Ultrastructural Changes in Final Instar Larvae of Papilio polytes (Lepidoptera: Papilionidae) Lead to Differences in Epidermal Spreading of Water and Adjuvants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Insects
2.1.2. Adjuvants
2.1.3. Sampling
2.1.4. Treatments
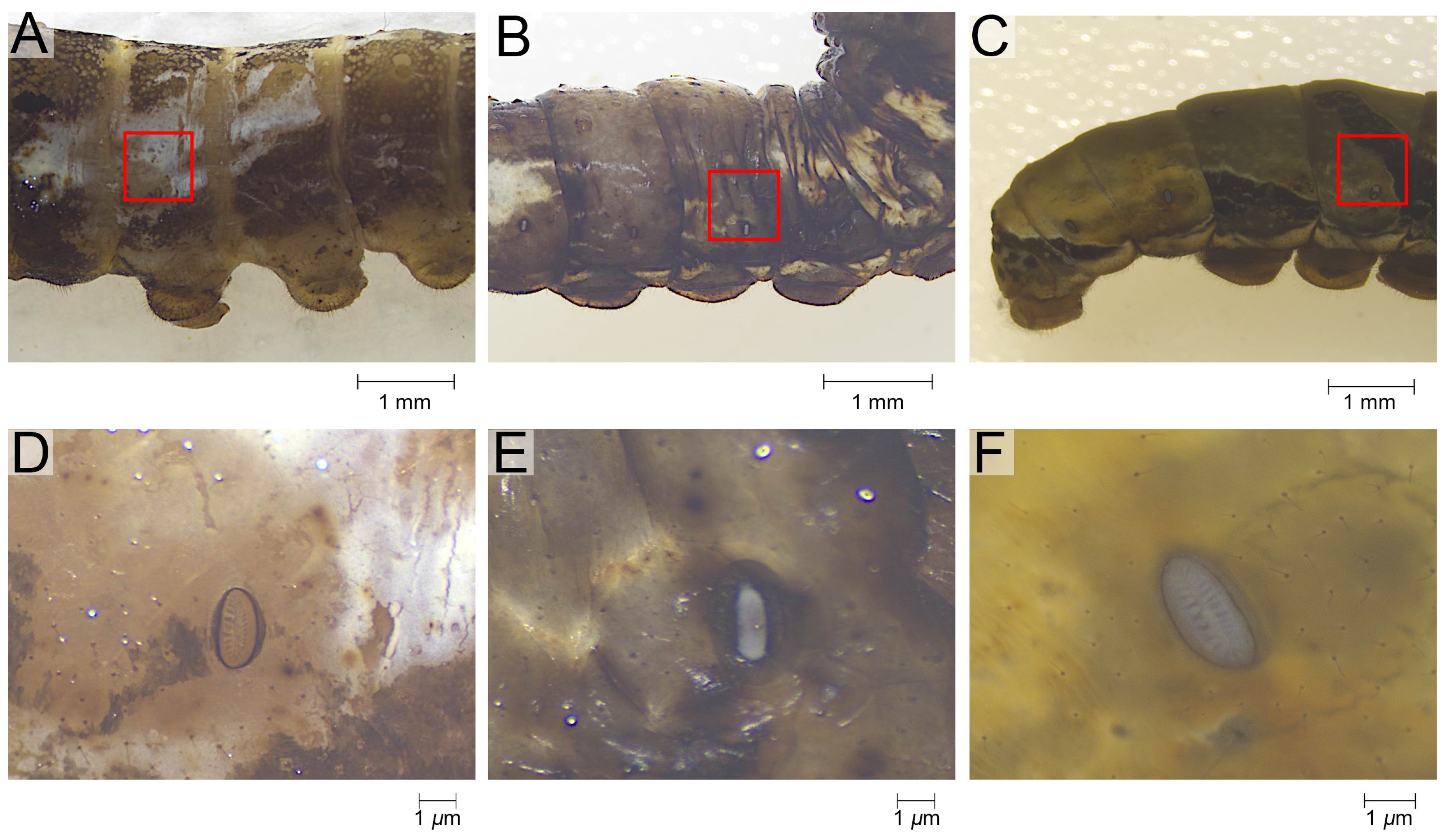
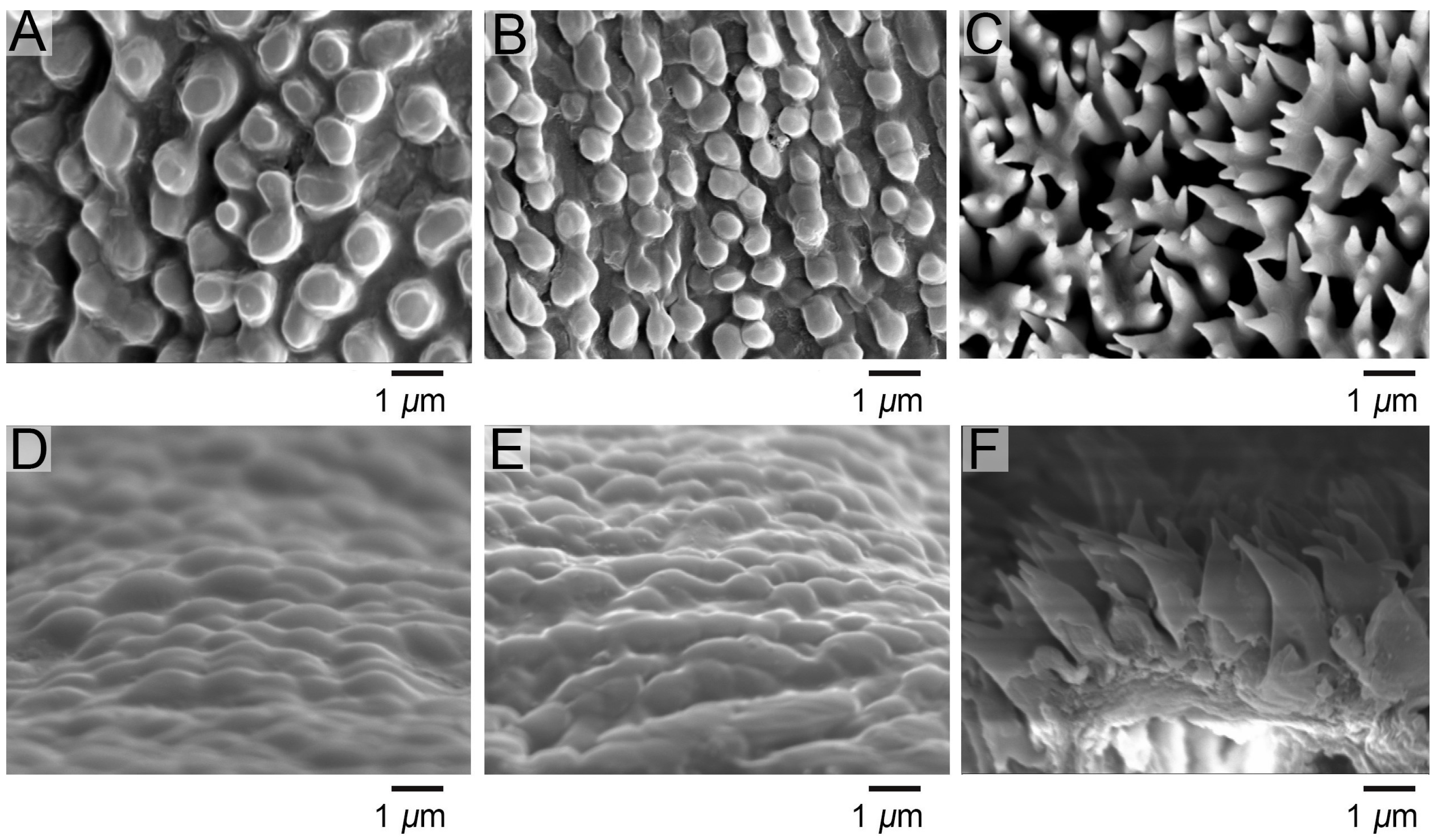
2.2. Microscopy
2.3. Morphometric Measurements
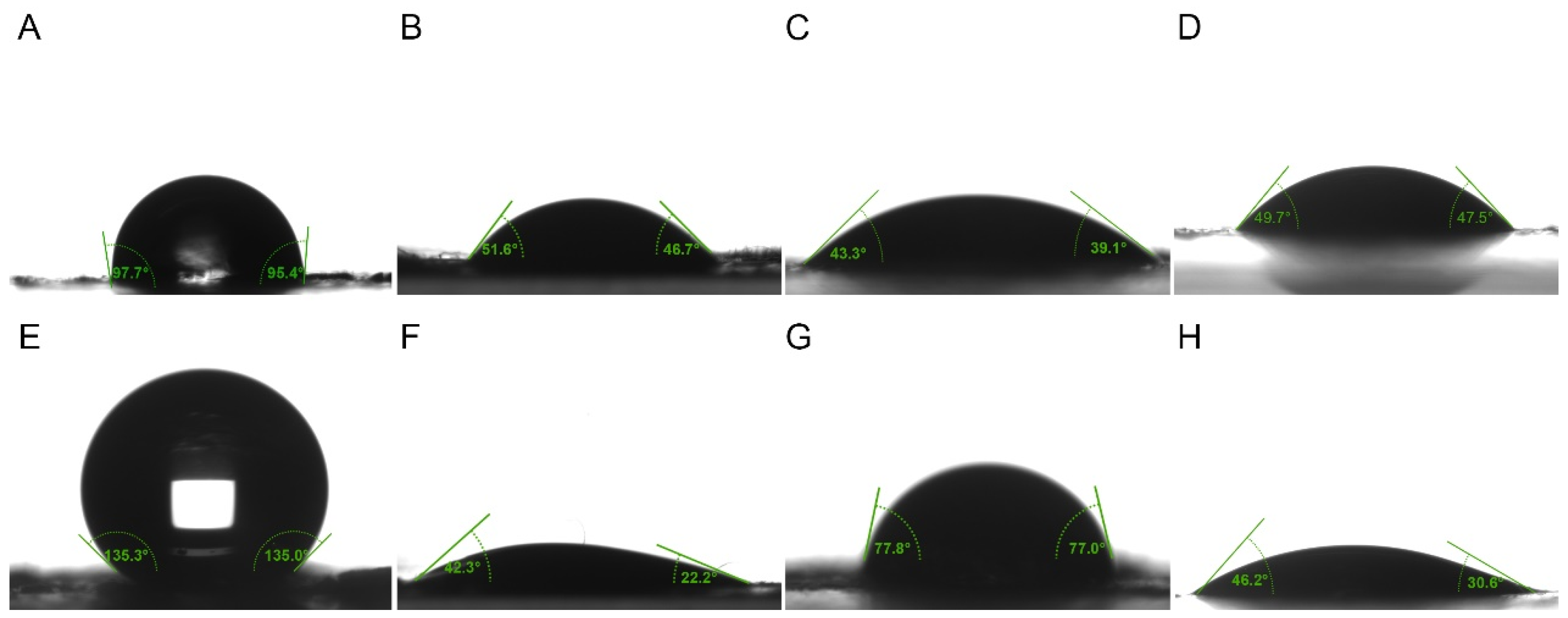
2.4. Wetting Property Measurements
2.5. Transmission Spectra Measurements
2.6. Data Analysis
2.6.1. Significance of Difference
2.6.2. Chemical Functional Groups Comparison
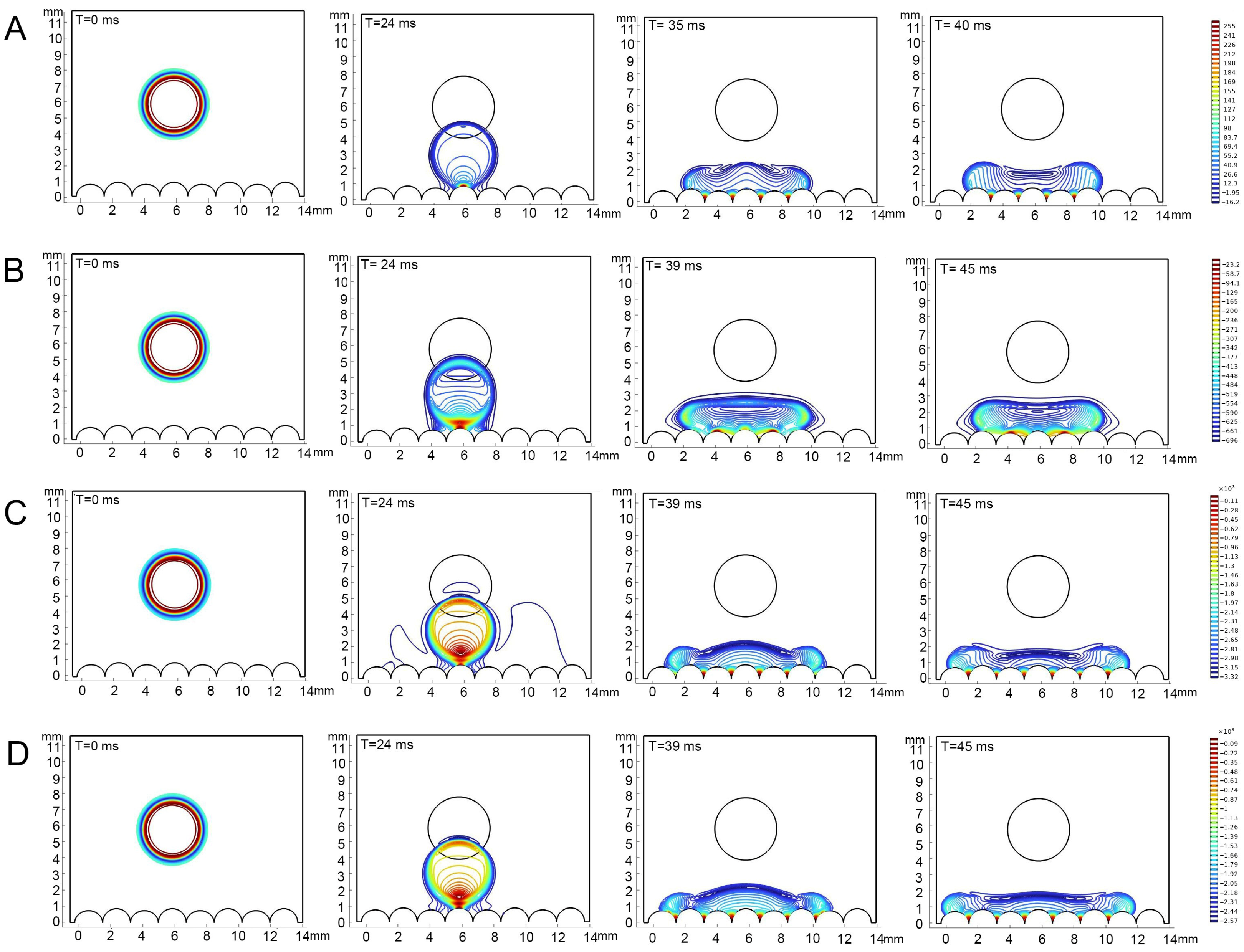
2.6.3. Simulation of Contact Angles
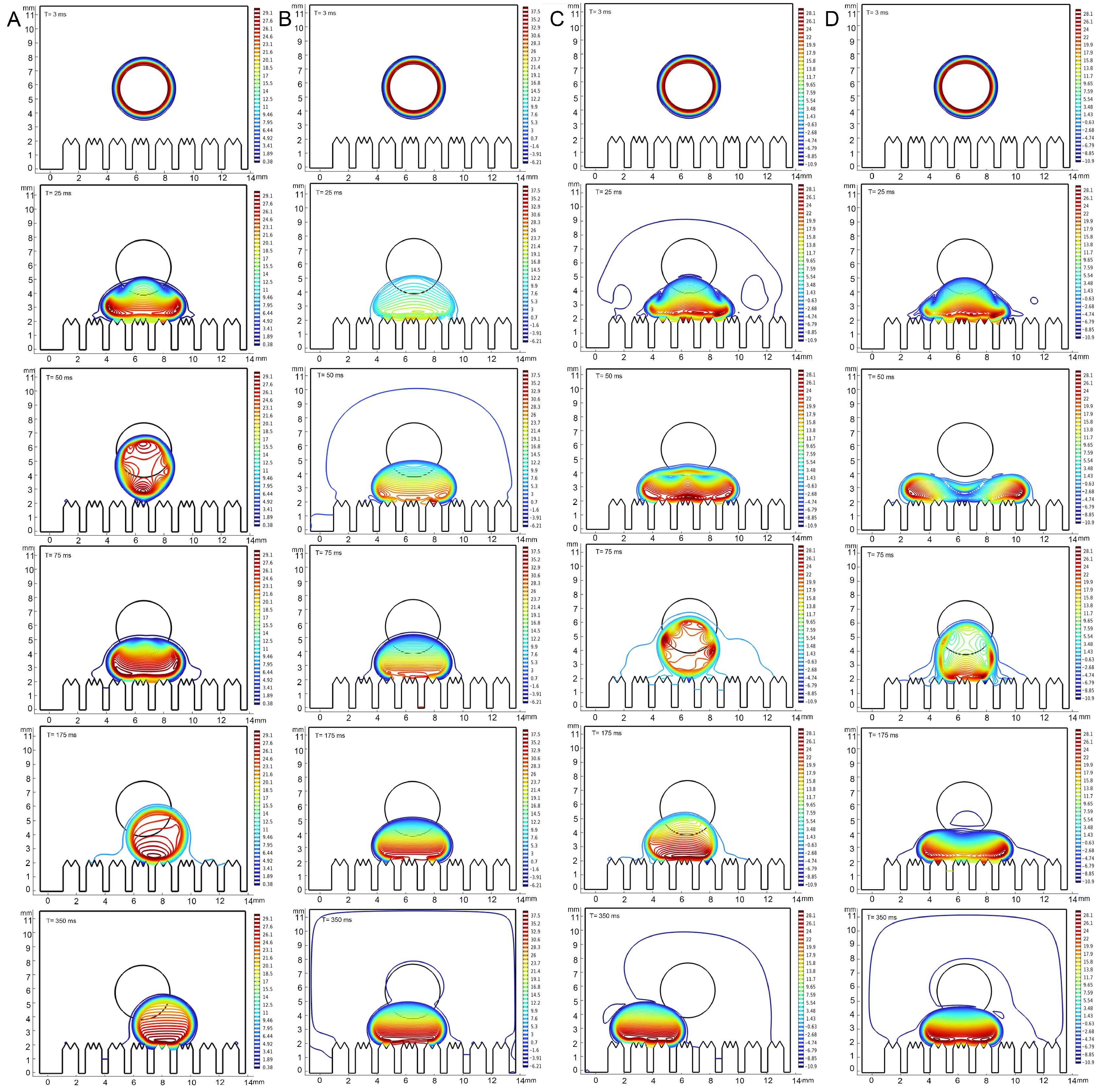
3. Results
3.1. Comparative Morphology of Epidermal Ultrastructure
3.2. Comparison of Wetting Properties of Epidermal Surface
3.3. Comparison of Epidermal Chemical Functional Groups
3.4. Correlation Between Epidermal Ultrastructure and Wetting Properties
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAH | Contact Angle Hysteresis |
| CHCs | Cuticle hydrocarbons |
| SEM | Scanning Electron Microscope |
| 20E | Ecdysone |
| JH | Juvenile hormone |
| CHS | Chalcone synthase gene |
| CPR | Cytochrome P450 reductase gene |
Appendix A
| Instar | Treatments | Base width (μm) | Height (μm) | Chamfer Angle (°) | Spacing (μm) |
|---|---|---|---|---|---|
| 3rd | Hexyl hydride | 0.73 ± 0.04 | 0.66 ± 0.03 | 38.65 ± 0.50 | 1.25 ± 0.18 |
| 1.0% methylated seed oil | 0.84 ± 0.05 | 0.62 ± 0.09 | 41.06 ± 9.73 | 1.16 ± 0.23 | |
| 0.9 g/L benzalkonium chloride | 0.74 ± 0.09 | 0.66 ± 0.14 | 37.17 ± 0.43 | 1.35 ± 0.13 | |
| 0.03% Silwet 618 | 0.67 ± 0.09 | 0.58 ± 0.04 | 36.98 ± 9.52 | 1.13 ± 0.11 | |
| 4th | Hexyl hydride | 0.91 ± 0.03 | 0.70 ± 0.01 | 39.97 ± 0.57 | 1.29 ± 0.03 |
| 1.0% methylated seed oil | 0.92 ± 0.10 | 0.69 ± 0.24 | 39.61 ± 11.19 | 1.14 ± 0.08 | |
| 0.9 g/L benzalkonium chloride | 0.90 ± 0.34 | 0.71 ± 0.16 | 39.28 ± 4.69 | 1.38 ± 0.06 | |
| 0.03% Silwet 618 | 0.92 ± 0.10 | 0.61 ± 0.08 | 40.53 ± 2.19 | 1.16 ± 0.14 |
| Treatments | Base Width (μm) | Tip Width (μm) | Base Height (μm) | Lobe Height (μm) | Angle Between Lobes (3-Lobed) | Angle Between Lobes (2-Lobed) |
|---|---|---|---|---|---|---|
| Hexyl hydride | 1.16 ± 0.01 | 1.19 ± 0.02 | 1.80 ± 0.08 | 0.50 ± 0.02 | 49.51 ± 0.52 | 60.17 ± 0.31 |
| 1.0% methylated seed oil | 1.17 ± 0.26 | 1.15 ± 0.42 | 1.69 ± 0.18 | 0.49 ± 0.01 | 48.35 ± 0.69 | 59.90 ± 1.84 |
| 0.9 g/L benzalkonium chloride | 1.16 ± 0.36 | 1.22 ± 0.02 | 1.71 ± 0.26 | 0.47 ± 0.25 | 49.07 ± 1.91 | 60.49 ± 0.28 |
| 0.03% Silwet 618 | 1.14 ± 0.16 | 1.18 ± 0.37 | 1.90 ± 0.37 | 0.55 ± 0.28 | 49.57 ± 1.20 | 60.62 ± 3.08 |
References
- Bonebrake, T.C.; Ponisio, L.C.; Boggs, C.L.; Ehrlich, P.R. More than just indicators: A review of tropical butterfly ecology and conservation. Biol. Conserv. 2010, 143, 1831–1841. [Google Scholar] [CrossRef]
- James, D.G.; Seymour, L.; Lauby, G.; Buckley, K. Beauty with benefits: Butterfly conservation in Washington State, USA, wine grape vineyards. J. Insect Conserv. 2015, 19, 341–348. [Google Scholar] [CrossRef]
- Kitamura, T.; Imafuku, M. Behavioural mimicry in flight path of Batesian intraspecific polymorphic butterfly. P. Roy. Soc. B-Biol. Sci. 2015, 282, 1809. [Google Scholar]
- Suwarno; Salmah, M.R.C.; Ali, A.; Abu Hassan, A. Oviposition preference of swallowtail butterfly, (Lepidoptera: Papilionidae) on four Rutaceae (Sapindales) host plant species. Insect Sci. 2010, 17, 369–378. [Google Scholar] [CrossRef]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef]
- Watson, G.S.; Watson, J.A.; Cribb, B.W. Diversity of Cuticular Micro- and Nanostructures on Insects: Properties, Functions, and Potential Applications. Annu. Rev. Entomol. 2017, 62, 185–205. [Google Scholar] [CrossRef]
- Shannon, B.; Jeon, H.; Johnson, R.M. Review: The risks of spray adjuvants to honey bees. J. Insect Sci. 2023, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fine, J.D.; Mullin, C.A. Are organosilicon surfactants safe for bees or humans? Sci. Total Environ. 2018, 612, 415–421. [Google Scholar] [CrossRef]
- Hu, X.Z.; Gong, H.N.; Hollowell, P.; Liao, M.R.; Li, Z.Y.; Ruane, S.; Liu, H.Y.; Pambou, E.; Mahmoudi, N.; Dalgliesh, R.M.; et al. What happens when pesticides are solubilised in binary ionic/zwitterionic-nonionic mixed micelles? J. Colloid. Interf. Sci. 2021, 586, 190–199. [Google Scholar] [CrossRef]
- Hamilton, R.J. Structure and General-Properties of Mineral and Vegetable-Oils Used as Spray Adjuvants. Pestic. Sci. 1993, 37, 141–146. [Google Scholar] [CrossRef]
- Wolfgang, W.J.; Riddiford, L.M. Cuticular Morphogenesis during Continuous Growth of the Final Instar Larva of a Moth. Tissue Cell 1981, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Hanna, L.; Lamouret, T.; Poças, G.M.; Mirth, C.K.; Moczek, A.P.; Nijhout, F.H.; Abouheif, E. Evaluating old truths: Final adult size in holometabolous insects is set by the end of larval development. J. Exp. Zool. Part. B 2023, 340, 270–276. [Google Scholar] [CrossRef]
- Lin, H.T.; Slate, D.J.; Paetsch, C.R.; Dorfmann, A.L.; Trimmer, B.A. Scaling of caterpillar body properties and its biomechanical implications for the use of a hydrostatic skeleton. J. Exp. Biol. 2011, 214, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Niehuis, O.; Yen, S.H.; Naumann, C.M.; Misof, B. Higher phylogeny of zygaenid moths (Insecta: Lepidoptera) inferred from nuclear and mitochondrial sequence data and the evolution of larval cuticular cavities for chemical defence. Mol. Phylogenetics Evol. 2006, 39, 812–829. [Google Scholar] [CrossRef]
- Futahashi, R.; Banno, Y.; Fujiwara, H. Caterpillar color patterns are determined by a two-phase melanin gene prepatterning process: New evidence from and. Evol. Dev. 2010, 12, 157–167. [Google Scholar] [CrossRef]
- Andersen, S.O.; Peter, M.G.; Roepstorff, P. Cuticular sclerotization in insects. Comp. Biochem. Phys. B 1996, 113, 689–705. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, G.; Bi, Y.H.; Zhi, H. Multiple-dimensional micro/nano structural models for hydrophobicity of butterfly wing surfaces and coupling mechanism. Sci. Bull. 2015, 60, 256–263. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.C.; Nestler, B. Wetting Effect on Patterned Substrates. Adv. Mater. 2023, 35, 25. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Blackman, B.R.K.; Eduardo, S. Mimicking nature to control bio-material surface wetting and adhesion. Int. Mater. Rev. 2022, 67, 658–681. [Google Scholar] [CrossRef]
- Wang, F.; Nestler, B. Wetting and Contact-Angle Hysteresis: Density Asymmetry and van der Waals Force. Phys. Rev. Lett. 2024, 132, 126202. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, H.D.; Wang, F.; Nestler, B. Exploration of contact angle hysteresis mechanisms: From microscopic to macroscopic. J. Chem. Phys. 2024, 161, 19. [Google Scholar] [CrossRef] [PubMed]
- Tretyakov, N.; Papadopoulos, P.; Vollmer, D.; Butt, H.J.; Dünweg, B.; Daoulas, K.C. The Cassie-Wenzel transition of fluids on nanostructured substrates: Macroscopic force balance versus microscopic density-functional theory. J. Chem. Phys. 2016, 145, 13. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, F.; Wang, F.; Nestler, B. Wetting phenomena of droplets and gas bubbles: Contact angle hysteresis based on varying liquid-solid and solid-gas interfacial tensions. J. Chem. Phys. 2024, 161, 164708. [Google Scholar] [CrossRef]
- Bormashenko, E. Wetting of real solid surfaces: New glance on well-known problems. Colloid. Polym. Sci. 2013, 291, 339–342. [Google Scholar] [CrossRef]
- Herminghaus, S.; Brinkmann, M.; Seemann, R. Wetting and dewetting of complex surface geometries. Annu. Rev. Mater. Res. 2008, 38, 101–121. [Google Scholar] [CrossRef]
- Thielemann, H.C.; Cardellini, A.; Fasano, M.; Bergamasco, L.; Alberghini, M.; Ciorra, G.; Chiavazzo, E.; Asinari, P. From GROMACS to LAMMPS: GRO2LAM: A converter for molecular dynamics software. J. Mol. Model. 2019, 25, 147. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Man, Z.Y.; Li, M.L.; Cha, J.; Dai, K. Numerical analysis of water injection in coal seams under mining influence. Phys. Fluids 2024, 36, 10. [Google Scholar] [CrossRef]
- Iyi, D.; Balogun, Y.; Oyeneyin, B.; Faisal, N. Numerical modelling of the effect of wettability, interfacial tension and temperature on oil recovery at pore-scale level. J. Petrol. Sci. Eng. 2021, 201, 108453. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Ghiradella, H. Insect Cuticular Surface Modifications: Scales and Other Structural Formations. Adv. Insect Physiol. 2010, 38, 135–180. [Google Scholar]
- Guillén, M.; Heraty, J.M. Instar differences in (Lepidoptera: Gracillariidae). Ann. Entomol. Soc. Am. 2004, 97, 1227–1232. [Google Scholar] [CrossRef]
- Zeng, M.Z.; Zhou, W.; Wen, S.S.; Wu, H.; Zhang, Q.; Fu, K.Y.; Guo, W.C.; Shi, J.F. Identification and Functional Insights of Knickkopf Genes in the Larval Cuticle of. Insects 2024, 15, 623. [Google Scholar] [CrossRef]
- Moussian, B. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem. Molec 2010, 40, 363–375. [Google Scholar] [CrossRef]
- Wolfgang, W.J.; Riddiford, L.M. Larval Cuticular Morphogenesis in the Tobacco Hornworm, Manduca-Sexta, and Its Hormonal-Regulation. Dev. Biol. 1986, 113, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.Y.; Wang, X.P.; Di, Y.Q.; Zhao, Y.M.; Wang, J.X.; Zhao, X.F. The transcription factor RUNT-like regulates pupal cuticle development via promoting a pupal cuticle protein transcription. PLoS Genet. 2024, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Molec 2010, 40, 166–178. [Google Scholar] [CrossRef]
- Ren, M.F.; Lu, J.J.; Li, D.Q.; Yang, J.; Zhang, Y.Y.; Dong, J.M.; Niu, Y.B.; Zhou, X.G.; Zhang, X.H. Identification and Functional Characterization of Two Chitin Synthases in the Black Cutworm, Agrotis ipsilon (Hufnagel) (Lepidoptera: Noctuidae). J. Econ. Entomol. 2023, 116, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Li, F.F.; Gao, H.L.; Zhang, Y.X.; Liu, Z.W. Characterization of cuticular proteins in CPR family in the wolf spider, and the response of one subfamily genes to environmental stresses. Insect Biochem. Molec 2022, 150, 103859. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Ginzel, M.D. Chemical Ecology, Biochemistry, and Molecular Biology of Insect Hydrocarbons. Annu. Rev. Entomol. 2021, 66, 45–60. [Google Scholar] [CrossRef]
- Ingleby, F.C. Insect Cuticular Hydrocarbons as Dynamic Traits in Sexual Communication. Insects 2015, 6, 732–742. [Google Scholar] [CrossRef]
- Wang, Z.N.; Andika, I.P.; Chung, H. Regulation of insect cuticular hydrocarbon biosynthesis. Curr. Opin. Insect Sci. 2025, 67, 101287. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, P.P.; Burkert, L.H.; Abou, B.; Federle, W.; Menzel, F. Coping with the climate: Cuticular hydrocarbon acclimation of ants under constant and fluctuating conditions. J. Exp. Biol. 2018, 221, 9. [Google Scholar] [CrossRef] [PubMed]

| Liquid Type | Liquid Name | 4th Instar | 5th Instar |
|---|---|---|---|
| Water | Water | 98.03 ± 3.37° Ba | 126.94 ± 6.47° Aa |
| Lipidic Adjuvants | 1.0% methylated seed oil | 48.44 ± 3.90° Acd | 32.99 ± 7.94° Bd |
| 0.1% octodecyl glucoside | 53.14 ± 5.77° Ac | 21.92 ± 3.59° Be | |
| 97% mineral oil | 65.40 ± 4.98° Ab | 44.65 ± 10.73° Ac | |
| Organosilicon Adjuvants | 0.03% Silwet 618 | 48.04 ± 5.77° Acd | 30.82 ± 7.68° Ac |
| 0.03% Silwet 408 | 44.55 ± 5.63° Ade | 15.50 ± 4.53° Be | |
| Cationic Adjuvants | 0.9 g/L benzalkonium chloride | 42.16 ± 6.29° Ae | 60.75 ± 12.40° Ab |
| 1.0 g/L tetradecyl benzyl dimethyl ammonium chloride | 33.06 ± 4.67° Bf | 44.65 ± 6.27° Ac |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Wu, X.; Zhang, T.; Tang, C. Ultrastructural Changes in Final Instar Larvae of Papilio polytes (Lepidoptera: Papilionidae) Lead to Differences in Epidermal Spreading of Water and Adjuvants. Biomimetics 2025, 10, 251. https://doi.org/10.3390/biomimetics10040251
Lu Z, Wu X, Zhang T, Tang C. Ultrastructural Changes in Final Instar Larvae of Papilio polytes (Lepidoptera: Papilionidae) Lead to Differences in Epidermal Spreading of Water and Adjuvants. Biomimetics. 2025; 10(4):251. https://doi.org/10.3390/biomimetics10040251
Chicago/Turabian StyleLu, Zhengyu, Xue Wu, Tingting Zhang, and Chufei Tang. 2025. "Ultrastructural Changes in Final Instar Larvae of Papilio polytes (Lepidoptera: Papilionidae) Lead to Differences in Epidermal Spreading of Water and Adjuvants" Biomimetics 10, no. 4: 251. https://doi.org/10.3390/biomimetics10040251
APA StyleLu, Z., Wu, X., Zhang, T., & Tang, C. (2025). Ultrastructural Changes in Final Instar Larvae of Papilio polytes (Lepidoptera: Papilionidae) Lead to Differences in Epidermal Spreading of Water and Adjuvants. Biomimetics, 10(4), 251. https://doi.org/10.3390/biomimetics10040251






