A Structural Optimization Framework for Biodegradable Magnesium Interference Screws
Abstract
:1. Introduction
2. Method
2.1. Design Parameters
2.2. Corrosion Model and Model Calibration
2.3. Objective Function
2.4. SQP Strategy
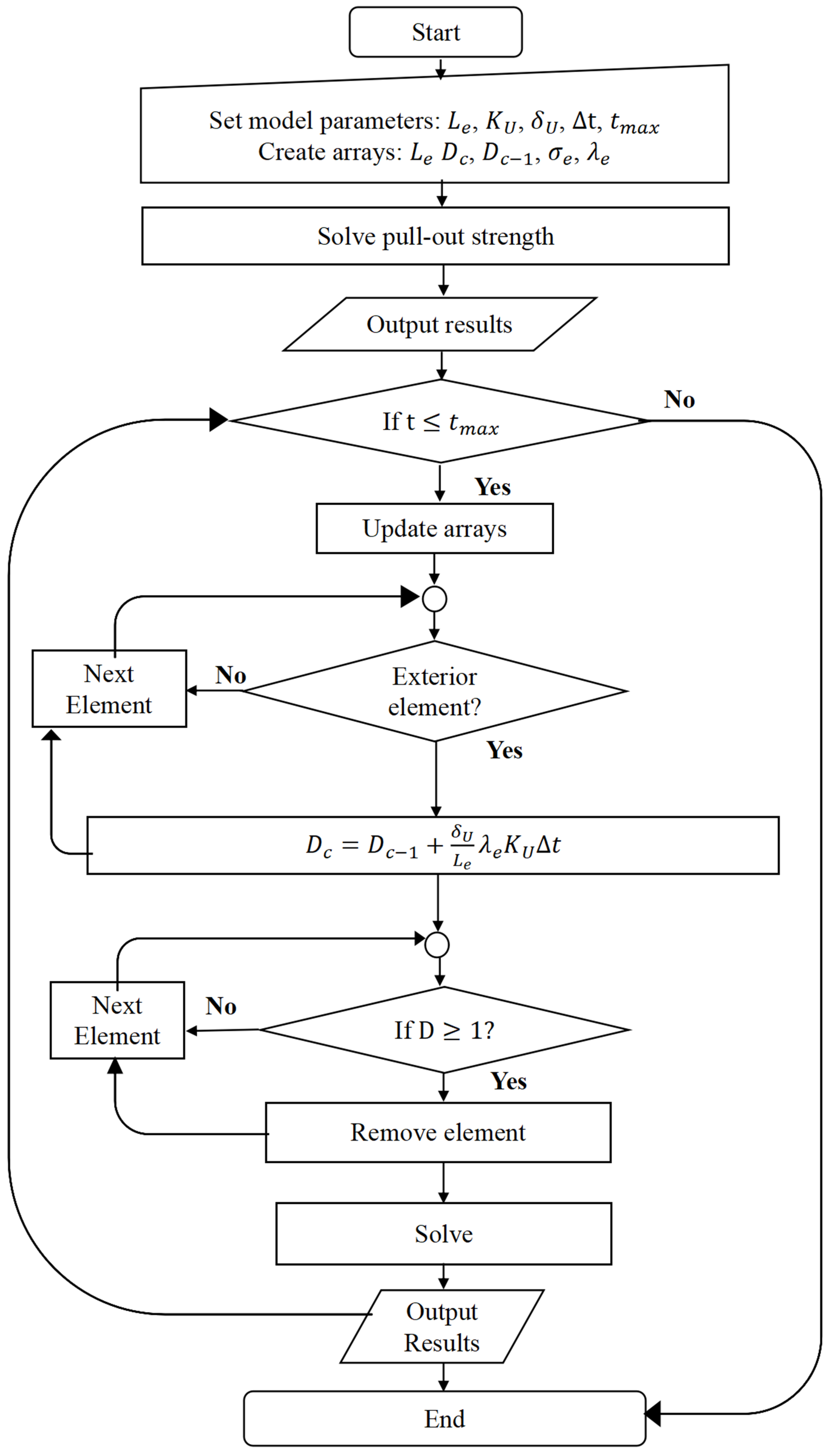
2.5. Optimization Flowchart
- (1)
- (2)
- Generating Corrosion Models: Corrosion models corresponding to each sample point were generated using Abaqus/CAE (6.14) pre-processor and Python (2.73) scripting. The main function of the Python scripting is to traverse the elements in the model, mark the surface elements, and generate random numbers following a Weibull distribution for them. It then writes the element information, adjacent element information, and the random numbers into an Abaqus initial conditions file to set the initial conditions for the entire model.
- (3)
- Evaluating the Objective Function: The objective function was assessed by running numerical simulations.
- (4)
- Building Surrogate Models: Response Surface Methodology (RSM) and Kriging models were used to construct surrogate models that approximate the relationship between the objective function and geometric parameters.
- (5)
- Optimization Using SQP Algorithm: The Sequential Quadratic Programming (SQP) algorithm was applied to explore the design space and determine the optimal solution.
- (6)
- Verifying the Optimal Solution: The objective function was re-evaluated using the corrosion model built from the optimal solution. If the new objective function value was strictly lower than those of other sample points, the optimization process was complete. Otherwise, the process looped back to Step 1 and repeated.

3. Results
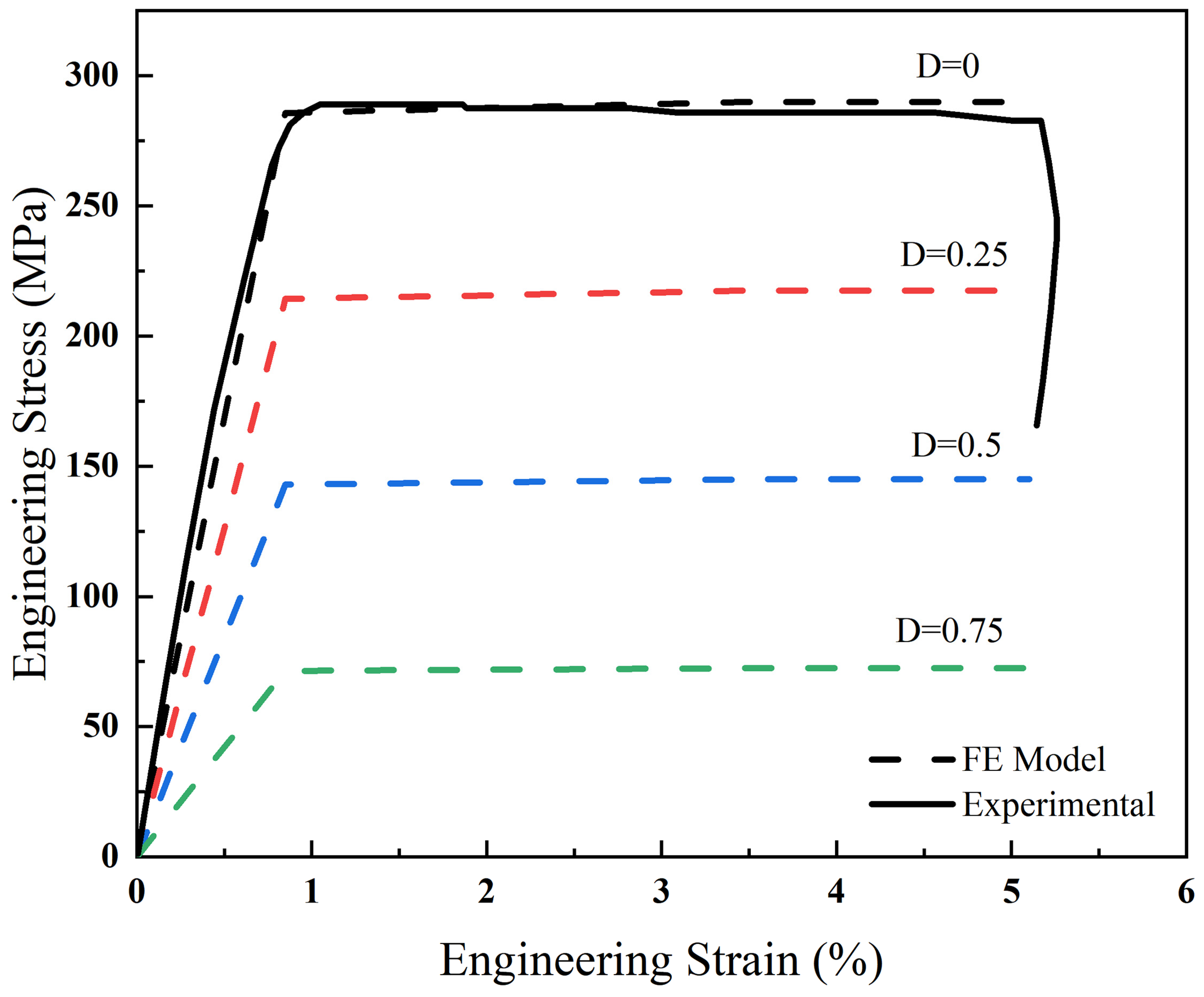
3.1. Calibration Results
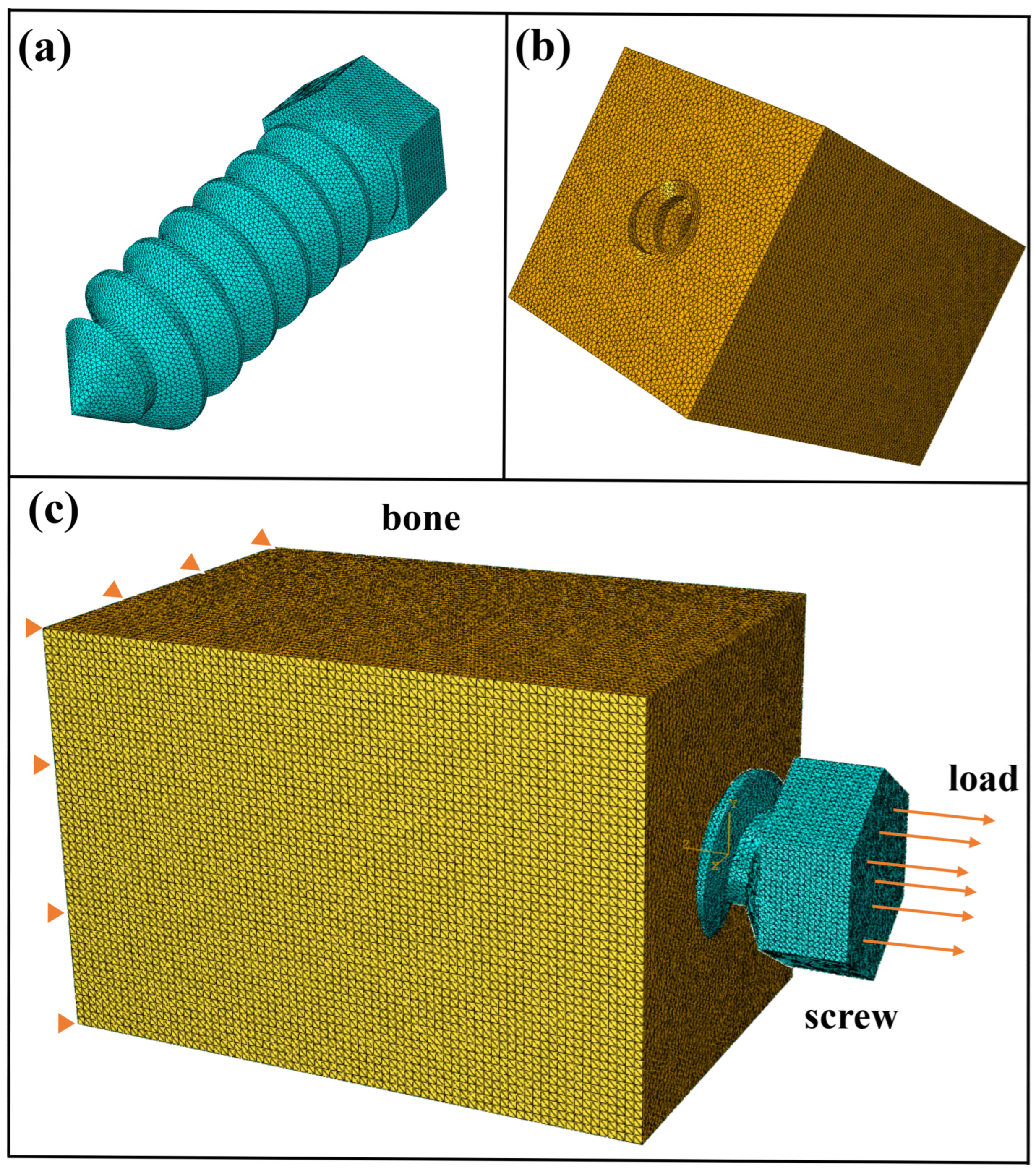
3.2. Mesh Sensitivity Analysis
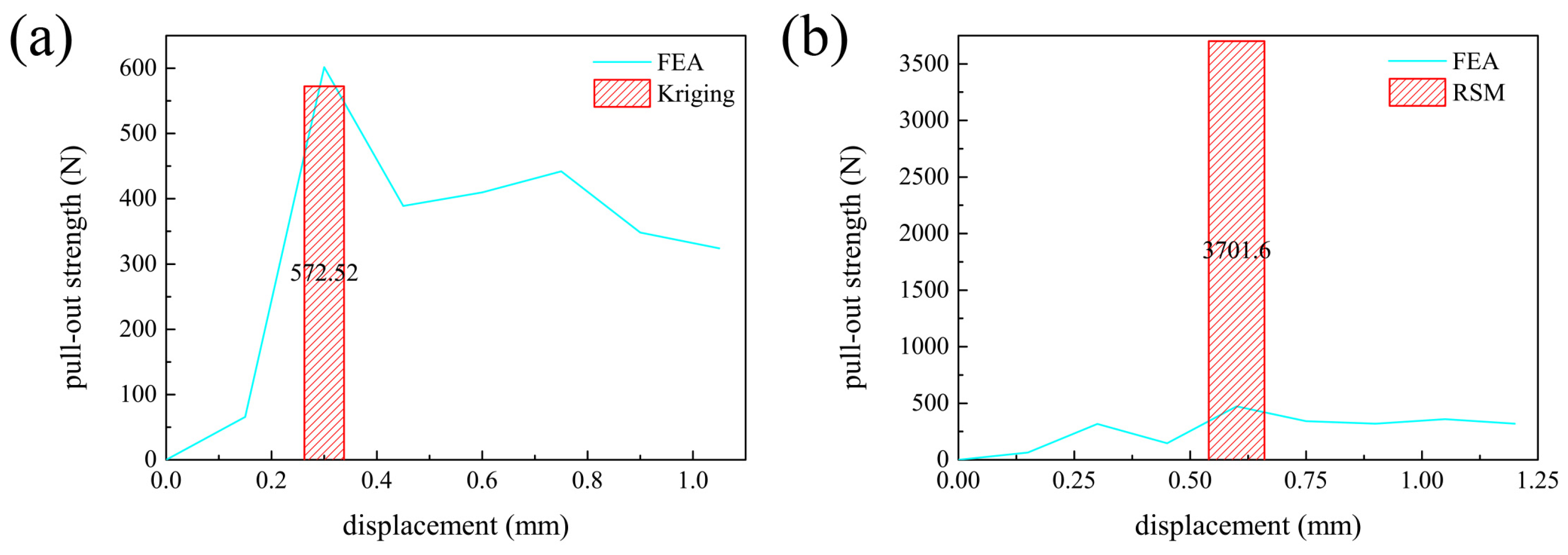
3.3. Optimal Solutions
4. Discussion
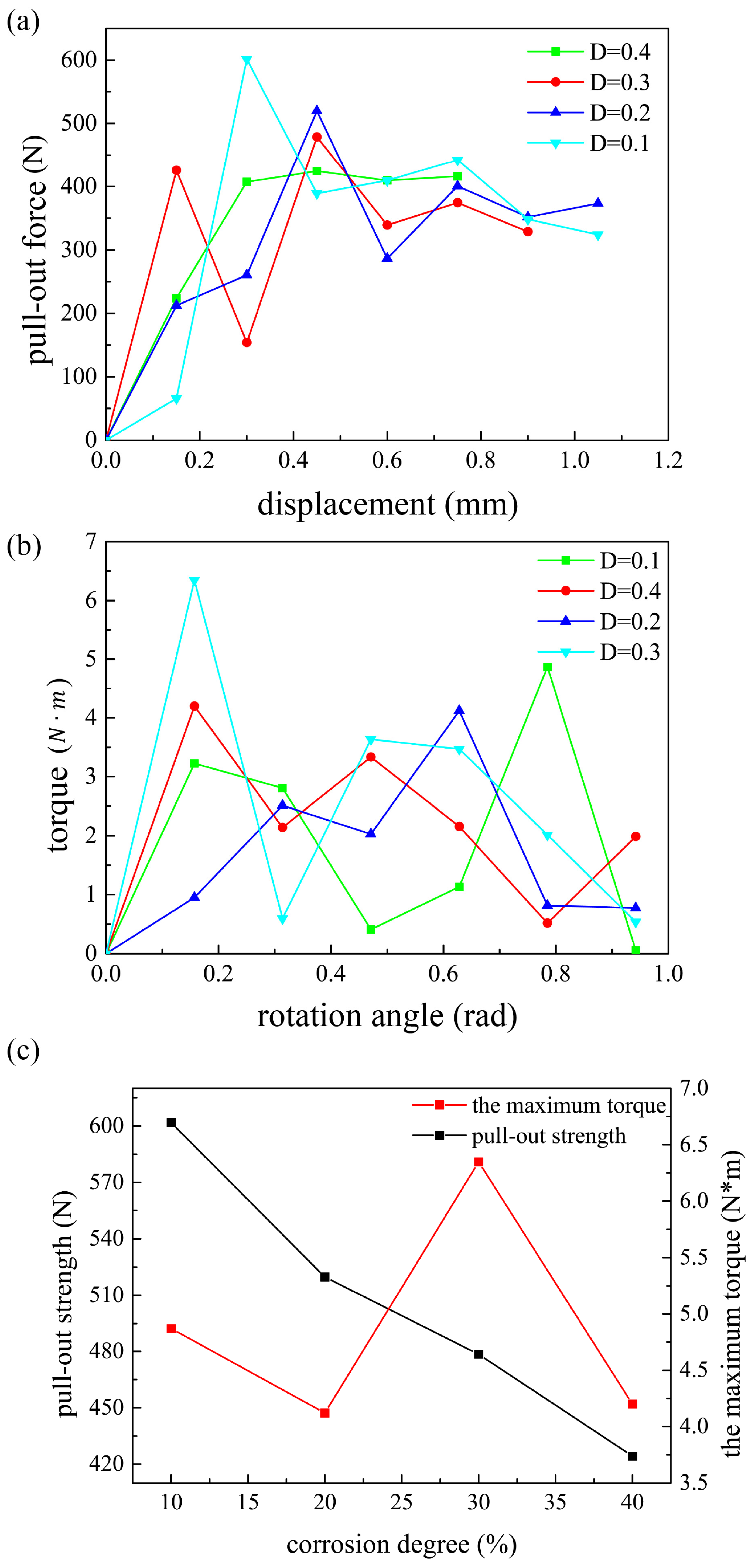
4.1. The Relationship Between Pull-Out Strength and Design Parameters
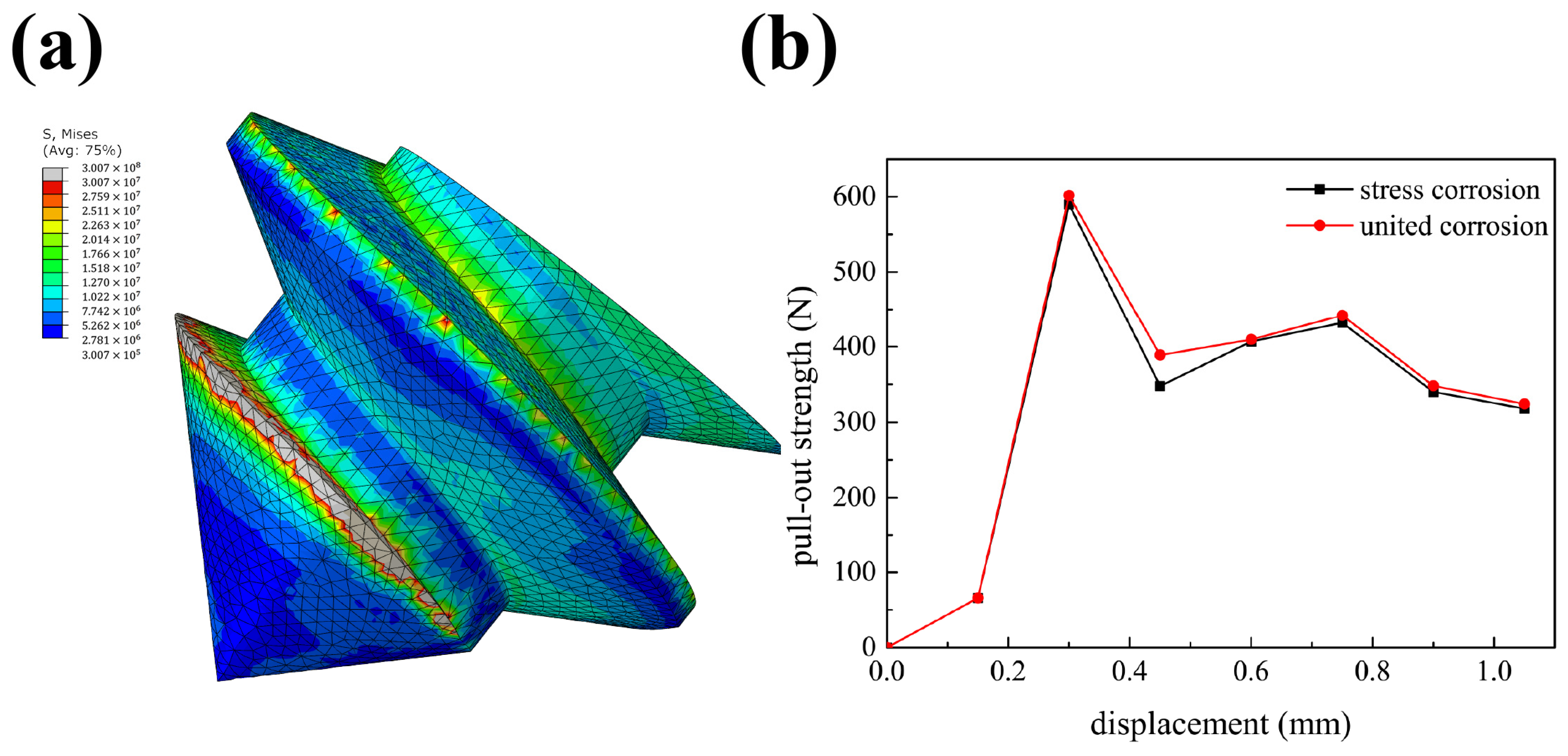
4.2. Verification
4.3. Rationality
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Dou, J.; Wang, Z.; Hu, C.; Yu, H.; Chen, C. Research progress of biodegradable magnesium-based biomedical materials: A review. J. Alloys Compd. 2022, 923, 166377. [Google Scholar]
- Siroros, N.; Merfort, R.; Liu, Y.; Praster, M.; Migliorini, F.; Maffulli, N.; Michalik, R.; Hildebrand, F.; Eschweiler, J. Mechanical properties of a bioabsorbable magnesium interference screw for anterior cruciate ligament reconstruction in various testing bone materials. Sci. Rep. 2023, 13, 12342. [Google Scholar]
- Siroros, N.; Merfort, R.; Liu, Y.; Praster, M.; Hildebrand, F.; Michalik, R.; Eschweiler, J. In vitro investigation of the fixation performance of a bioabsorbable magnesium ACL interference screw compared to a conventional interference screw. Life 2023, 13, 484. [Google Scholar] [CrossRef]
- Rider, P.; Kačarević, Ž.P.; Elad, A.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.; Molnar, B.; Hesse, B. Biodegradation of a magnesium alloy fixation screw used in a guided bone regeneration model in beagle dogs. Materials 2022, 15, 4111. [Google Scholar] [CrossRef]
- AlBaraghtheh, T.; Römer, R.W.; Plumhoff, B.Z. Best Practices in Developing a Workflow for Uncertainty Quantification for Modeling the Biodegradation of Mg-Based Implants. Adv. Sci. 2024, 11, 2403543. [Google Scholar]
- Shen, Z.; Zhao, M.; Zhou, X.; Yang, H.; Liu, J.; Guo, H.; Zheng, Y.; Yang, J.A. A numerical corrosion-fatigue model for biodegradable Mg alloy stents. Acta Biomater. 2019, 97, 671–680. [Google Scholar]
- Li, Y.; Wang, Y.; Shen, Z.; Miao, F.; Wang, J.; Sun, Y.; Zhu, S.; Zheng, Y.; Guan, S. A biodegradable magnesium alloy vascular stent structure: Design, optimisation and evaluation. Acta Biomater. 2022, 142, 402–412. [Google Scholar]
- Shen, Z.; Zhao, M.; Bian, D.; Shen, D.; Zhou, X.; Liu, J.; Liu, Y.; Guo, H.; Zheng, Y. Predicting the degradation behavior of magnesium alloys with a diffusion-based theoretical model and in vitro corrosion testing. J. Mater. Sci. Technol. 2019, 35, 1393–1402. [Google Scholar]
- Luo, Y.; Liu, F.; Chen, Z.; Luo, Y.; Li, W.; Wang, J. A magnesium screw with optimized geometry exhibits improved corrosion resistance and favors bone fracture healing. Acta Biomater. 2024, 178, 320–329. [Google Scholar]
- Luo, Y.; Wang, J.; Ong, M.T.Y.; Yung, P.S.-H.; Wang, J.; Qin, L. Update on the research and development of magnesium-based biodegradable implants and their clinical translation in orthopaedics. Biomater. Transl. 2021, 2, 188–196. [Google Scholar]
- Erbel, R.; Di Mario, C.; Bartunek, J.; Bonnier, J.; de Bruyne, B.; Eberli, F.R.; Erne, P.; Haude, M.; Heublein, B.; Horrigan, M.; et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: A prospective, non-randomised multicentre trial. Lancet 2007, 369, 1869–1875. [Google Scholar]
- Grogan, J.A.; Leen, S.B.; McHugh, P.E. Optimizing the design of a bioabsorbable metal stent using computer simulation methods. Biomaterials 2013, 34, 8049–8060. [Google Scholar]
- Chen, C.; Chen, J.; Wu, W.; Shi, Y.; Jin, L.; Petrini, L.; Shen, L.; Yuan, G.; Ding, W.; Ge, J.; et al. In vivo and in vitro evaluation of a biodegradable magnesium vascular stent designed by shape optimization strategy. Biomaterials 2019, 221, 119414. [Google Scholar]
- Wu, W.; Petrini, L.; Gastaldi, D.; Villa, T.; Vedani, M.; Lesma, E.; Previtali, B.; Migliavacca, F. Finite Element Shape Optimization for Biodegradable Magnesium Alloy Stents. Ann. Biomed. Eng. 2010, 38, 2829–2840. [Google Scholar]
- Farraro, K.F.; Kim, K.E.; Woo, S.L.; Flowers, J.R.; McCullough, M.B. Revolutionizing orthopaedic biomaterials: The potential of biodegradable and bioresorbable magnesium-based materials for functional tissue engineering. J. Biomech. 2014, 47, 1979–1986. [Google Scholar]
- Kennady, M.C.; Tucker, M.R.; Lester, G.E.; Buckley, M.J. Stress shielding effect of rigid internal fixation plates on mandibular bone grafts. A photon absorption densitometry and quantitative computerized tomographic evaluation. Int. J. Oral Max. Surg. 1989, 18, 307–310. [Google Scholar]
- Sha, M.; Guo, Z.; Fu, J.; Li, J.; Yuan, C.F.; Shi, L.; Li, S.J. The effects of nail rigidity on fracture healing in rats with osteoporosis. Acta Orthop. 2009, 80, 135–138. [Google Scholar]
- Minkowitz, R.B.; Bhadsavle, S.; Walsh, M.; Egol, K.A. Removal of painful orthopaedic implants after fracture union. J. Bone Jt. Surg. 2007, 89, 1906–1912. [Google Scholar]
- Amini, A.R.; Wallace, J.S.; Nukavarapu, S.P. Short-term and long-term effects of orthopedic biodegradable implants. J. Long-Term Eff. Med. Implant. 2011, 21, 93–122. [Google Scholar]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar]
- Huang, S.; Wang, B.; Zhang, X.; Lu, F.; Wang, Z.; Tian, S.; Li, D.; Yang, J.; Cao, F.; Cheng, L. High-purity weight-bearing magnesium screw: Translational application in the healing of femoral neck fracture. Biomaterials 2020, 238, 119829. [Google Scholar]
- Atrens, A.; Liu, M.; Abidin, N.I.Z. Corrosion mechanism applicable to biodegradable magnesium implants. Mater. Sci. Eng. B 2011, 176, 1609–1636. [Google Scholar]
- Barber, F.A.; Elrod, B.F.; Mcguire, D.A.; Paulos, L.E. Preliminary results of an absorbable interference screw. Arthroscopy 1995, 11, 537–548. [Google Scholar]
- Brown, C.H.; Hecker, A.T.; Hipp, J.A.; Myers, E.R.; Hayes, W.C. The biomechanics of interference screw fixation of patellar tendon anterior cruciate ligament grafts. Am. J. Sports Med. 1993, 21, 880–886. [Google Scholar]
- Gastaldi, D.; Sassi, V.; Petrini, L.; Vedani, M.; Trasatti, S.; Migliavacca, F. Continuum damage model for bioresorbable magnesium alloy devices—Application to coronary stents. J. Mech. Behav. Biomed. Mater. 2011, 4, 352–365. [Google Scholar]
- Wu, W.; Gastaldi, D.; Yang, K.; Tan, L.; Petrini, L.; Migliavacca, F. Finite element analyses for design evaluation of biodegradable magnesium alloy stents in arterial vessels. Mater. Sci. Eng. B 2011, 176, 1733–1740. [Google Scholar]
- Grogan, J.A.; O’Brien, B.J.; Leen, S.B.; McHugh, P.E. A corrosion model for bioabsorbable metallic stents. Acta Biomater. 2011, 7, 3523–3533. [Google Scholar]
- Galvin, E.; O’Brien, D.; Cummins, C.; Donald, B.J.M.; Lally, C. A strain-mediated corrosion model for bioabsorbable metallic stents. Acta Biomater. 2017, 55, 505–517. [Google Scholar]
- Weiler, A.; Strobel, M.; Timmermans, A. Interference screw. U.S. Patent EP1093774A1, 2003. [Google Scholar]
- Fang, H.; Rais-Rohani, M.; Liu, Z.; Horstemeyer, M.F. A comparative study of metamodeling methods for multiobjective crashworthiness optimization. Comput. Struct. 2005, 83, 2121–2136. [Google Scholar]
- Simpson, T.W.; Poplinski, J.D.; Koch, P.N.; Allen, J.K. Metamodels for Computer-based Engineering Design: Survey and recommendations. Eng. Comput. 2001, 17, 129–150. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Tan, S.H.; Chou, S.M. Effects of bone materials on the screw pull-out strength in human spine. Med. Eng. Phys. 2006, 28, 795–801. [Google Scholar] [CrossRef]
- Hibbitt, H.; Karlsson, B.; Sorensen, P. Abaqus Analysis User’s Manual Version 6.10; Dassault Systèmes Simulia Corp.: Providence, RI, USA, 2011. [Google Scholar]
- Liu, C.; Chen, H.; Cheng, C.; Kao, H.; Lo, W. Biomechanical evaluation of a new anterior spinal implant. Clin. Biomech. 1998, 13, S40–S45. [Google Scholar] [CrossRef]
- Goel, V.K.; Kim, Y.E.; Lim, T.; Weinstein, J.N. An Analytical Investigation of the Mechanics of Spinal Instrumentation. Spine 1988, 13, 1003–1011. [Google Scholar] [CrossRef]
- Silva, M.J.; Gibson, L.J. Modeling the mechanical behavior of vertebral trabecular bone: Effects of age-related changes in microstructure. Bone 1997, 21, 191–199. [Google Scholar] [CrossRef]
- Dassault Systèmes Simulia Corp. Abaqus Analysis Manual v6.10; DS Simulia: Providence, RI, USA, 2010. [Google Scholar]
- Cheng, P.; Han, P.; Zhao, C.; Zhang, S.; Wu, H.; Ni, J.; Hou, P.; Zhang, Y.; Liu, J.; Xu, H.; et al. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF. Biomaterials 2016, 81, 14–26. [Google Scholar] [CrossRef]
- Tammareddi, S.; Sun, G.; Li, Q. Multiobjective robust optimization of coronary stents. Mater. Des. 2016, 90, 682–692. [Google Scholar] [CrossRef]
- Sandia National Laboratories. DAKOTA Theory Manual v5.2; Sandia National Laboratories: Albuquerque, NM, USA, 2012. [Google Scholar]
- Cressie, N.A.C. Statistics for Spatial Data, Revised ed.; J. Wiley: Hoboken, NJ, USA, 1993. [Google Scholar]
- Simpson, T.W.; Mauery, T.M.; Korte, J.J.; Mistree, F. Kriging Models for Global Approximation in Simulation-Based Multidisciplinary Design Optimization. AIAA J. 2001, 39, 2233–2241. [Google Scholar] [CrossRef]
- Schittkowski, K. A robust implementation of a sequential quadratic programming algorithm with successive error restoration. Optim. Lett. 2010, 5, 283–296. [Google Scholar] [CrossRef]
- Andrei, N. Continuous Nonlinear Optimization for Engineering Applications in GAMS Technology; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Santer, T.J.; Williams, B.; Notz, W. The Design and Analysis of Computer Experiments; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]



| Group | |||||
|---|---|---|---|---|---|
| 1 | 4.5 | 15.0 | 30.0 | 2.75 | 0.40 |
| 2 | 3.9 | 16.4 | 32.3 | 2.85 | 0.42 |
| 3 | 5.0 | 15.0 | 30.0 | 2.75 | 0.40 |
| 4 | 4.0 | 16.1 | 33.5 | 2.88 | 0.45 |
| 5 | 4.5 | 15.0 | 30.0 | 2.75 | 0.50 |
| 6 | 4.3 | 16.6 | 32.5 | 2.80 | 0.39 |
| 7 | 4.5 | 15.0 | 30.0 | 2.60 | 0.40 |
| 8 | 4.2 | 18.7 | 30.1 | 2.76 | 0.30 |
| 9 | 4.5 | 15.0 | 35.0 | 2.75 | 0.40 |
| 10 | 4.3 | 16.6 | 31.9 | 2.55 | 0.32 |
| 11 | 4.5 | 20.0 | 35.0 | 2.75 | 0.40 |
| 12 | 4.0 | 15.8 | 30.4 | 2.80 | 0.45 |
| 13 | 4.5 | 20.0 | 30.0 | 2.75 | 0.40 |
| 14 | 4.6 | 16.8 | 33.8 | 2.64 | 0.43 |
| 15 | 5.0 | 20.0 | 30.0 | 2.90 | 0.30 |
| 16 | 4.0 | 15.0 | 30.0 | 2.75 | 0.40 |
| 17 | 4.2 | 17.4 | 32.5 | 2.83 | 0.39 |
| 18 | 4.5 | 15.0 | 30.0 | 2.75 | 0.30 |
| 19 | 4.7 | 15.2 | 32.5 | 2.84 | 0.36 |
| 20 | 4.5 | 15.0 | 30.0 | 2.75 | 0.60 |
| 21 | 4.1 | 18.2 | 34.2 | 2.79 | 0.31 |
| 22 | 4.5 | 15.0 | 30.0 | 2.90 | 0.40 |
| Mg-1Ca | Young Modulus (MPa) | Poisson’s Ratio | Density (kg/m3) | Yield Stress (MPa) | Plastic Strain (%) |
| 43,480 | 0.3 | 1738 | 277 | 0 | |
| 290 | 0.0106 | ||||
| Bone | Young Modulus (MPa) | Poisson’s Ratio | Density (kg/m3) | Yield Stress (MPa) | Plastic Strain (%) |
| 100 | 0.2 | 1200 | 2 | 0 |
| Pull-Out Strength (N) | ||||||
|---|---|---|---|---|---|---|
| Kriging | ||||||
| RSM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Zhou, X.; Zhao, M.; Li, Y. A Structural Optimization Framework for Biodegradable Magnesium Interference Screws. Biomimetics 2025, 10, 210. https://doi.org/10.3390/biomimetics10040210
Shen Z, Zhou X, Zhao M, Li Y. A Structural Optimization Framework for Biodegradable Magnesium Interference Screws. Biomimetics. 2025; 10(4):210. https://doi.org/10.3390/biomimetics10040210
Chicago/Turabian StyleShen, Zhenquan, Xiaochen Zhou, Ming Zhao, and Yafei Li. 2025. "A Structural Optimization Framework for Biodegradable Magnesium Interference Screws" Biomimetics 10, no. 4: 210. https://doi.org/10.3390/biomimetics10040210
APA StyleShen, Z., Zhou, X., Zhao, M., & Li, Y. (2025). A Structural Optimization Framework for Biodegradable Magnesium Interference Screws. Biomimetics, 10(4), 210. https://doi.org/10.3390/biomimetics10040210






