, 6268–6277. [Google Scholar] [CrossRef]
- Gonzalez Solveyra, E.; Thompson, D.H.; Szleifer, I. Proteins Adsorbing onto Surface-Modified Nanoparticles: Effect of Surface Curvature, PH, and the Interplay of Polymers and Proteins Acid–Base Equilibrium. Polymers 2022, 14, 739. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Raz, P.; Zhao, G.; Chen, J.; Wieland, M.; Cochran, D.L.; Chaudhri, R.A.; Ornoy, A.; Boyan, B.D.; Schwartz, Z. Integrin A2β1 Plays a Critical Role in Osteoblast Response to Micron-Scale Surface Structure and Surface Energy of Titanium Substrates. Proc. Nat. Acad. Sci. USA 2008, 105, 15767–15772. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Navarrete, R.; Rodil, S.E.; Hyzy, S.L.; Dunn, G.R.; Almaguer-Flores, A.; Schwartz, Z.; Boyan, B.D. Role of Integrin Subunits in Mesenchymal Stem Cell Differentiation and Osteoblast Maturation on Graphitic Carbon-Coated Microstructured Surfaces. Biomaterials 2015, 51, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Raines, A.L.; Berger, M.B.; Schwartz, Z.; Boyan, B.D. Osteoblasts Grown on Microroughened Titanium Surfaces Regulate Angiogenic Growth Factor Production through Specific Integrin Receptors. Acta Biomater. 2019, 97, 578–586. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, W.; Fang, C.; Wang, N.; Zhuang, Y.; Gao, S. Research on the Regulatory Mechanism of Ginseng on the Tumor Microenvironment of Colorectal Cancer Based on Network Pharmacology and Bioinformatics Validation. Curr. Comput. Aided Drug Des. 2024, 20, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, C.; Lee, S.; Siu, A.; Ramos, D.M. The Cytoplasmic Extension of the Integrin Β6 Subunit Regulates Epithelial-to-Mesenchymal Transition. Anticancer Res. 2014, 34, 659–664. [Google Scholar] [PubMed]
- Schnapp, L.M.; Hatch, N.; Ramos, D.M.; Klimanskaya, I.V.; Sheppard, D.; Pytela, R. The Human Integrin A8β1 Functions as a Receptor for Tenascin, Fibronectin, and Vitronectin. J. Biol. Chem. 1995, 270, 23196–23202. [Google Scholar] [CrossRef]
- Kinoshita, M.; Yamada, A.; Sasa, K.; Ikezaki, K.; Shirota, T.; Kamijo, R. Phorbol-12-Myristate 13-Acetate Inhibits Nephronectin Gene Expression via Protein Kinase C Alpha and c-Jun/c-Fos Transcription Factors. Sci. Rep. 2021, 11, 20360. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of Skeletal Development and Maintenance by Runx2 and Sp7. Int. J. Mol. Sci. 2024, 25, 10102. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Molecular Mechanism of Runx2-Dependent Bone Development. Mol. Cells 2020, 43, 168–175. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, H.-N.; Park, E.-K.; Lee, B.-H.; Ryoo, H.-M.; Kim, S.-Y.; Kim, I.-S.; Stein, J.L.; Lian, J.B.; Stein, G.S.; et al. The Bone-Related Zn Finger Transcription Factor Osterix Promotes Proliferation of Mesenchymal Cells. Gene 2006, 366, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Cohen, D.J.; Sabalewski, E.L.; Van Duyn, C.; Wilson, D.S.; Schwartz, Z.; Boyan, B.D. Semaphorin 3A Delivered by a Rapidly Polymerizing Click Hydrogel Overcomes Impaired Implant Osseointegration in a Rat Type 2 Diabetes Model. Acta Biomater. 2023, 157, 236–251. [Google Scholar] [CrossRef] [PubMed]
| Canonical Pathway | |||||||||||||||
| MN vs. TCPS | RSPO | FRP | CycD | Frizzled | LRP5/6 | Notum | AXIM | BAMBI | P53 | ICAT | GBP | APC | PRARD | CK2 | SIP |
| OM vs. TCPS | RSPO | FRP | CycD | Frizzled | LRP5/6 | Notum | AXIM | BAMBI | P53 | ICAT | APC | PKA | NKD | Catenin | C-JUN |
| Planar Cell Polarity | |||||||||||||||
| MN vs. TCPS | WNT11 | Frizzled | Daam1 | ROR1/2 | Knypek | Prickle | ROCK2 | ||||||||
| OM vs. TCPS | WNT11 | Frizzled | Daam1 | DVL | JNK | Prickle | ROCK2 | ||||||||
| Wnt/Ca (Non-Canonical Pathway) | |||||||||||||||
| MN vs. TCPS | Frizzled | CaMKII | NFAT | PLC | PKC | ||||||||||
| OM vs. TCPS | Frizzled | CaMKII | NFAT | PLC | PKC | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, D.J.; Van Duyn, C.M.; Deng, J.; Lodi, M.K.; Gallagher, M.B.; Sugar, J.T.; Rawlinson, J.J.; Ghosh, P.; Boyan, B.D.; Schwartz, Z. Bone Marrow Stromal Cells Generate a Pro-Healing Inflammasome When Cultured on Titanium–Aluminum–Vanadium Surfaces with Microscale/Nanoscale Structural Features. Biomimetics 2025, 10, 66. https://doi.org/10.3390/biomimetics10010066
Cohen DJ, Van Duyn CM, Deng J, Lodi MK, Gallagher MB, Sugar JT, Rawlinson JJ, Ghosh P, Boyan BD, Schwartz Z. Bone Marrow Stromal Cells Generate a Pro-Healing Inflammasome When Cultured on Titanium–Aluminum–Vanadium Surfaces with Microscale/Nanoscale Structural Features. Biomimetics. 2025; 10(1):66. https://doi.org/10.3390/biomimetics10010066
Chicago/Turabian StyleCohen, David J., Christine M. Van Duyn, Jingyao Deng, Musaddiq K. Lodi, Michelle B. Gallagher, James T. Sugar, Jeremy J. Rawlinson, Preetam Ghosh, Barbara D. Boyan, and Zvi Schwartz. 2025. "Bone Marrow Stromal Cells Generate a Pro-Healing Inflammasome When Cultured on Titanium–Aluminum–Vanadium Surfaces with Microscale/Nanoscale Structural Features" Biomimetics 10, no. 1: 66. https://doi.org/10.3390/biomimetics10010066
APA StyleCohen, D. J., Van Duyn, C. M., Deng, J., Lodi, M. K., Gallagher, M. B., Sugar, J. T., Rawlinson, J. J., Ghosh, P., Boyan, B. D., & Schwartz, Z. (2025). Bone Marrow Stromal Cells Generate a Pro-Healing Inflammasome When Cultured on Titanium–Aluminum–Vanadium Surfaces with Microscale/Nanoscale Structural Features. Biomimetics, 10(1), 66. https://doi.org/10.3390/biomimetics10010066








