Extraction and Purification of the FrHb1 Fraction from Commercial Natural Latex of Hevea brasiliensis for Biomedical Applications
Abstract
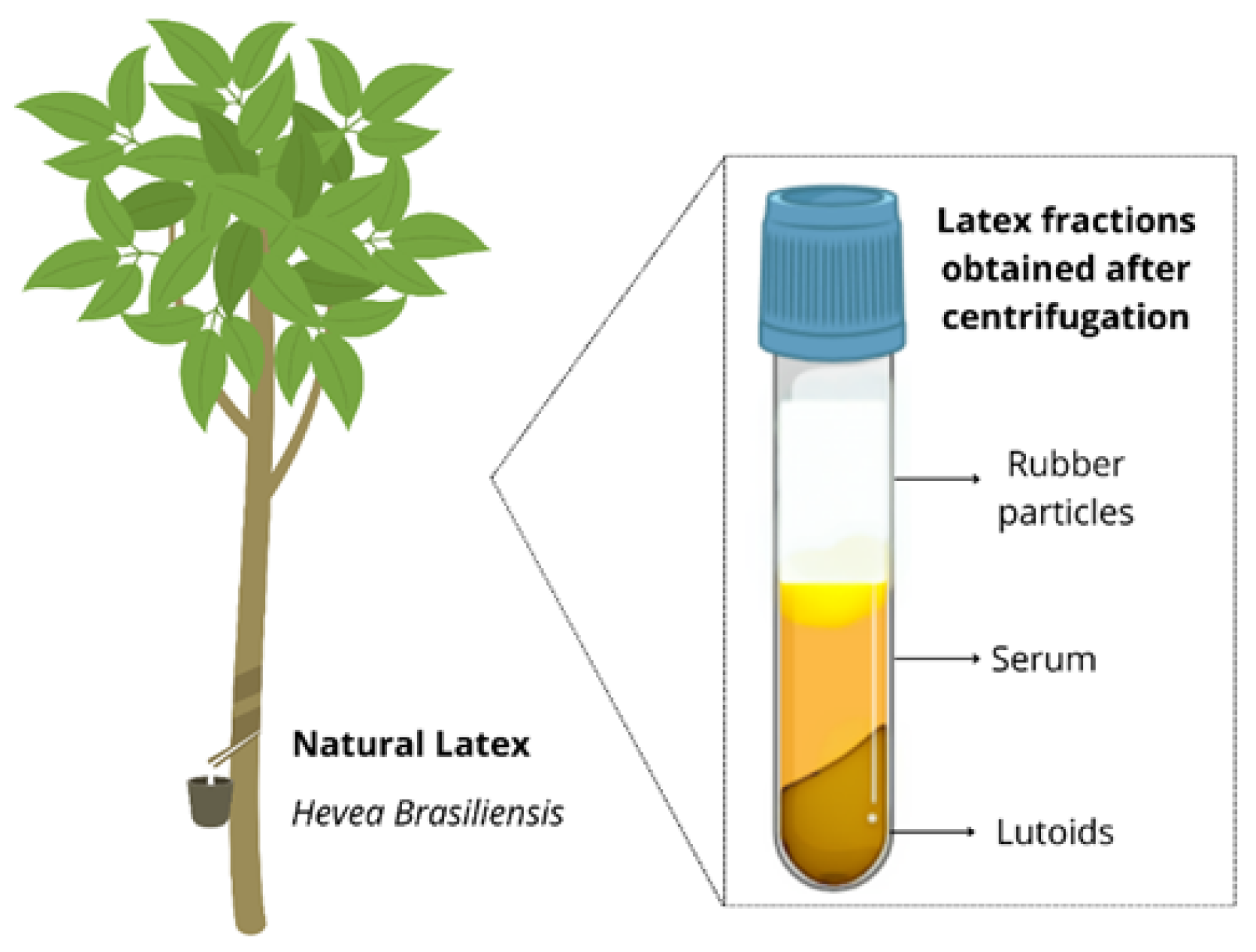
1. Introduction
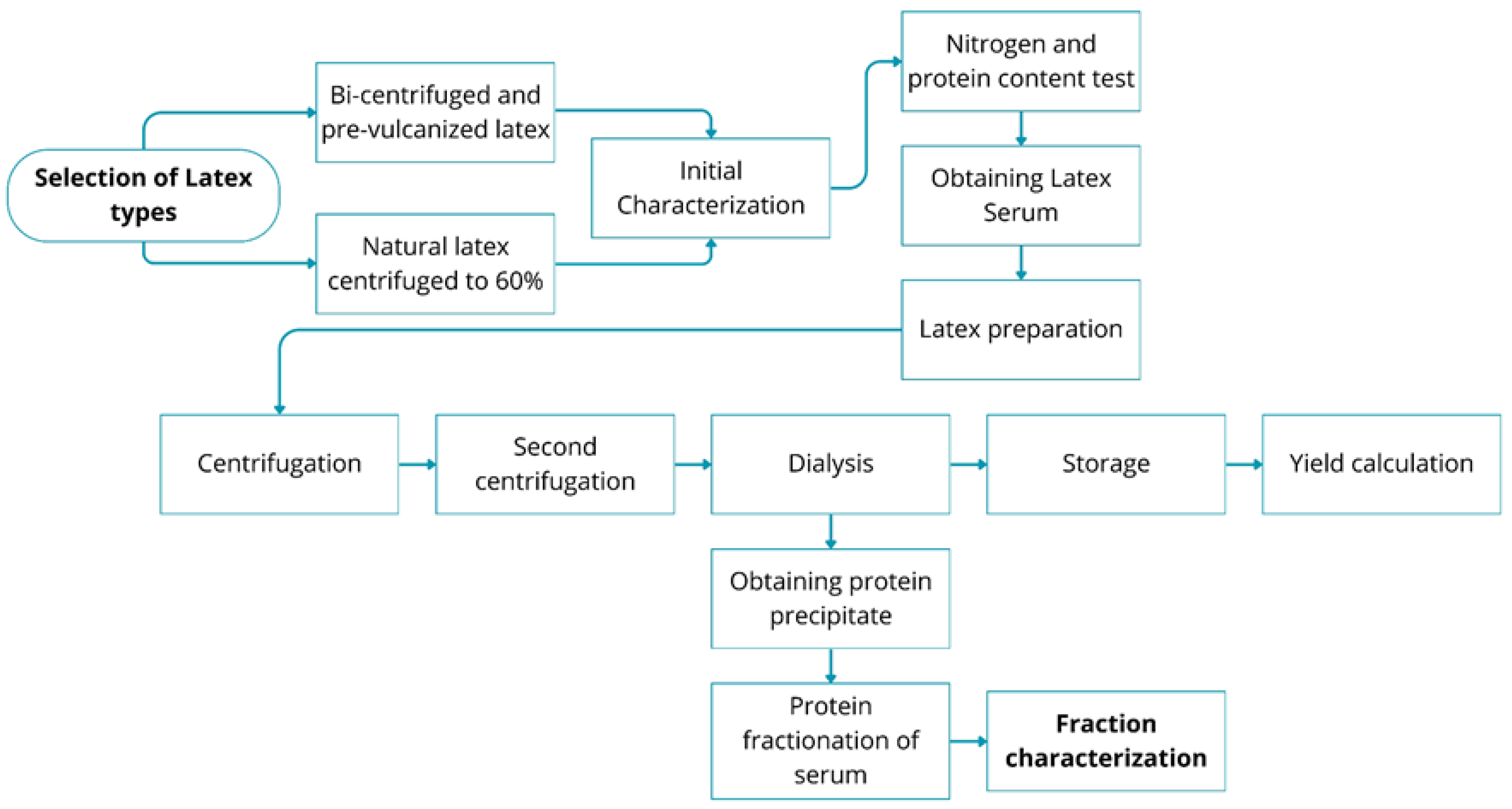
2. Materials and Methods
2.1. Characterization of the Sample by Determination of Nitrogen and Proteins
- = Concentration of the standardized sulfuric acid solution;
- = Volume used in the titration.
2.2. Obtaining the Latex Serum
2.3. Obtaining the Protein Precipitate
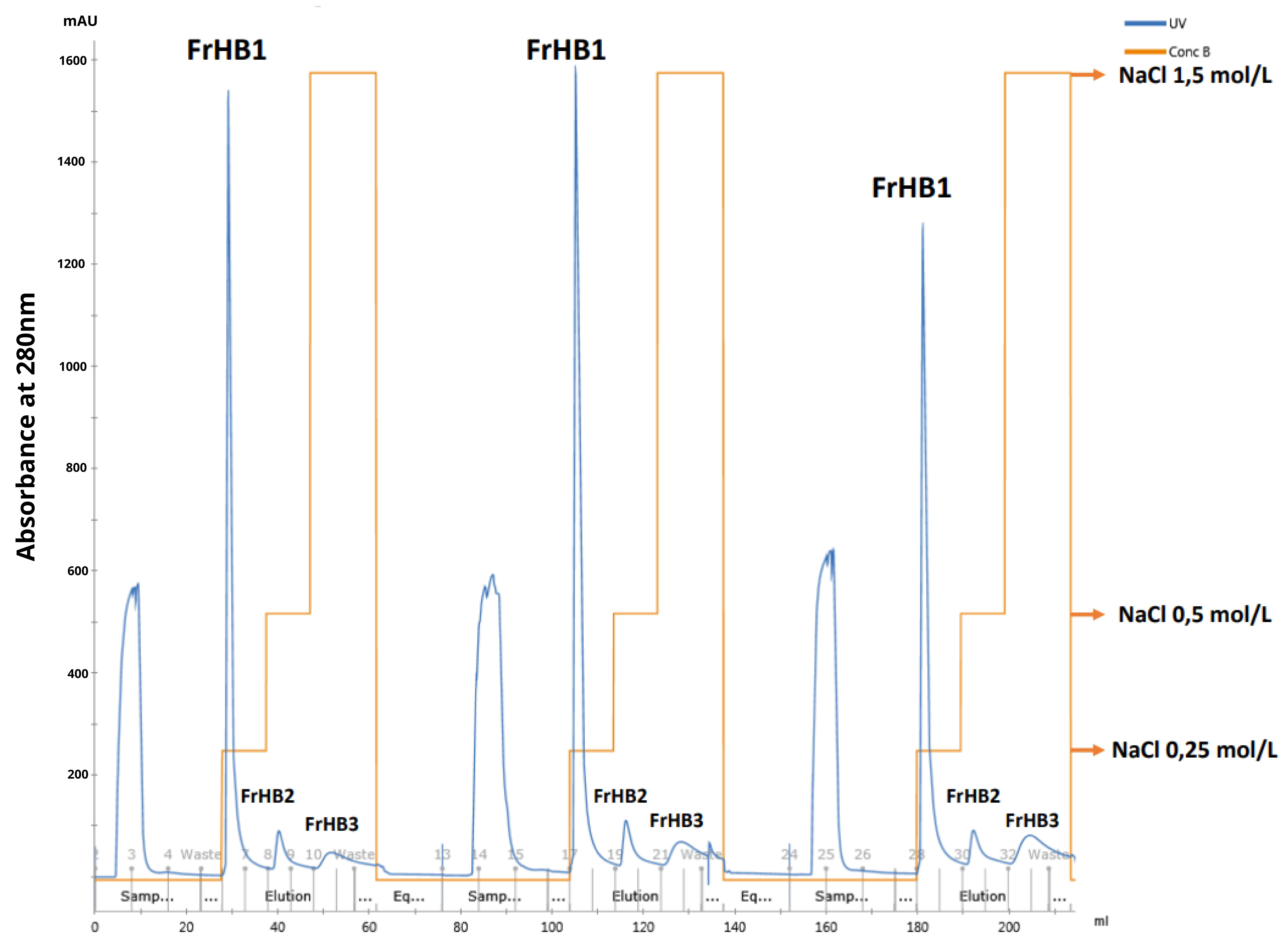
2.4. Protein Fractionation of the Serum
2.5. Characterization of the Fractions
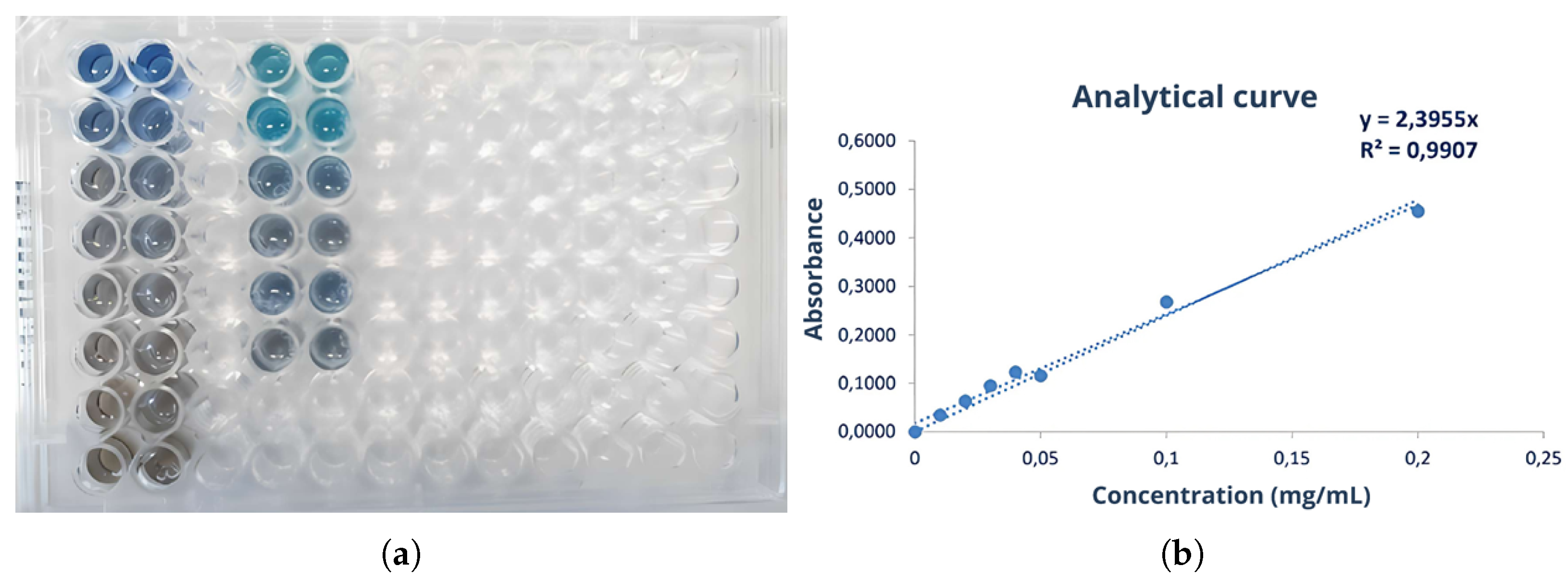
2.5.1. Bradford Assay
2.5.2. Electrophoresis
3. Results
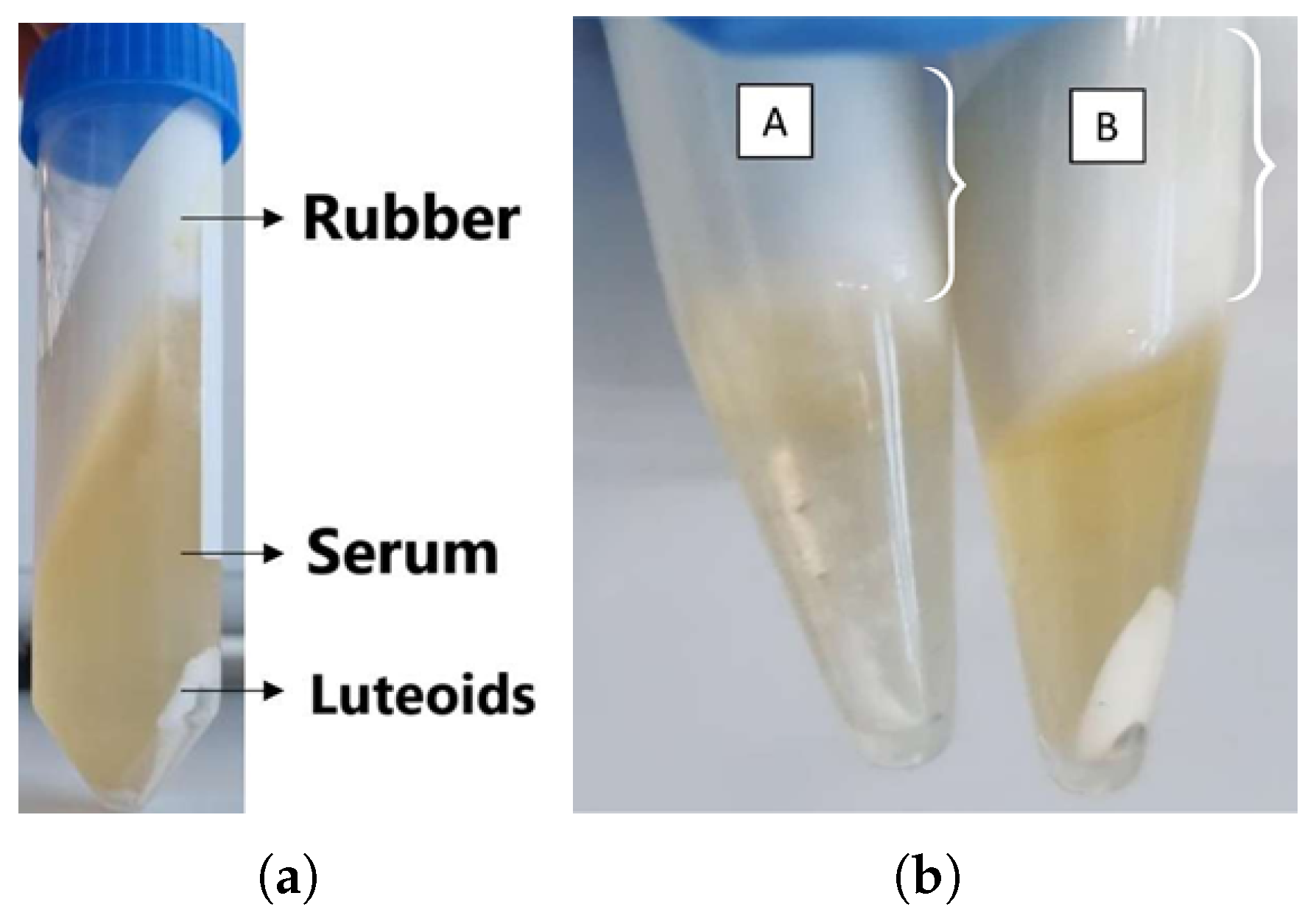
3.1. Characterization of Latex
3.2. Serum
3.3. Protein Precipitate
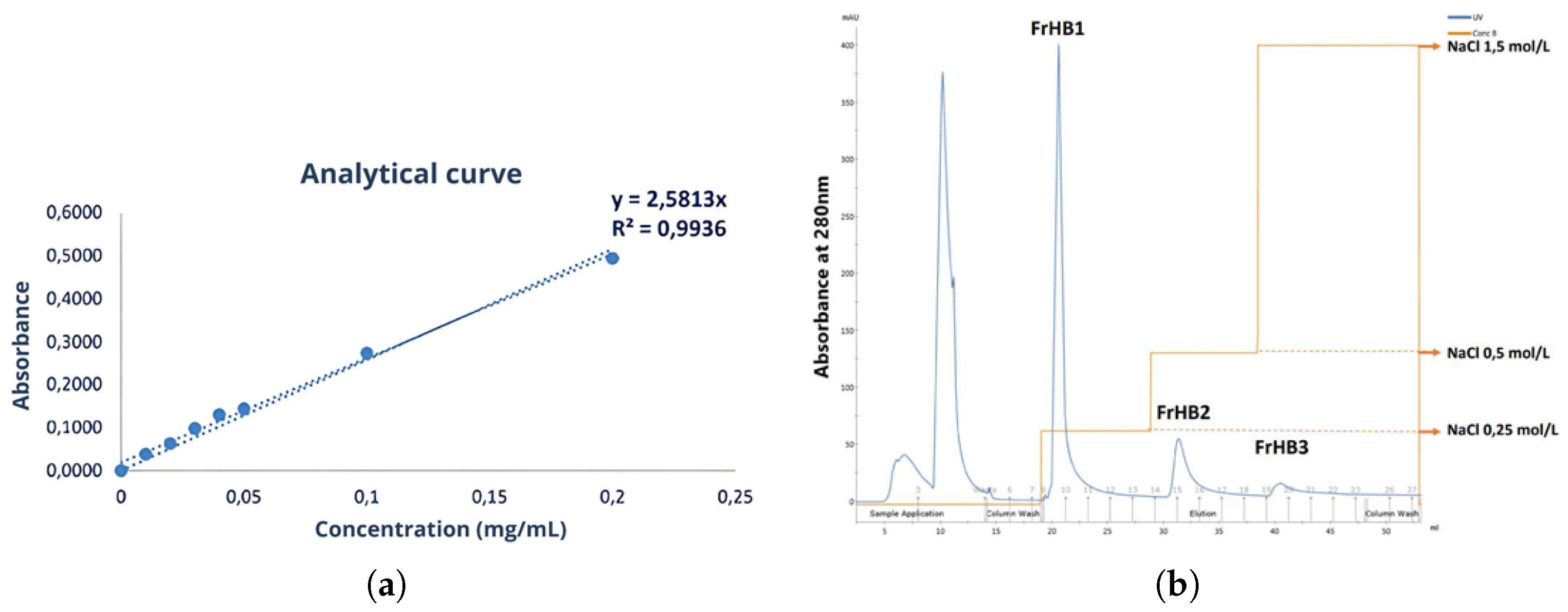
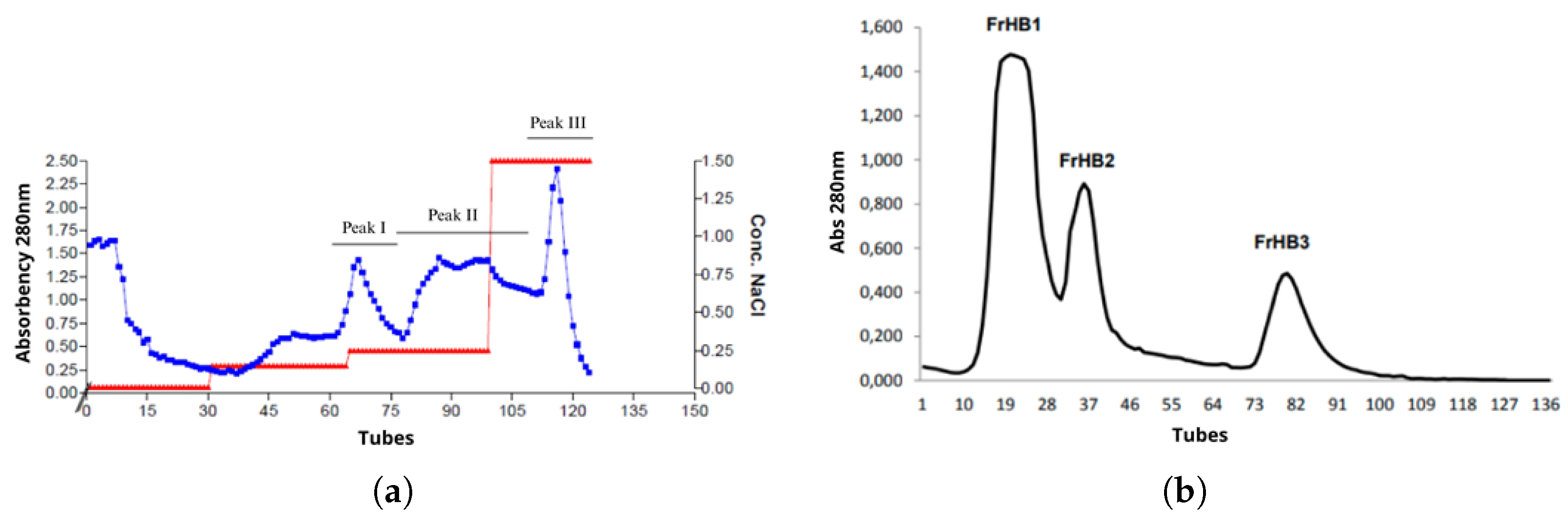
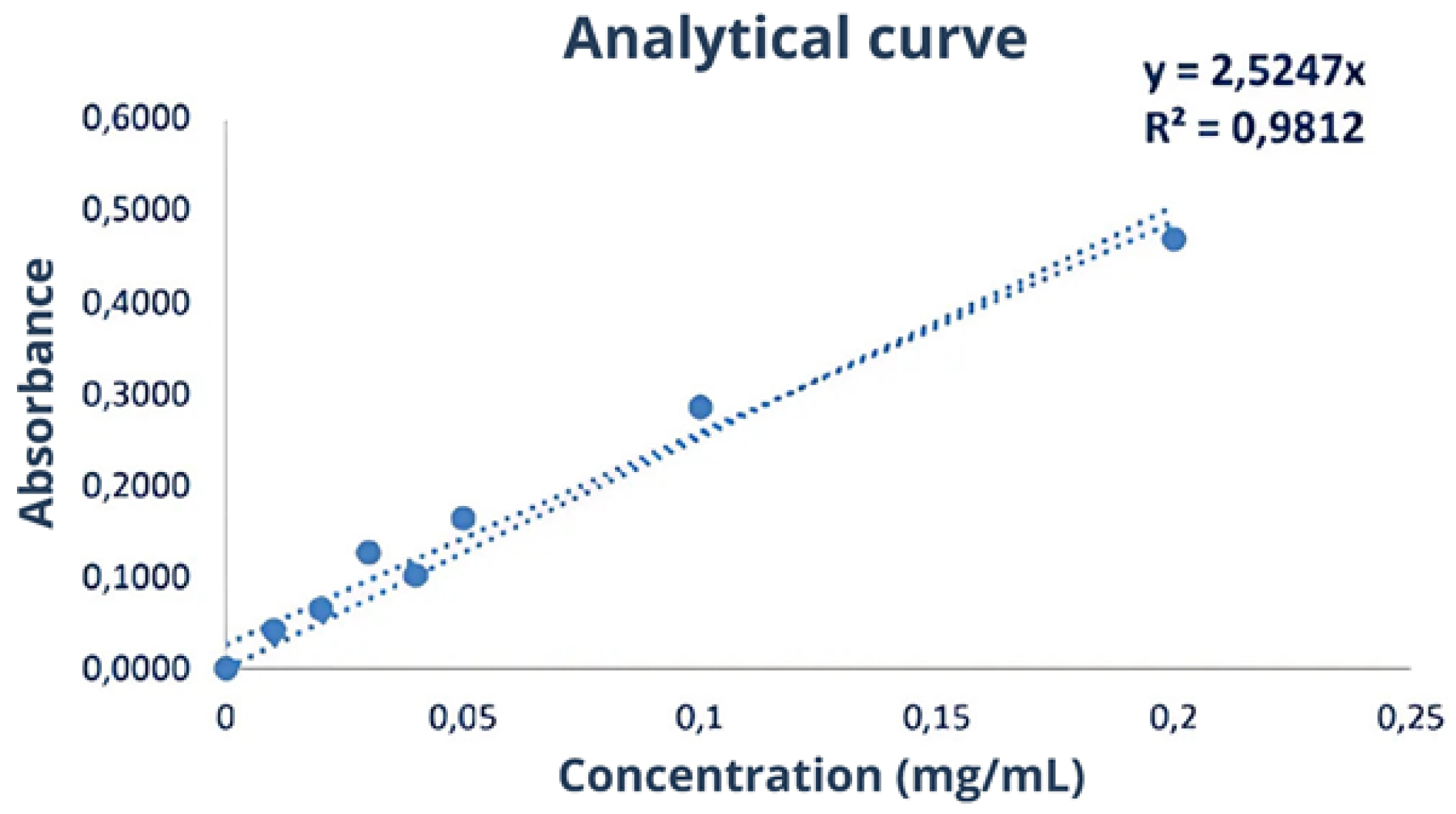
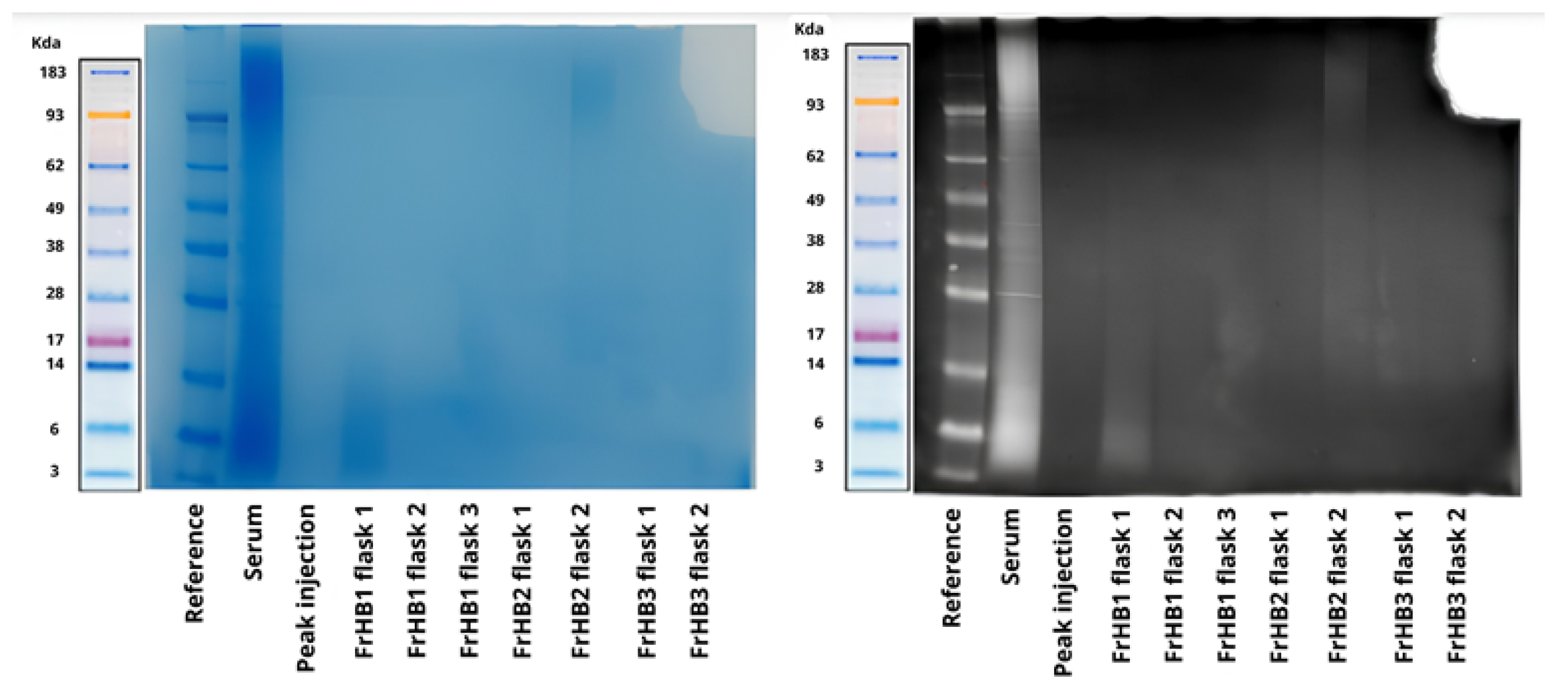
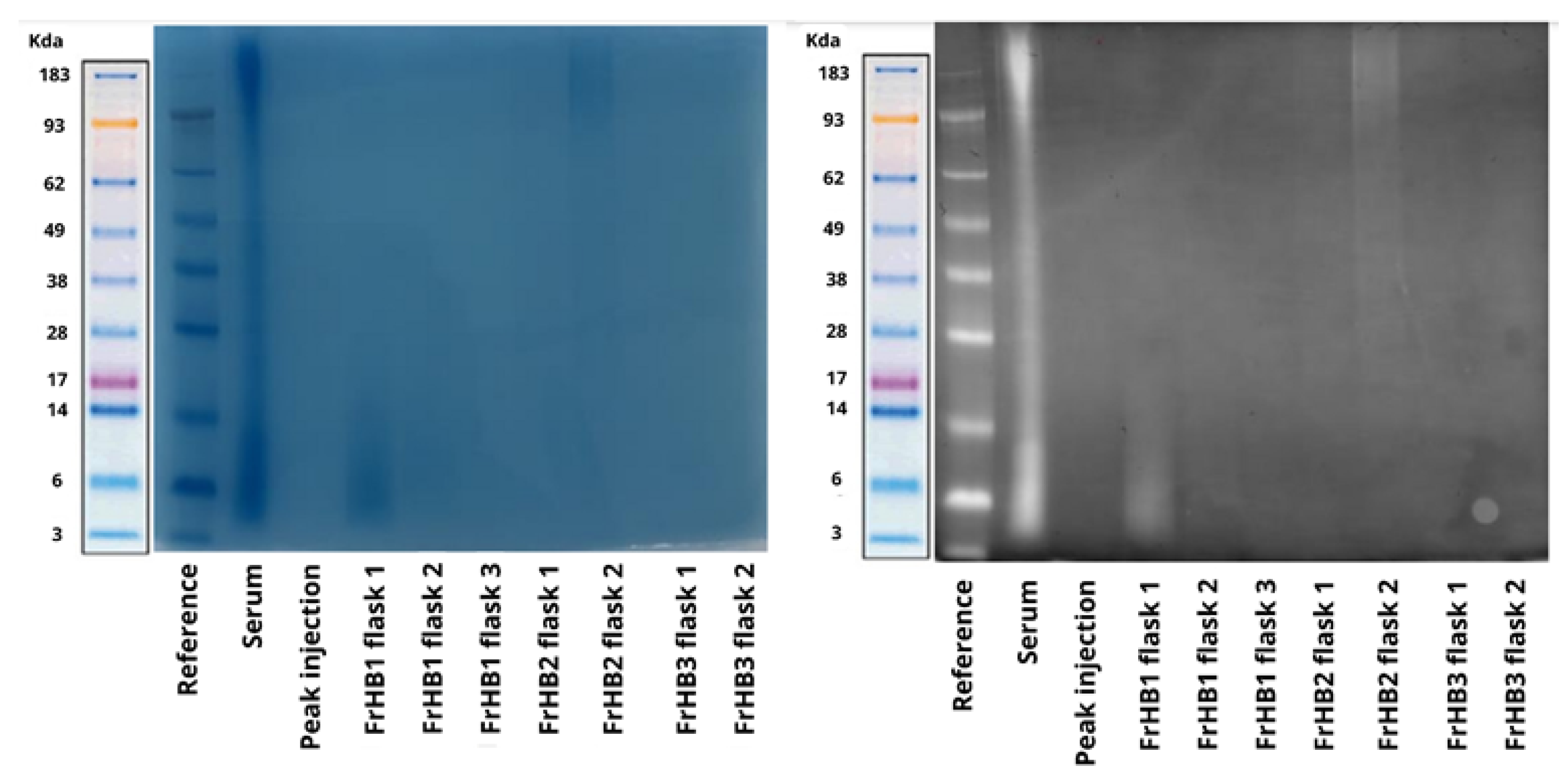
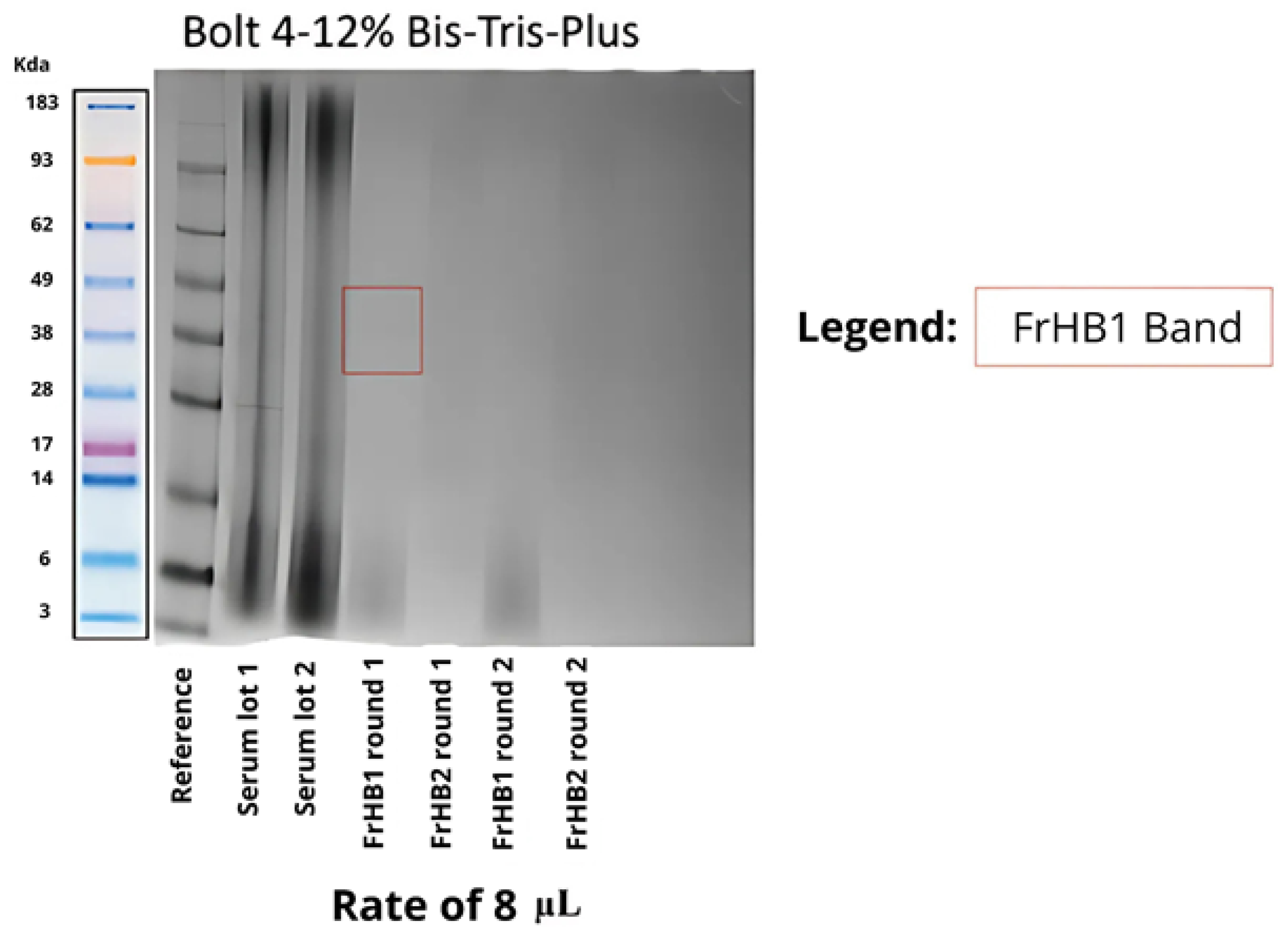
3.4. Protein Fractions and Characterization
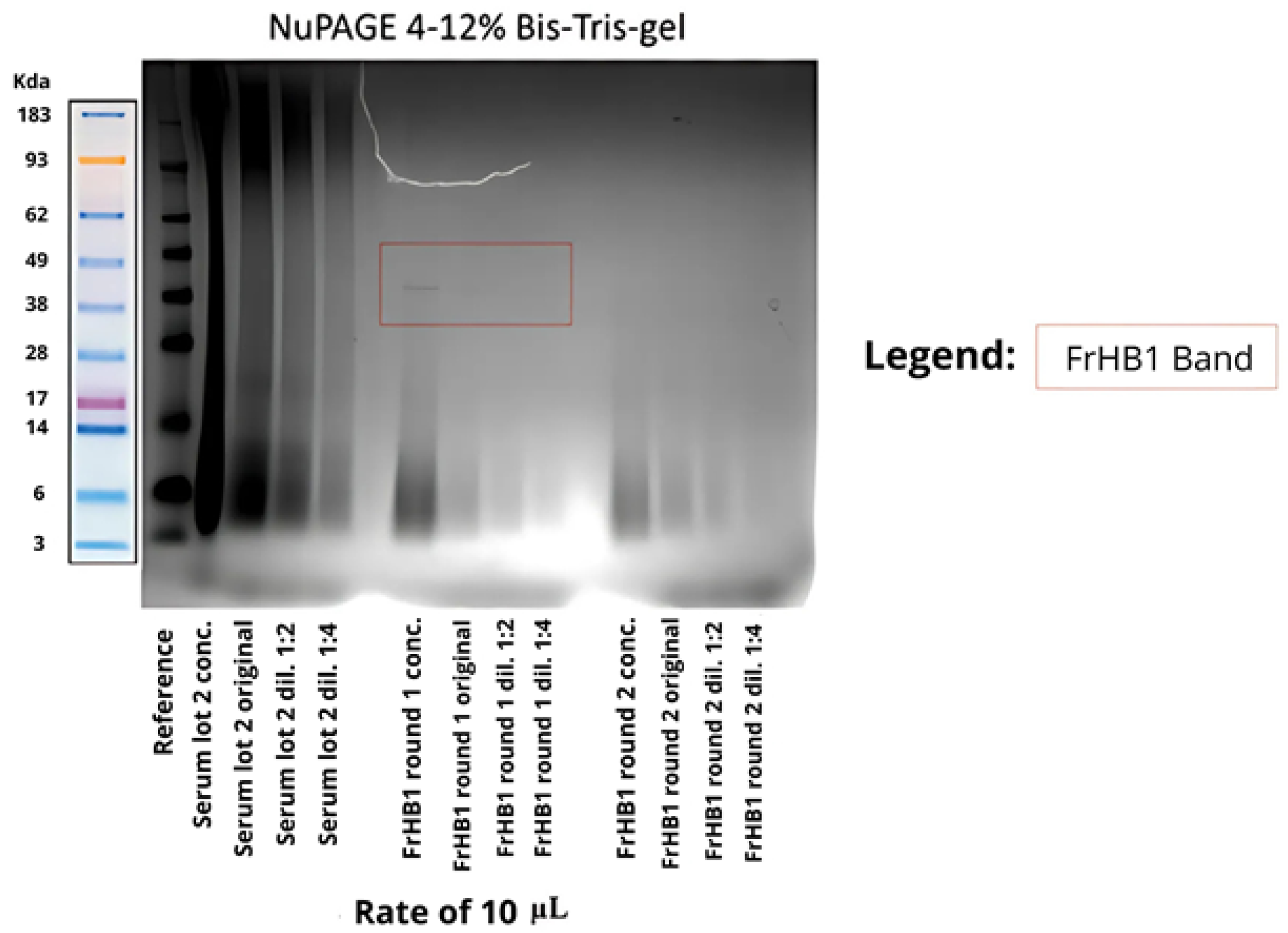
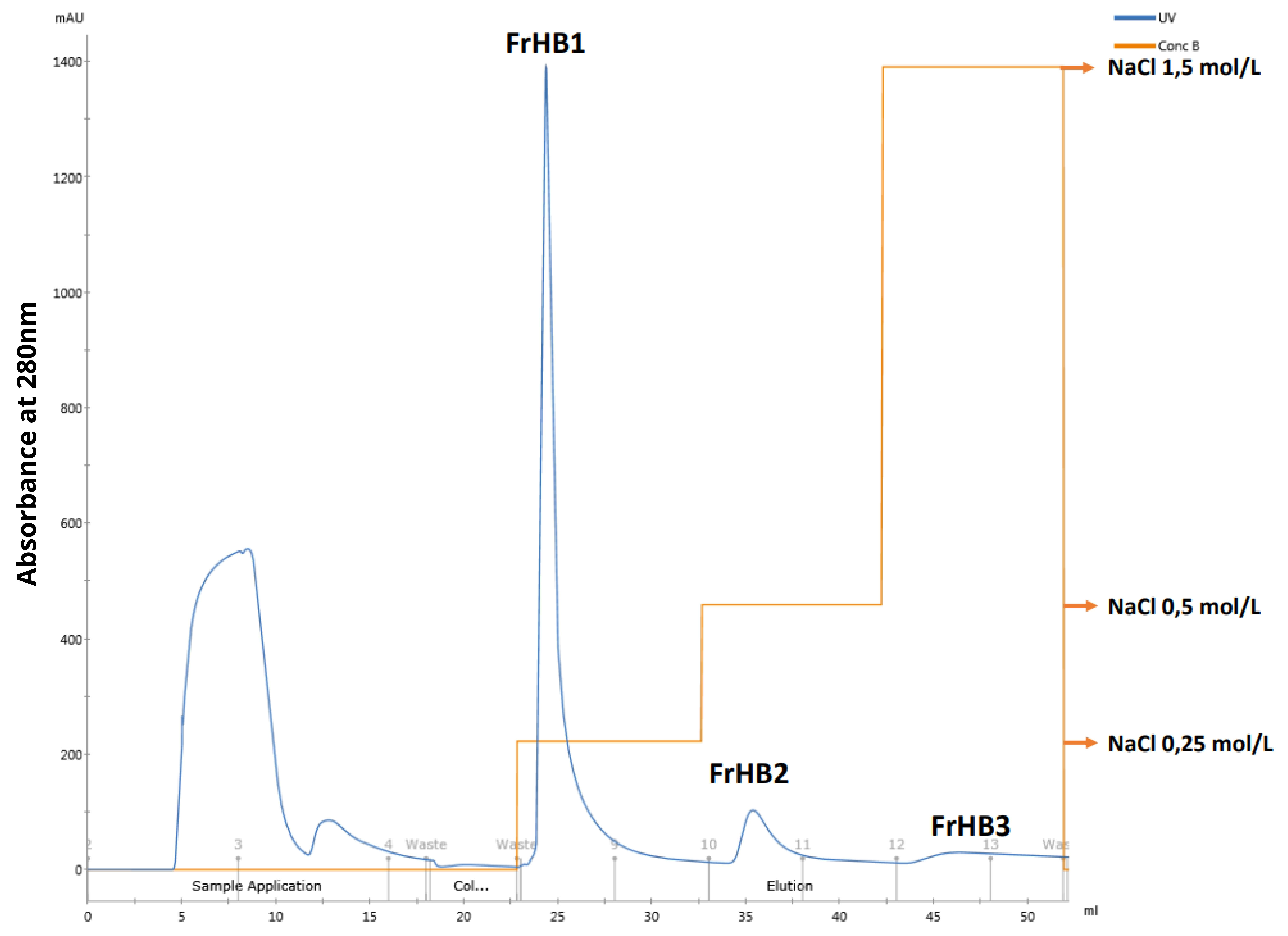
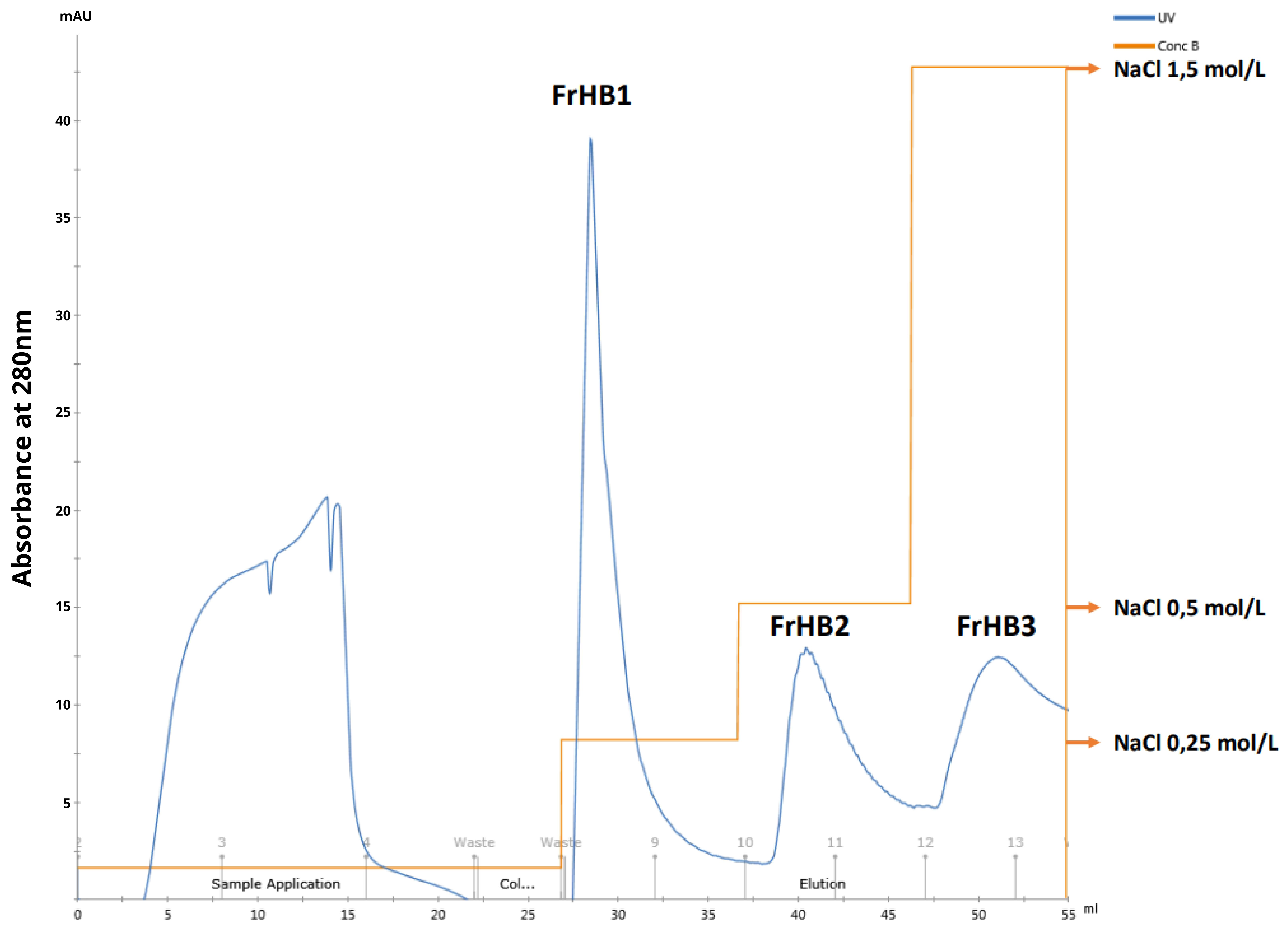
3.4.1. Fractionation 1
3.4.2. Fractionation 2
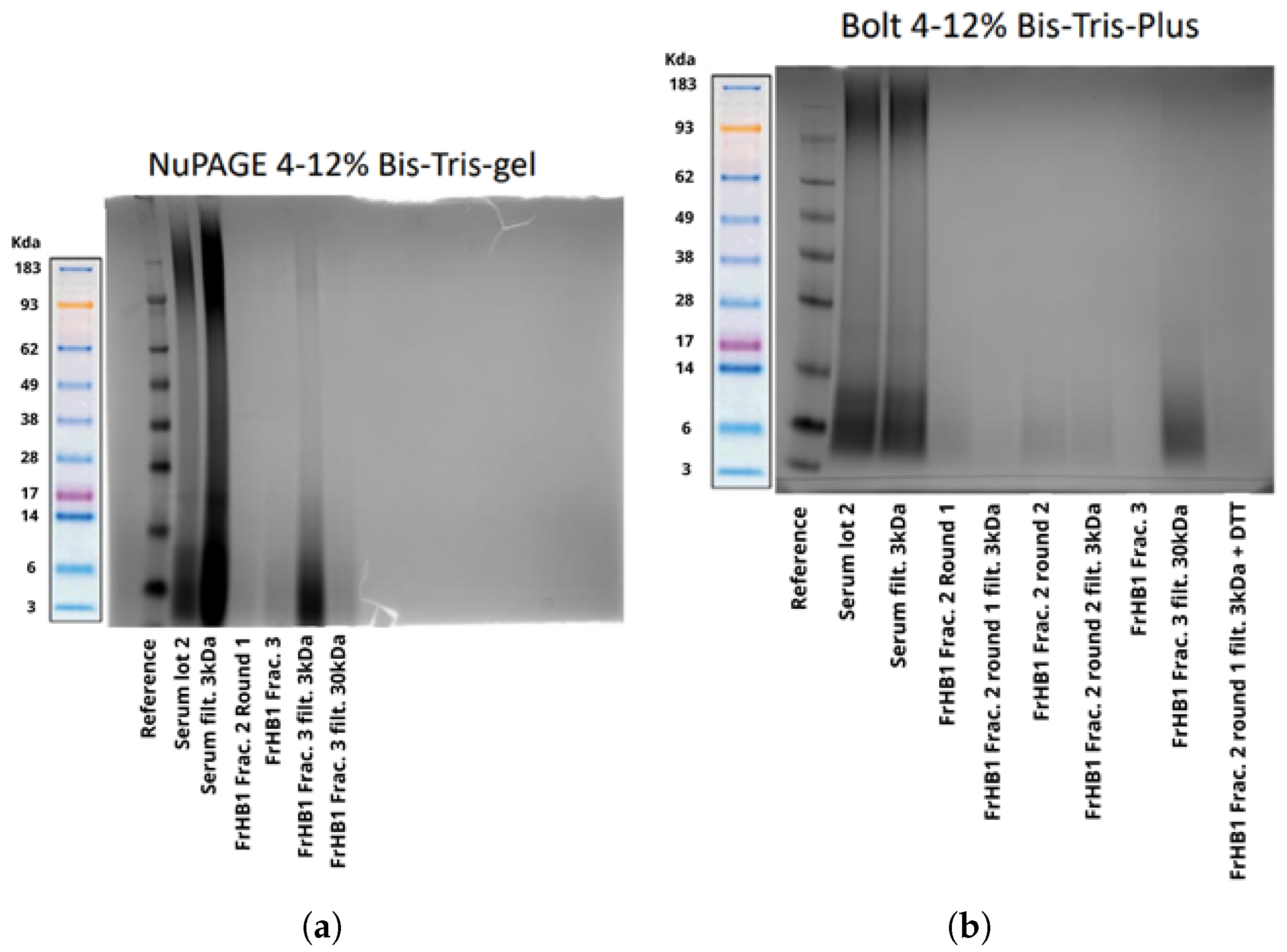
3.4.3. Fractionation 3
3.4.4. Fractionation 4
3.4.5. Fractionations 5 to 9
4. Discussion
Extraction of FrHb1 from Natural Latex
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marques, P.A.C.; Guerra, N.B.; Santos, L.S.D.; Mussagy, C.U.; Brasil, G.S.P.; Burd, B.S.; Su, Y.; da Silva Sasaki, J.C.; Scontri, M.; de Lima Lopes Filho, P.E.; et al. Natural rubber latex-based biomaterials for drug delivery and regenerative medicine: Trends and directions. Int. J. Biol. Macromol. 2024, 267, 131666. [Google Scholar] [CrossRef]
- Herculano, R.D.; Mussagy, C.U.; Guerra, N.B.; Brasil, G.S.P.; Floriano, J.F.; Burd, B.S.; Su, Y.; da Silva Sasaki, J.C.; Marques, P.A.C.; Scontri, M.; et al. Recent advances and perspectives on natural latex serum and its fractions for biomedical applications. Biomater. Adv. 2024, 157, 213739. [Google Scholar] [CrossRef]
- Wadeesirisak, K.; Castano, S.; Berthelot, K.; Vaysse, L.; Bonfils, F.; Peruch, F.; Rattanaporn, K.; Liengprayoon, S.; Lecomte, S.; Bottier, C. Rubber particle proteins REF1 and SRPP1 interact differently with native lipids extracted from Hevea brasiliensis latex. Biochim. Biophys. Acta. Biomembr. 2017, 1859, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Guerra, N.B.; Pegorin, G.S.; Boratto, M.H.; de Barros, N.R.; de Oliveira Graeff, C.F.; Herculano, R.D. Biomedical applications of natural rubber latex from the rubber tree Hevea brasiliensis. Mater. Sci. Eng. Mater. Biol. Appl. 2021, 126, 112126. [Google Scholar] [CrossRef] [PubMed]
- Asami, J.; Quevedo, B.V.; Santos, A.R., Jr.; Giorno, L.P.; Komatsu, D.; de Rezende Duek, E.A. The impact of non-deproteinization on physicochemical and biological properties of natural rubber latex for biomedical applications. Int. J. Biol. Macromol. 2023, 253, 126782. [Google Scholar] [CrossRef] [PubMed]
- Mrue, F.; Netto, J.C.; Ceneviva, R.; Lachat, J.J.; Thomazini, J.A.; Tambelini, H. Evaluation of the biocompatibility of a new biomembrane. Mater. Res. 2004, 7, 277–283. [Google Scholar] [CrossRef]
- Sader, S.L.; Netto, J.C.; Neto, J.B.; Mazzetto, S.A.; Alves, P.R.A.; Vanni, J.C.; Sader, A.A. Substituição parcial do pericárdio de cães por membrana de látex natural. Braz. J. Cardiovasc. Surg. 2000, 15, 338–344. [Google Scholar] [CrossRef]
- Paulo, N.M.; de Lima, F.G.; de Siqueira Júnior, J.T.; Fleury, L.F.F.; de Sant’Ana, F.J.F.; Borges, A.C.; da Costa Telles, T. Seringueira’s latex membrane (Hevea brasiliensis) with and without polylysine 0.1% and marlex mesh for the reconstruction of iatrogenics abdominal wall defects in rats. Acta Cir. Bras. 2005, 20, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.A.; Iyer, A.; Das, P.K.; Foss, N.T.; Garcia, S.B.; Coutinho-Netto, J.; Jordão, A.A.J.; Frade, M.A. The inflammatory stimulus of a natural latex biomembrane improves healing in mice. Braz. J. Med. Biol. Res. 2011, 40, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Spin, J.R. Efeito da membrana de látex natural sobre o reparo de áreas doadoras do palato para enxerto gengival livre: Estudo clínico, controlado e randomizado. Ph.D. Thesis, State University of São Paulo, Araraquara, Brazil, 2018. [Google Scholar]
- Rosa, S.S.R.F.; Rosa, M.F.F.; Marques, M.P.; Guimarães, G.A.; Motta, B.C.; Macedo, Y.C.L.; Inazawa, P.; Dominguez, A.; Macedo, F.S.; Lopes, C.A.P.; et al. Regeneration of Diabetic Foot Ulcers Based on Therapy with Red LED Light and a Natural Latex Biomembrane. Ann. Biomed. Eng. 2019, 47, 1153–1164. [Google Scholar] [CrossRef]
- Dias, F.J.; Fazan, V.P.S.; Cury, D.P.; de Almeida, S.R.Y.; Borie, E.; Fuentes, R.; Coutinho-Netto, J.; Watanabe, I.S. Growth factors expression and ultrastructural morphology after application of low-level laser and natural latex protein on a sciatic nerve crush-type injury. PloS ONE 2019, 14, e0210211. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça, R.J. Caracterização Biológica de Uma Fração Angiogênica do Latex Natural da Seringueira Hevea brasiliensis. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2004. [Google Scholar]
- Morais, P.C.R.; Floriano, J.F.; Garcia, C.G.P.; Chagas, A.L.D.; Mussagy, C.U.; Guerra, N.B.; Brasil, G.S.P.; Vicentine, K.F.D.; Rocha, L.B.; Oliveira, C.J.F.; et al. Comparing the wound healing potential of natural rubber latex serum and F1-protein: An in vivo approach. Biomater. Adv. 2024, 157, 213754. [Google Scholar] [CrossRef]
- Leite, M.N.; Leite, S.N.; Caetano, G.F.; de Andrade, T.A.M.; Fronza, M.; Frade, M.A.C. Healing effects of natural latex serum 1% from Hevea brasiliensis in an experimental skin abrasion wound model. An. Bras. Dermatol. 2020, 95, 418–427. [Google Scholar] [CrossRef]
- Paini, S.; Bighetti, A.C.C.; Cestari, T.M.; Arantes, R.V.N.; Santos, P.S.; Mena-Laura, E.E.; Garlet, G.P.; Taga, R.; Assis, G.F. Concentration-dependent effects of latex F1-protein fraction incorporated into deproteinized bovine bone and biphasic calcium phosphate on the repair of critical-size bone defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 3270–3285. [Google Scholar] [CrossRef]
- Dias, F.J.; Cury, D.P.; Dias, P.E.; Borie, E.; Alarcón-Apablaza, J.; Lezcano, M.F.; Martínez-Rodríguez, P.; Vargas, D.; Gutiérrez, B.; Fazan, V.P.S. Effects of Low-Level Laser Therapy and Purified Natural Latex (Hevea brasiliensis) Protein on Injured Sciatic Nerve in Rodents: Morpho-Functional Analysis. Int. J. Mol. Sci. 2023, 24, 14031. [Google Scholar] [CrossRef]
- Wongkhat, J.; Pattamaprom, C. Enzymatic approach in accelerating colloidal stability of concentrated natural rubber latex and its effect on rubber products. Ind. Crops Prod. 2022, 182, 114892. [Google Scholar] [CrossRef]
- Elumalai, S.; Ling, S.K.; Arija, S.; Arif, M.; Yeang, H.Y. Deciphering Rubber Particle Destabilisation by Hevea Bark Extract. J. Rubber Res. 2007, 10, 97–105. [Google Scholar]
- ISO 1656:2019; Rubber, Raw Natural, and Rubber Latex, Natural—Determination of Nitrogen Content. International Organization for Standardization: Geneva, Switzerland, 2019.
- Marciano, J.S.P. Identificação de Proteínas Presentes No Soro do Látex da Himatanthus Sucuuba (Apocynaceae). Ph.D. Thesis, Universidade de Ribeirão Preto, Ribeirão Preto, Brazil, 2017. [Google Scholar]
- Habib, M.A.H.; Ismail, M.N. Hevea brasiliensis latex proteomics: A review of analytical methods and the way forward. J. Plant Res. 2020, 134, 3. [Google Scholar] [CrossRef]
- Morais, P.C.R. Avaliação do Efeito do Soro Total e de Frações Obtidas do Látex da Seringueira (Hevea brasiliensis) Na Cicatrização de Feridas em Modelo Experimental. Ph.D. Thesis, Universidade Federal do Triângulo Mineiro, Uberaba, Brazil, 2017. [Google Scholar]
- de Souza, V.F. Liberação Controlada do Fator de Crescimento angiogêNico Extraído do Látex Natural da Seringueira Hevea brasiliensis, Encapsulado em Microesferas de ácido Hialurônico Para Utilização Na Terapia Angiogênica e Engenharia Tecidual. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2007. [Google Scholar]
- Li, D.; Yan, J.; Deng, Z.; Chen, C.; He, P.; Wu, M.; Chen, S. Evaluation of Three Methods For Protein Extraction Suitable for 2-DE. Chin. Agric. Sci. Bull. 2009, 25, 273–279. [Google Scholar]
- Andrade, K.L.; Ramlow, H.; Floriano, J.F.; Acosta, E.D.; Faita, F.; Machado, R.A.F. Latex and natural rubber: Processing techniques for biomedical applications. Braz. J. Chem. Eng. 2023, 40. [Google Scholar] [CrossRef]
- Srisomboon, S.; Wadeesirisak, K.; Sauvage, F.X.; Rattanaporn, K.; Sriroth, K.; Vaysse, L.; Bonfils, F.; Sainte-Beuve, J.; Liengprayoon, S.; Bottier, C. Optimization of Protein Extraction from Different Latex Samples of Hevea brasiliensis. Thaksin Univ. J. 2014, 14, 418–427. [Google Scholar]
- Yeo, K.I.; Park, I.; Lee, S.H.; Lee, S.Y.; Chang, W.J.; Bashir, R.; Choi, S.; Lee, S.W. Ultra-sensitive dielectrophoretic surface charge multiplex detection inside a micro-dielectrophoretic device. Biosens. Bioelectron. 2022, 210, 114235. [Google Scholar] [CrossRef]
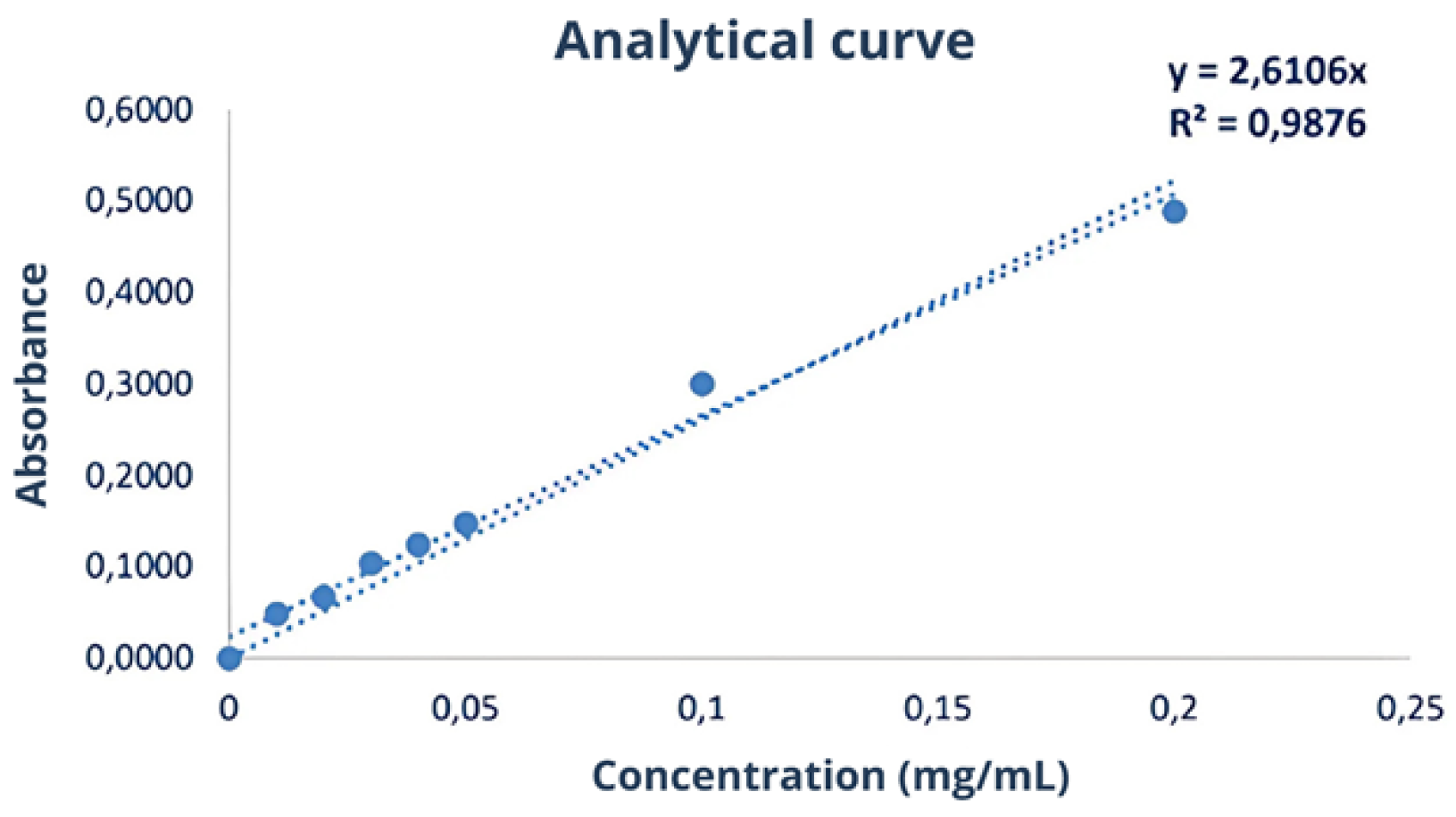
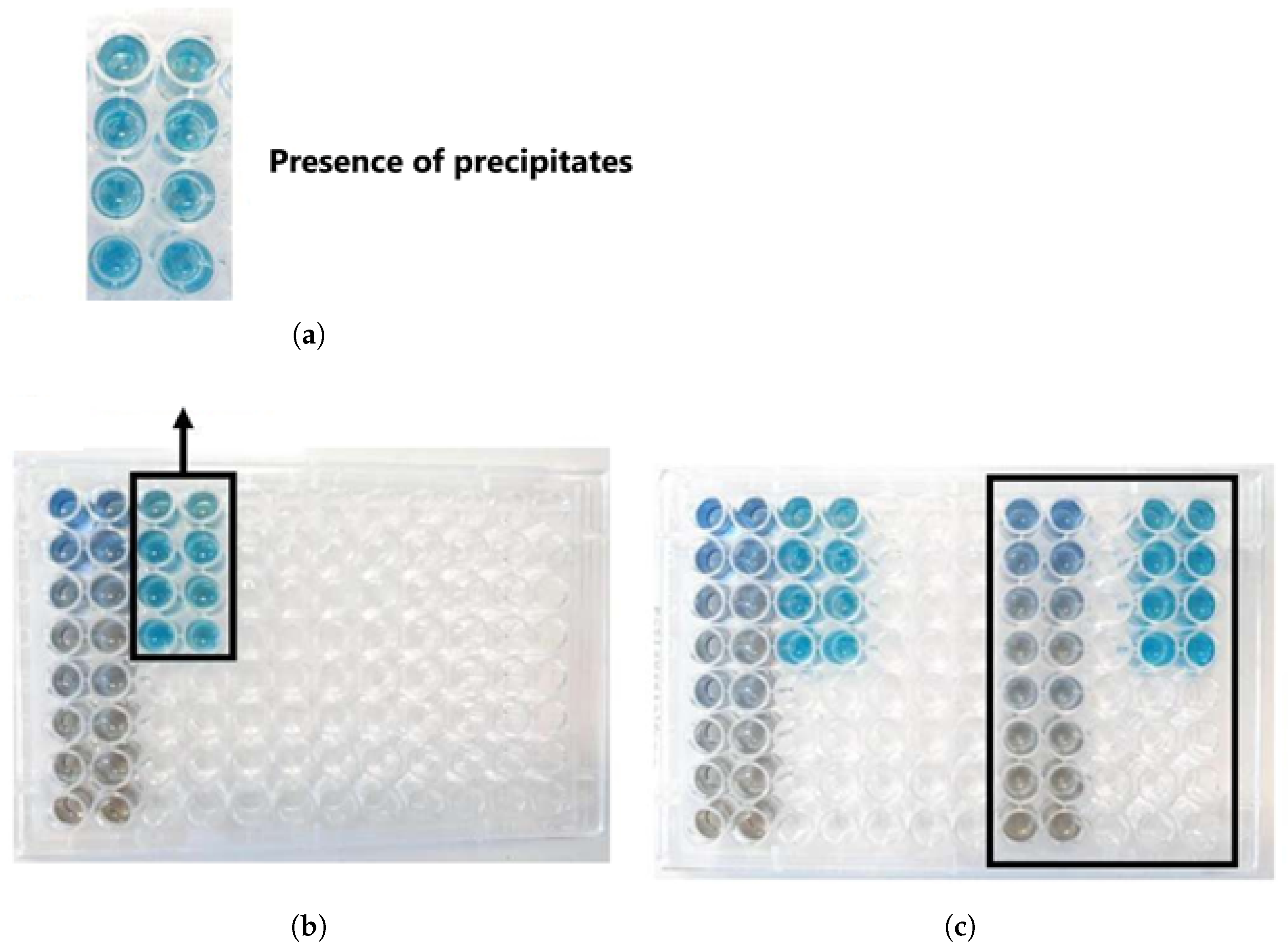
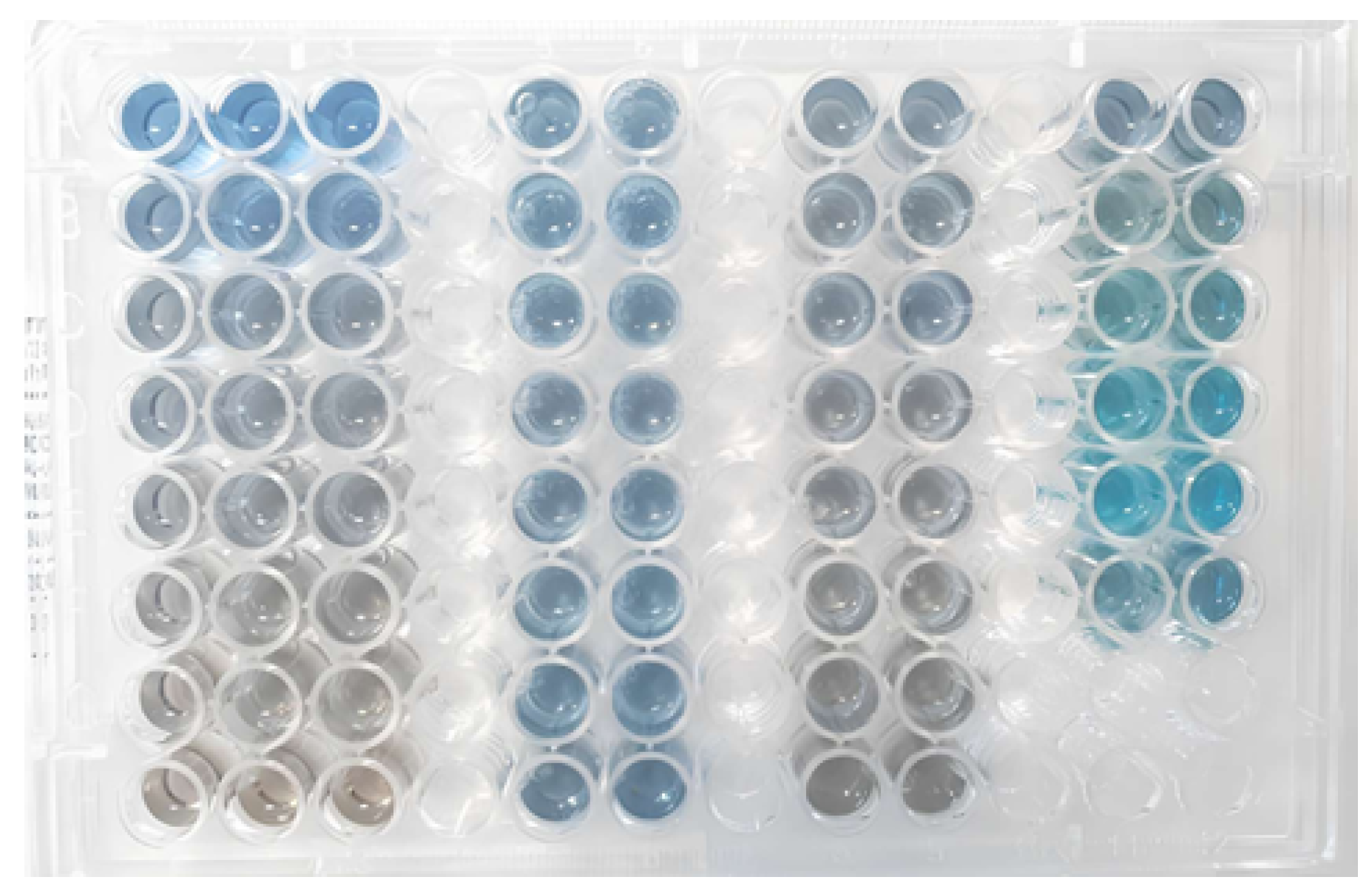

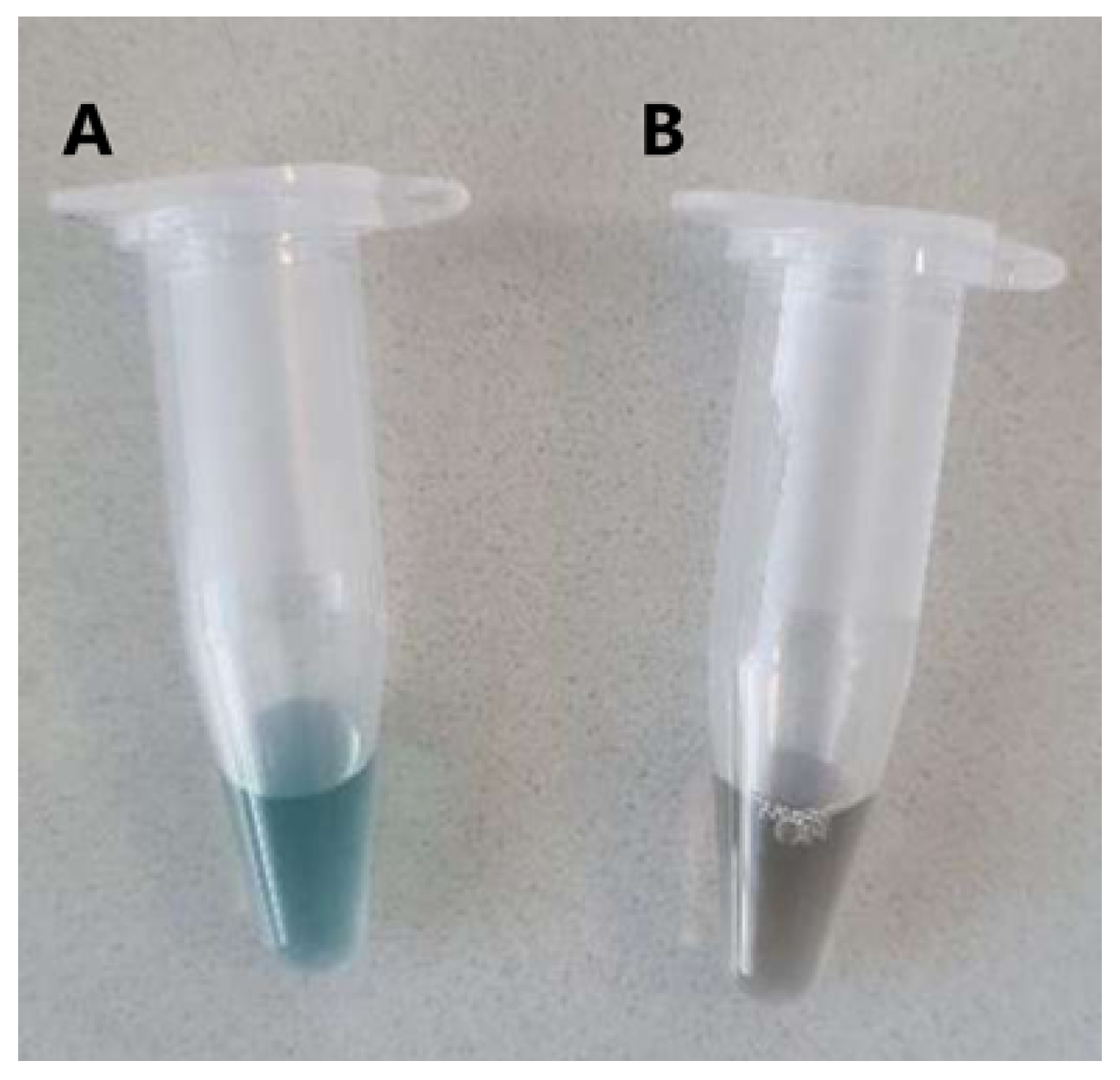

| Sample | Protein Content (g/100 g) Elemental Analysis | Protein Content (g/100 g) Kjeldahl |
|---|---|---|
| Natural latex | 3.49 ± 0.14 | 3.05 ± 0.68 |
| Pre-vulcanized latex | 3.37 ± 0.11 | 3.95 ± 0.75 |
| Sample | Nitrogen Content (g/100 g) Elemental Analysis | Nitrogen Content (g/100 g) Kjeldahl |
|---|---|---|
| Natural latex serum | 0.283 ± 0.028 | 0.998 ± 0.088 |
| Pre-vulcanized latex serum | 0.434 ± 0.022 | 1.070 ± 0.022 |
| Sample | Absorbance |
|---|---|
| Serum Batch 1 | 0.7734 ± 0.0025 |
| Serum Batch 2 refrigerated dialysate | 0.8085 ± 0.0093 |
| Serum Batch 2 frozen dialysate | 0.8176 ± 0.0155 |
| Serum Batch 2 non-dialysate | 0.9128 ± 0.0040 |
| Sample | Concentration mg/mL |
|---|---|
| FrHB1 flask 1 | 0.180 ± 0.002 |
| FrHB1 flask 2 | 0.135 ± 0.0002 |
| FrHB1 flask 3 | 0.123 ± 0.002 |
| FrHB2 flask 1 | 0.130 ± 0.0002 |
| FrHB2 flask 2 | 0.157 ± 0.007 |
| FrHB2 flask 3 | 0.123 ± 0.00005 |
| FrHB3 flask 1 | 0.119 ± 0.0004 |
| FrHB3 flask 2 | 0.115 ± 0.0006 |
| FrHB3 flask 3 | 0.114 ± 0.002 |
| Sample | Concentration (mg/mL) |
|---|---|
| FrHB1 run 1 | 0.227 ± 0.002 |
| FrHB2 run 1 | 0.199 ± 0.002 |
| FrHB1 run 2 | 0.224 ± 0.001 |
| FrHB2 run 2 | 0.191 ± 0.006 |
| Sample | Concentration (mg/mL) * |
|---|---|
| Soro Lot 2 concentrated | 1.994 |
| Soro Lot 2 original | 0.319 |
| Soro Lot 2 dilution 1:2 | 0.995 |
| Soro Lot 2 dilution 1:4 | 0.49 |
| FrHB1 round 1 concentrated | 1.42 |
| FrHB1 round 1 original | 0.227 |
| FrHB1 round 1 dilution 1:2 | 0.114 |
| FrHB1 round 1 dilution 1:4 | 0.057 |
| FrHB1 round 2 concentrated | 1.4 |
| FrHB1 round 2 original | 0.224 |
| FrHB1 round 2 dilution 1:2 | 0.112 |
| FrHB1 round 2 dilution 1:4 | 0.056 |
| Sample | Concentration (mg/mL) | Average Concentration (mg/mL) |
|---|---|---|
| FrHB1 15/03 1 | 0.231 ± 0.009 | |
| FrHB1 15/03 2 | 0.233 ± 0.008 | |
| FrHB1 15/03 3 | 0.208 ± 0.008 | |
| FrHB1 19/03 1 | 0.202 ± 0.008 | |
| FrHB1 19/03 2 | 0.214 ± 0.009 | |
| FrHB1 19/03 3 | 0.219 ± 0.007 | |
| FrHB1 19/03 4 | 0.216 ± 0.006 | |
| FrHB1 19/03 5 | 0.205 ± 0.001 | |
| FrHB1 20/03 1 | 0.211 ± 0.016 | 0.196 ± 0.030 |
| FrHB1 20/03 2 | 0.197 ± 0.003 | |
| FrHB1 20/03 3 | 0.182 ± 0.002 | |
| FrHB1 26/03 1 | 0.192 ± 0.013 | |
| FrHB1 26/03 2 | 0.163 ± 0.001 | |
| FrHB1 26/03 3 | 0.161 ± 0.002 | |
| FrHB1 01/04 1 | 0.155 ± 0.007 | |
| FrHB1 01/04 2 | 0.142 ± 0.001 | |
| FrHB1 01/04 3 | 0.131 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.K.A.d.; Nunes, G.A.M.d.A.; Faria, R.M.; Favilla, A.L.C.; Dornelas, J.; Fonseca, M.A.M.; Benavides, A.D.I.; da Costa, L.B.M.; Fleury Rosa, M.F.; da Rocha, A.F.; et al. Extraction and Purification of the FrHb1 Fraction from Commercial Natural Latex of Hevea brasiliensis for Biomedical Applications. Biomimetics 2025, 10, 85. https://doi.org/10.3390/biomimetics10020085
Silva AKAd, Nunes GAMdA, Faria RM, Favilla ALC, Dornelas J, Fonseca MAM, Benavides ADI, da Costa LBM, Fleury Rosa MF, da Rocha AF, et al. Extraction and Purification of the FrHb1 Fraction from Commercial Natural Latex of Hevea brasiliensis for Biomedical Applications. Biomimetics. 2025; 10(2):85. https://doi.org/10.3390/biomimetics10020085
Chicago/Turabian StyleSilva, Ana Karoline Almeida da, Gustavo Adolfo Marcelino de Almeida Nunes, Rafael Mendes Faria, Ana Luiza Coutinho Favilla, Jéssica Dornelas, Marcos Augusto Mountinho Fonseca, Angie Daniela Ibarra Benavides, Lindemberg Barreto Mota da Costa, Mário Fabrício Fleury Rosa, Adson Ferreira da Rocha, and et al. 2025. "Extraction and Purification of the FrHb1 Fraction from Commercial Natural Latex of Hevea brasiliensis for Biomedical Applications" Biomimetics 10, no. 2: 85. https://doi.org/10.3390/biomimetics10020085
APA StyleSilva, A. K. A. d., Nunes, G. A. M. d. A., Faria, R. M., Favilla, A. L. C., Dornelas, J., Fonseca, M. A. M., Benavides, A. D. I., da Costa, L. B. M., Fleury Rosa, M. F., da Rocha, A. F., & Rosa, S. d. S. R. F. (2025). Extraction and Purification of the FrHb1 Fraction from Commercial Natural Latex of Hevea brasiliensis for Biomedical Applications. Biomimetics, 10(2), 85. https://doi.org/10.3390/biomimetics10020085








