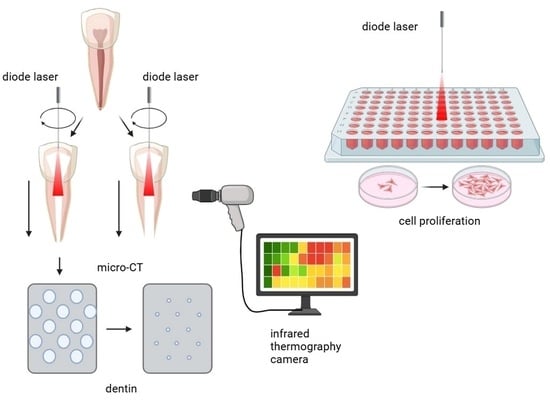
A Biomimetic Approach to Diode Laser Use in Endodontic Treatment of Immature Teeth: Thermal, Structural, and Biological Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Diode Laser System
2.2. Sample Size
2.3. Samples’ Preparation
2.3.1. Preparation of Mature Root Samples
2.3.2. Preparation of Immature Root Samples
2.4. Simulation of Endodontic Laser Treatment
2.5. Temperature Measurements
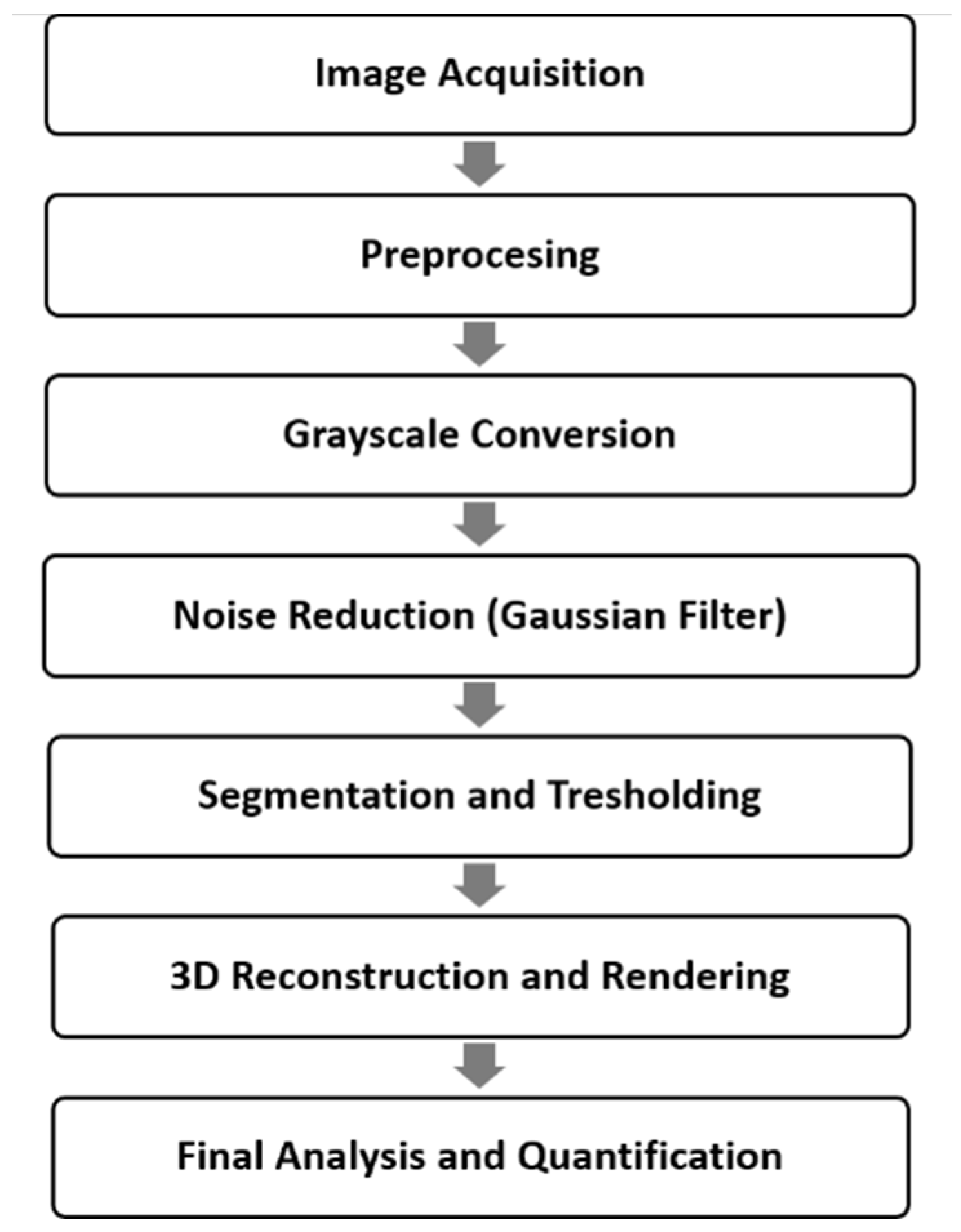
2.6. Micro-CT Scanning
2.6.1. Micro-CT Image Reconstructions
2.6.2. Volumetric Rendering Software Analysis
2.6.3. Measurements
2.7. In Vitro Biocompatibility on Stem Cells from Apical Papilla (SCAPs)
2.7.1. Isolation and Characterization
2.7.2. Mitochondrial Activity Assay (MTT)
2.8. Statistical Analyses
3. Results
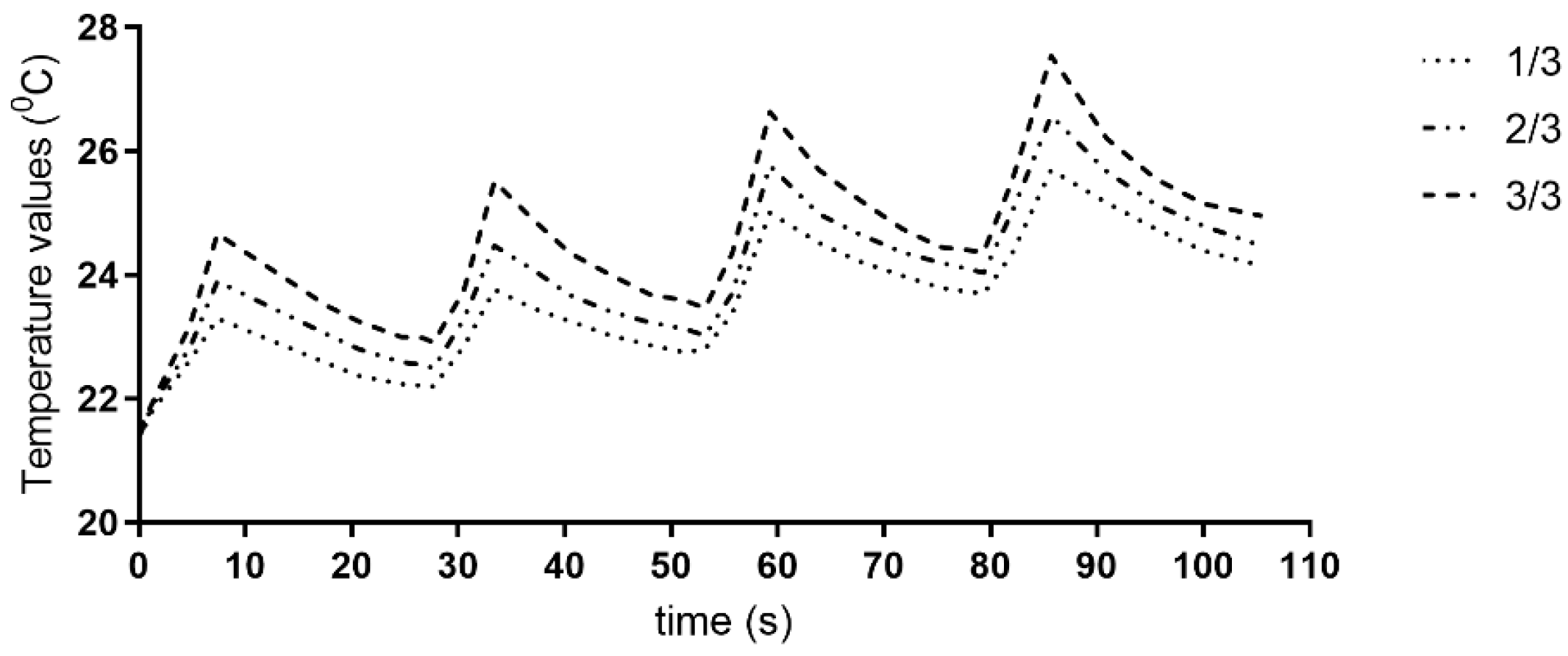
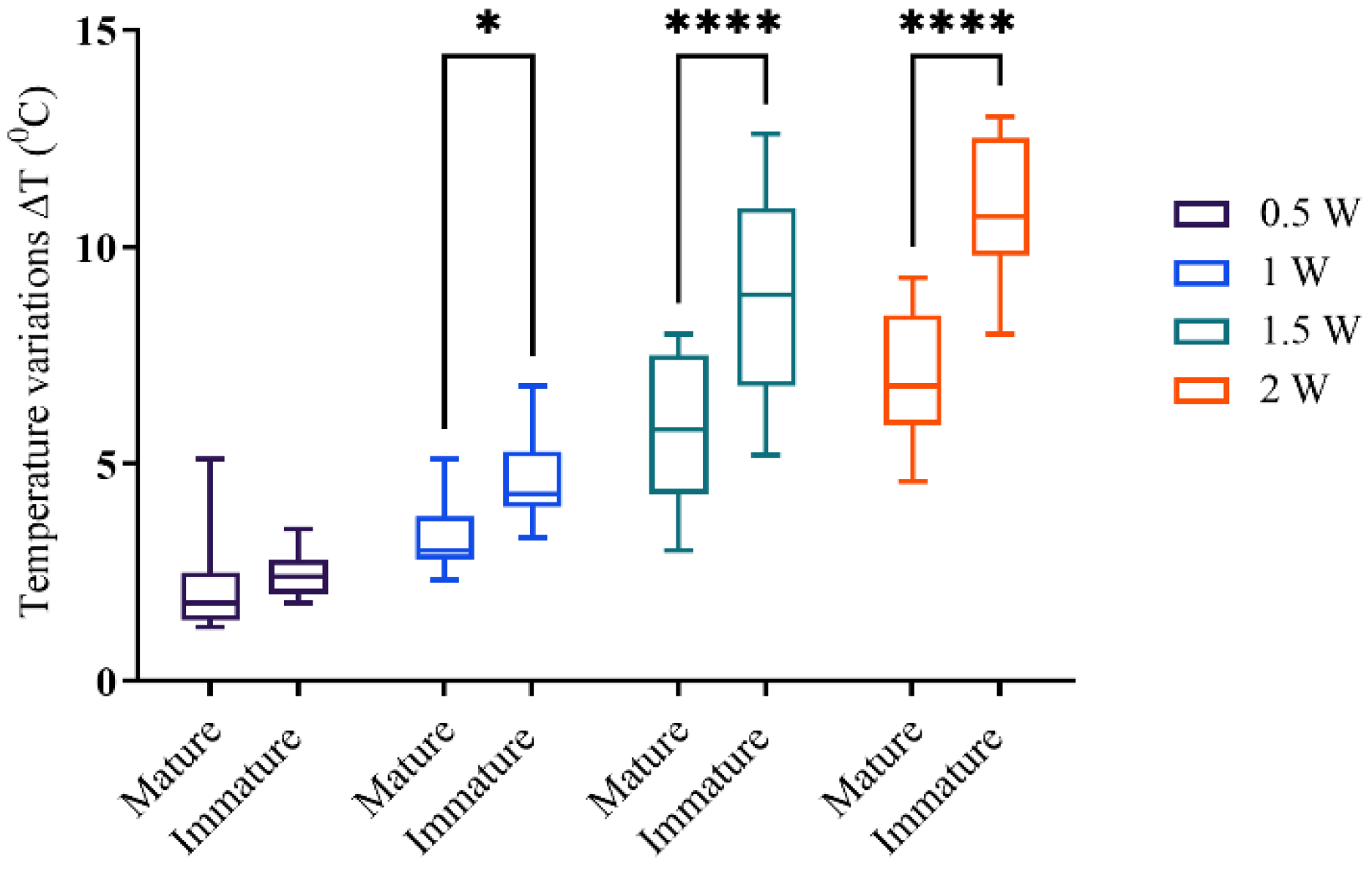
3.1. Temperature Measurements
3.2. Micro-CT Scanning
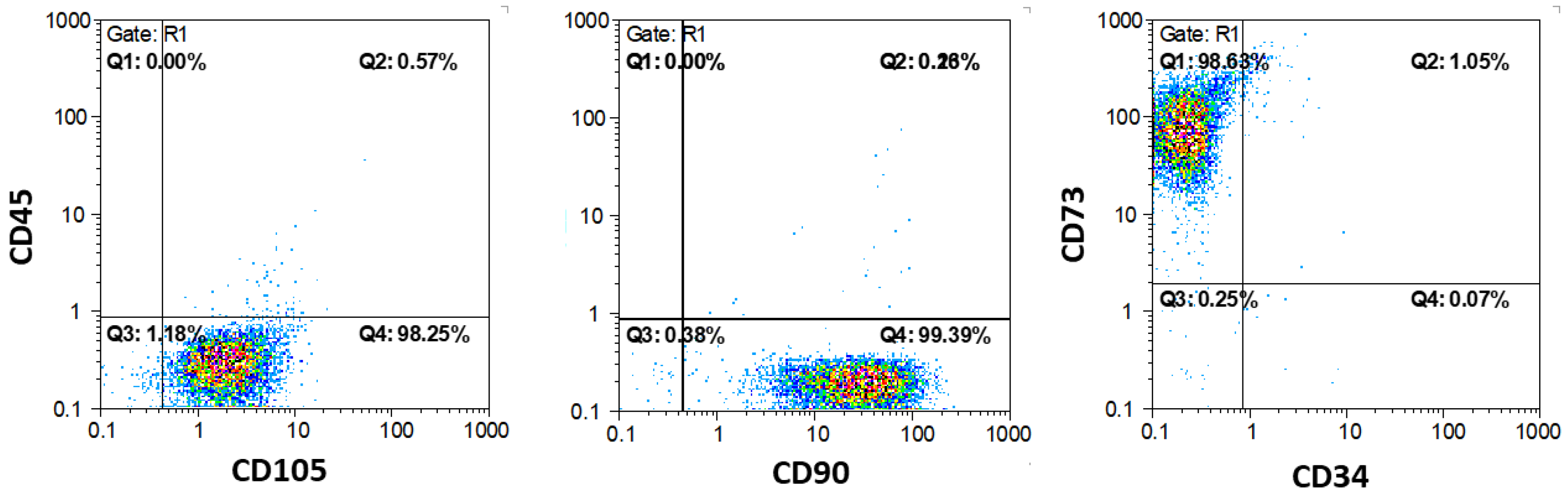
3.3. Flow Cytometry
3.4. Mitochondrial Activity Assay (MTT)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Calculations of Temperature on the Inner Dentinal Walls
Appendix A.2. Calculation of Laser Irradiation Parameters
Appendix A.2.1. Power Density/Irradiance at Target (mW/cm2)
Appendix A.2.2. Radiant Energy (J)
Appendix A.2.3. Energy Density (J/cm2)
Appendix A.2.4. Total Radiant Energy (J)
| Parameter | Teeth Sample Irradiation | SCAPs Irradiation |
|---|---|---|
| Laser type | Diode laser (InGaAsP) | Diode laser (InGaAsP) |
| Wavelength (nm) | 940 ± 10 | 940 ± 10 |
| Operating mode | Continuous wave | Continuous wave |
| Average radiant power (mW) | 500, 1000, 1500, 2000 | 500, 1000, 1500, 2000 |
| Beam spot size/area at target (cm2) | 1.873 | 0.33 |
| Distance from target (mm) | / | 13 |
| Exposure duration (s) | 5 | 5 |
| Power density/irradiance at target (mW/cm2) | 267, 534, 801, 1068 | 1515, 3030, 4545, 6060 |
| Radiant energy (J) | 2.5, 5, 7.5, 10 | 2.5, 5, 7.5, 10 |
| Energy density/radiant exposure (J/cm2) | 1.33, 2.67, 4, 5.34 | 7.6, 15, 22.7, 30.3 |
| Number and frequency of treatment sessions | 4 cycles, with 20 s resting periods | 4 cycles, with 20 s resting periods |
| Total radiant energy (J) | 10, 20, 30, 40 | 10, 20, 30, 40 |
References
- Pinto, K.P.; Barbosa, A.F.A.; Silva, E.J.N.L.; Santos, A.P.P.; Sassone, L.M. What Is the Microbial Profile in Persistent Endodontic Infections? A Scoping Review. J. Endod. 2023, 49, 786–798.e7. [Google Scholar] [CrossRef]
- Godoi-Jr, E.P.; Bronzato, J.D.; Francisco, P.A.; Bícego-Pereira, E.C.; Lopes, E.M.; Passini, M.R.Z.; de-Jesus-Soares, A.; Almeida, J.F.A.; Marciano, M.A.; Ferraz, C.C.R.; et al. Microbiological Profile of Root Canals Indicated for Endodontic Retreatment Due to Secondary Endodontic Infections or for Prosthetic Reasons. Clin. Oral Investig. 2023, 27, 2049–2064. [Google Scholar] [CrossRef] [PubMed]
- Gunes, B.; Yeter, K.Y.; Altay, Y. Impact of Different Activation Procedures on Sodium Hypochlorite Penetration into Dentinal Tubules after Endodontic Retreatment via Confocal Laser Scanning Microscopy. BMC Oral Health 2024, 24, 1103. [Google Scholar] [CrossRef]
- Stauffacher, S.; Lussi, A.; Nietzsche, S.; Neuhaus, K.W.; Eick, S. Bacterial Invasion into Radicular Dentine—An in Vitro Study. Clin. Oral Investig. 2017, 21, 1743–1752. [Google Scholar] [CrossRef]
- Tsesis, I.; Lokshin, M.; Littner, D.; Goldberger, T.; Rosen, E. Depth of Bacterial Penetration into Dentinal Tubules after Use of Different Irrigation Solutions: A Systematic Review of In Vitro Studies. Appl. Sci. 2022, 13, 496. [Google Scholar] [CrossRef]
- Harlamb, S. Management of Incompletely Developed Teeth Requiring Root Canal Treatment. Aust. Dent. J. 2016, 61, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.F.; Diogenes, A.R.; Torabinejad, M.; Hargreaves, K.M. Microbiome Changes during Regenerative Endodontic Treatment Using Different Methods of Disinfection. J. Endod. 2022, 48, 1273–1284. [Google Scholar] [CrossRef]
- Bordea, I.R.; Hanna, R.; Chiniforush, N.; Grădinaru, E.; Câmpian, R.S.; Sîrbu, A.; Amaroli, A.; Benedicenti, S. Evaluation of the Outcome of Various Laser Therapy Applications in Root Canal Disinfection: A Systematic Review. Photodiagn. Photodyn. Ther. 2020, 29, 101611. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, Z.; Lyu, P.; Zhou, X.; Fan, Y. Current Applications and Future Directions of Lasers in Endodontics: A Narrative Review. Bioengineering 2023, 10, 296. [Google Scholar] [CrossRef]
- Hsieh, S.-C.; Teng, N.-C.; Chu, C.C.; Chu, Y.-T.; Chen, C.-H.; Chang, L.-Y.; Hsu, C.-Y.; Huang, C.-S.; Hsiao, G.Y.-W.; Yang, J.-C. The Antibacterial Efficacy and In Vivo Toxicity of Sodium Hypochlorite and Electrolyzed Oxidizing (EO) Water-Based Endodontic Irrigating Solutions. Materials 2020, 13, 260. [Google Scholar] [CrossRef]
- Mármora, B.C.; Brochado, F.T.; Schmidt, T.R.; Santos, L.G.; Araújo, A.A.D.; Medeiros, C.A.C.X.D.; Ribeiro, S.B.; Martins, M.A.T.; Pilar, E.F.S.; Wagner, V.P.; et al. Defocused High-Power Diode Laser Accelerates Skin Repair in a Murine Model through REDOX State Modulation and Reepithelization and Collagen Deposition Stimulation. J. Photochem. Photobiol. B 2021, 225, 112332. [Google Scholar] [CrossRef] [PubMed]
- Divya, D.; Naik, S.; Raju, O.; Shivani, B.; Basappa, N.; Betur, A. Conceptual Combination of Disinfection in Regenerative Endodontics: Conventional versus Laser-Assisted Disinfection. J. Conserv. Dent. 2021, 24, 252. [Google Scholar] [CrossRef]
- Borges, C.C.; Estrela, C.; Lopes, F.C.; Palma-Dibb, R.G.; Pecora, J.D.; De Araújo Estrela, C.R.; Sousa-Neto, M.D.D. Effect of Different Diode Laser Wavelengths on Root Dentin Decontamination Infected with Enterococcus Faecalis. J. Photochem. Photobiol. B 2017, 176, 1–8. [Google Scholar] [CrossRef]
- Mathew, T.; Bm, S.; Gv, P.; Jose, J. Comparative Evaluation of the Antibacterial Efficacy of Chlorhexidine and 810 Nm Diode Laser in the Disinfection of Root Canals Contaminated With Enterococcus Faecalis: An In Vitro Study. Cureus 2022, 14, e28596. [Google Scholar] [CrossRef] [PubMed]
- Saydjari, Y.; Kuypers, T.; Gutknecht, N. Laser Application in Dentistry: Irradiation Effects of Nd:YAG 1064 Nm and Diode 810 Nm and 980 Nm in Infected Root Canals—A Literature Overview. BioMed Res. Int. 2016, 2016, 8421656. [Google Scholar] [CrossRef]
- Gutknecht, N.; Franzen, R.; Schippers, M.; Lampert, F. Bactericidal Effect of a 980-Nm Diode Laser in the Root Canal Wall Dentin of Bovine Teeth. J. Clin. Laser Med. Surg. 2004, 22, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Asnaashari, M.; Ebad, L.T.; Shojaeian, S. Comparison of Antibacterial Effects of 810 and 980- Nanometer Diode Lasers on Enterococcus Faecalis in the Root Canal System-An in Vitro Study. LASER Ther. 2016, 25, 209–214. [Google Scholar] [CrossRef]
- Lopes, F.C.; Roperto, R.; Akkus, A.; Silva Sousa, Y.T.C.; Sousa-Neto, M.D. Evaluation of Chemical and Morphological Changes in Radicular Dentin after Different Final Surface Treatments. Microsc. Res. Tech. 2018, 81, 973–979. [Google Scholar] [CrossRef]
- Rödig, T.; Müller, C.; Hoch, M.; Haupt, F.; Schulz, X.; Wiegand, A.; Rizk, M. Moisture Content of Root Canal Dentine Affects Detection of Microcracks Using Micro-computed Tomography. Int. Endod. J. 2018, 51, 357–363. [Google Scholar] [CrossRef]
- Alfredo, E.; Marchesan, M.A.; Sousa-Neto, M.D.; Brugnera-Júnior, A.; Silva-Sousa, Y.T.C. Temperature Variation at the External Root Surface during 980-Nm Diode Laser Irradiation in the Root Canal. J. Dent. 2008, 36, 529–534. [Google Scholar] [CrossRef]
- Driesen, R.B.; Gervois, P.; Vangansewinkel, T.; Lambrichts, I. Unraveling the Role of the Apical Papilla During Dental Root Maturation. Front. Cell Dev. Biol. 2021, 9, 665600. [Google Scholar] [CrossRef] [PubMed]
- Haidary, D.; Franzen, R.; Gutknecht, N. Root Surface Temperature Changes During Root Canal Laser Irradiation with Dual Wavelength Laser (940 and 2780 Nm): A Preliminary Study. Photomed. Laser Surg. 2016, 34, 336–344. [Google Scholar] [CrossRef]
- Gutknecht, N.; Franzen, R.; Meister, J.; Vanweersch, L.; Mir, M. Temperature Evolution on Human Teeth Root Surface after Diode Laser Assisted Endodontic Treatment. Lasers Med. Sci. 2005, 20, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Althubaiti, A. Sample Size Determination: A Practical Guide for Health Researchers. J. Gen. Fam. Med. 2023, 24, 72–78. [Google Scholar] [CrossRef]
- Trevino, E.G.; Patwardhan, A.N.; Henry, M.A.; Perry, G.; Dybdal-Hargreaves, N.; Hargreaves, K.M.; Diogenes, A. Effect of Irrigants on the Survival of Human Stem Cells of the Apical Papilla in a Platelet-Rich Plasma Scaffold in Human Root Tips. J. Endod. 2011, 37, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Dacostaribeiro, A.; Nogueira, G.; Antoniazzi, J.; Moritz, A.; Zezell, D. Effects of Diode Laser (810 Nm) Irradiation on Root Canal Walls: Thermographic and Morphological Studies. J. Endod. 2007, 33, 252–255. [Google Scholar] [CrossRef]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical Cone-Beam Algorithm. J. Opt. Soc. Am. A 1984, 1, 612. [Google Scholar] [CrossRef]
- Micic, M.; Antonijevic, D.; Milutinovic-Smiljanic, S.; Trisic, D.; Colovic, B.; Kosanovic, D.; Prokic, B.; Vasic, J.; Zivkovic, S.; Milasin, J.; et al. Developing a Novel Resorptive Hydroxyapatite-Based Bone Substitute for over-Critical Size Defect Reconstruction: Physicochemical and Biological Characterization and Proof of Concept in Segmental Rabbit’s Ulna Reconstruction. Biomed. Eng. Biomed. Tech. 2020, 65, 491–505. [Google Scholar] [CrossRef]
- Hilkens, P.; Gervois, P.; Fanton, Y.; Vanormelingen, J.; Martens, W.; Struys, T.; Politis, C.; Lambrichts, I.; Bronckaers, A. Effect of Isolation Methodology on Stem Cell Properties and Multilineage Differentiation Potential of Human Dental Pulp Stem Cells. Cell Tissue Res. 2013, 353, 65–78. [Google Scholar] [CrossRef]
- Basso, F.G.; Turrioni, A.P.S.; Almeida, L.F.; Soares, D.G.; Oliveira, C.F.; Hebling, J.; De Souza Costa, C.A. Nutritional Deprivation and LPS Exposure as Feasible Methods for Induction of Cellular—A Methodology to Validate for Vitro Photobiomodulation Studies. J. Photochem. Photobiol. B 2016, 159, 205–210. [Google Scholar] [CrossRef]
- Castiglioni, S. Short- and Long-Term Effects of Silver Nanoparticles on Human Microvascular Endothelial Cells. World J. Biol. Chem. 2014, 5, 457. [Google Scholar] [CrossRef]
- Dickers, B.; Lamard, L.; Peremans, A.; Geerts, S.; Lamy, M.; Limme, M.; Rompen, E.; De Moor, R.J.G.; Mahler, P.; Rocca, J.P.; et al. Temperature Rise during Photo-Activated Disinfection of Root Canals. Lasers Med. Sci. 2009, 24, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Lipski, M. In Vitro Infrared Thermographic Assessment of Root Surface Temperatures Generated by High-Temperature Thermoplasticized Injectable Gutta-Percha Obturation Technique. J. Endod. 2006, 32, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca Alvarez, A.; Moura-Netto, C.; Daliberto Frugoli, A.; Fernando, C.; Correa Aranha, A.C.; Davidowicz, H. Temperature Changes on the Root Surfaces of Mandibular Incisors after an 810-Nm High-Intensity Intracanal Diode Laser Irradiation. J. Biomed. Opt. 2012, 17, 015006. [Google Scholar] [CrossRef] [PubMed]
- Stefanidou, M.; Kostenkova, A.; Siudikienė, J.; Lodienė, G. Evaluation of Outcomes in Immature Teeth After Revitalization or Apexification Procedures: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e60357. [Google Scholar] [CrossRef]
- Seraj, B.; Moosavi Garmaroodi, Z.; Chiniforush, N.; Ghadimi, S. Thermal Changes in Root Surface of Primary Teeth During Root Canal Treatment With Diode Lasers: An In Vitro Study. J. Lasers Med. Sci. 2018, 9, 237–242. [Google Scholar] [CrossRef]
- Kimura, Y.; Yonaga, K.; Yokoyama, K.; Kinoshita, J.; Ogata, Y.; Matsumoto, K. Root Surface Temperature Increase during Er:YAG Laser Irradiation of Root Canals. J. Endod. 2002, 28, 76–78. [Google Scholar] [CrossRef]
- Shehab, N.; Al-Sabawi, N.; Alkhalidi, E. Influence of an 810-Nm Diode Laser on the Temperature Changes of the External Root Surface: An in Vitro Study. J. Int. Soc. Prev. Community Dent. 2020, 10, 445. [Google Scholar] [CrossRef]
- Steiner, R. Laser-Tissue Interactions. In Laser and IPL Technology in Dermatology and Aesthetic Medicine; Raulin, C., Karsai, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 23–36. ISBN 978-3-642-03437-4. [Google Scholar]
- Hmud, R.; Kahler, W.A.; Walsh, L.J. Temperature Changes Accompanying Near Infrared Diode Laser Endodontic Treatment of Wet Canals. J. Endod. 2010, 36, 908–911. [Google Scholar] [CrossRef]
- Otsuki, M.; Kijima, M.; Tagami, J. Transmission of Diode Laser through Dentin. J. Jpn. Soc. Laser Dent. 2010, 21, 18–21. [Google Scholar] [CrossRef]
- Behniafar, B.; Noori, F.; Chiniforoush, N.; Raee, A. The Effect of Lasers in Occlusion of Dentinal Tubules and Reducing Dentinal Hypersensitivity, a Scoping Review. BMC Oral Health 2024, 24, 1407. [Google Scholar] [CrossRef]
- Maenosono, R.M.; Bim Júnior, O.; Duarte, M.A.H.; Palma-Dibb, R.G.; Wang, L.; Ishikiriama, S.K. Diode Laser Irradiation Increases Microtensile Bond Strength of Dentin. Braz. Oral Res. 2014, 29, 01–05. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.I.A.; Sousa-Neto, M.D.; Souza-Gabriel, A.E.; Alfredo, E.; Romeo, U.; Silva-Sousa, Y.T.C. Effects of 980-Nm Diode Laser on the Ultrastructure and Fracture Resistance of Dentine. Lasers Med. Sci. 2013, 28, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Borzabadi-Farahani, A. Effect of Low-Level Laser Irradiation on Proliferation of Human Dental Mesenchymal Stem Cells; a Systemic Review. J. Photochem. Photobiol. B 2016, 162, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Migliario, M.; Pittarella, P.; Fanuli, M.; Rizzi, M.; Renò, F. Laser-Induced Osteoblast Proliferation Is Mediated by ROS Production. Lasers Med. Sci. 2014, 29, 1463–1467. [Google Scholar] [CrossRef]
- Gholami, L.; Hendi, S.S.; Saidijam, M.; Mahmoudi, R.; Tarzemany, R.; Arkian, A.; Afshar, S.; Fekrazad, R. Near-Infrared 940-Nm Diode Laser Photobiomodulation of Inflamed Periodontal Ligament Stem Cells. Lasers Med. Sci. 2022, 37, 449–459. [Google Scholar] [CrossRef]
- Bakhsh, T.A.; Alfaifi, A.; Alghamdi, Y.; Nassar, M.; Abuljadyel, R.A. Thermal Sensing of Photo-Activated Dental Resin Composites Using Infrared Thermography. Polymers 2023, 15, 4117. [Google Scholar] [CrossRef]

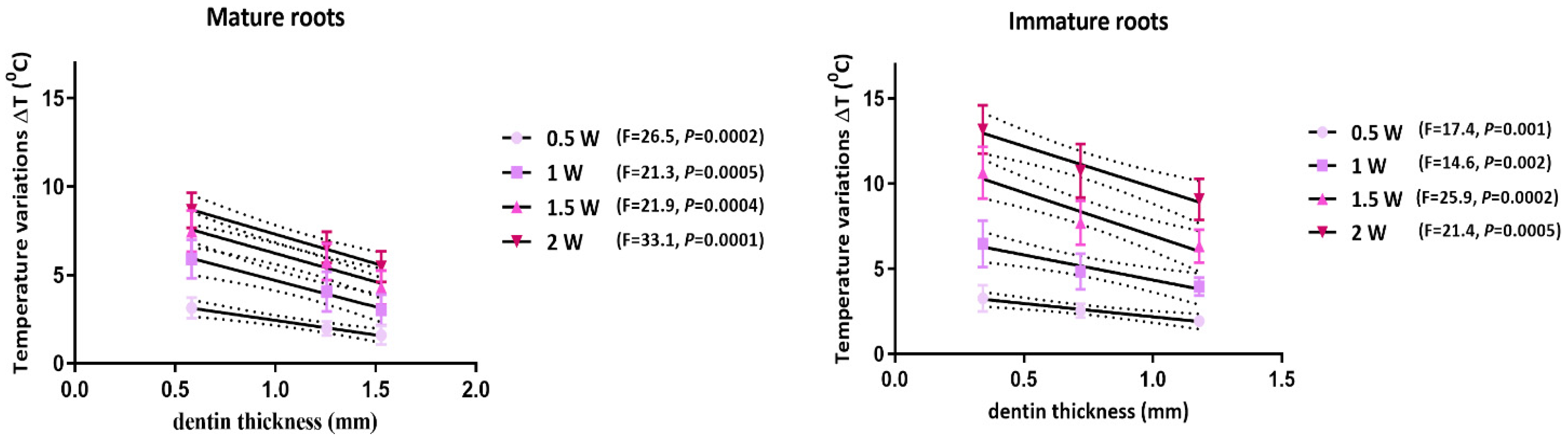


| Mature Roots (mm) | Immature Roots (mm) | |||||
|---|---|---|---|---|---|---|
| root third | cervical | middle | apical | cervical | middle | apical |
| Mean ± SD | 1.53 ± 0.17 | 1.25 ± 0.11 | 0.58 ± 0.08 | 1.18 ± 0.22 | 0.72 ± 0.11 | 0.34 ± 0.05 |
| Output Power (W) | Root Third | Mature Roots (°C) | Immature Roots (°C) |
|---|---|---|---|
| 0.5 | cervical | 1.67 ± 0.56 | 1.94 ± 0.17 |
| middle | 1.9 ± 0.44 | 2.54 ± 0.42 | |
| apical | 2.69 ± 1.44 | 2.86 ± 0.42 | |
| 1 | cervical | 2.82 ± 0.46 | 3.96 ± 0.52 |
| middle | 3.36 ± 0.66 | 4.84 ± 1.04 | |
| apical | 3.6 ± 1.44 | 5.06 ± 1.09 | |
| 1.5 | cervical | 4.49 ± 1.58 | 6.72 ± 1.19 |
| middle | 5.94 ± 1.55 | 8.70 ± 2.27 | |
| apical | 7.21 ± 1.64 | 10.43 ± 1.60 | |
| 2 | cervical | 5.48 ± 0.86 | 9.48 ± 0.99 |
| middle | 7.52 ± 1.76 | 10.96 ± 1.41 | |
| apical | 8.11 ± 1.67 | 12.08 ± 0.7 |
| Output Power (W) | Root Third | Mature Roots (°C) | Immature Roots (°C) |
|---|---|---|---|
| 0.5 | cervical | 1.97 | 3.24 |
| middle | 3.2 | 3.94 | |
| apical | 3.99 | 4.16 | |
| 1 | cervical | 5.42 | 6.56 |
| middle | 5.95 | 7.44 | |
| apical | 6.2 | 7.66 | |
| 1.5 | cervical | 8.39 | 10.62 |
| middle | 9.84 | 12.6 | |
| apical | 11.11 | 14.3 | |
| 2 | cervical | 10.68 | 14.68 |
| middle | 12.72 | 16.6 | |
| apical | 13.31 | 17.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitic, D.D.; Milosevic Markovic, M.S.; Jovanovic, I.D.; Mancic, D.D.; Orhan, K.; Jokanovic, V.R.; Markovic, D.L. A Biomimetic Approach to Diode Laser Use in Endodontic Treatment of Immature Teeth: Thermal, Structural, and Biological Analysis. Biomimetics 2025, 10, 216. https://doi.org/10.3390/biomimetics10040216
Mitic DD, Milosevic Markovic MS, Jovanovic ID, Mancic DD, Orhan K, Jokanovic VR, Markovic DL. A Biomimetic Approach to Diode Laser Use in Endodontic Treatment of Immature Teeth: Thermal, Structural, and Biological Analysis. Biomimetics. 2025; 10(4):216. https://doi.org/10.3390/biomimetics10040216
Chicago/Turabian StyleMitic, Dijana D., Maja S. Milosevic Markovic, Igor D. Jovanovic, Dragan D. Mancic, Kaan Orhan, Vukoman R. Jokanovic, and Dejan Lj. Markovic. 2025. "A Biomimetic Approach to Diode Laser Use in Endodontic Treatment of Immature Teeth: Thermal, Structural, and Biological Analysis" Biomimetics 10, no. 4: 216. https://doi.org/10.3390/biomimetics10040216
APA StyleMitic, D. D., Milosevic Markovic, M. S., Jovanovic, I. D., Mancic, D. D., Orhan, K., Jokanovic, V. R., & Markovic, D. L. (2025). A Biomimetic Approach to Diode Laser Use in Endodontic Treatment of Immature Teeth: Thermal, Structural, and Biological Analysis. Biomimetics, 10(4), 216. https://doi.org/10.3390/biomimetics10040216