The Right Tool for the Job: A Review of Insect Mouthparts as a Tool Kit for Biomimetic Studies
Abstract
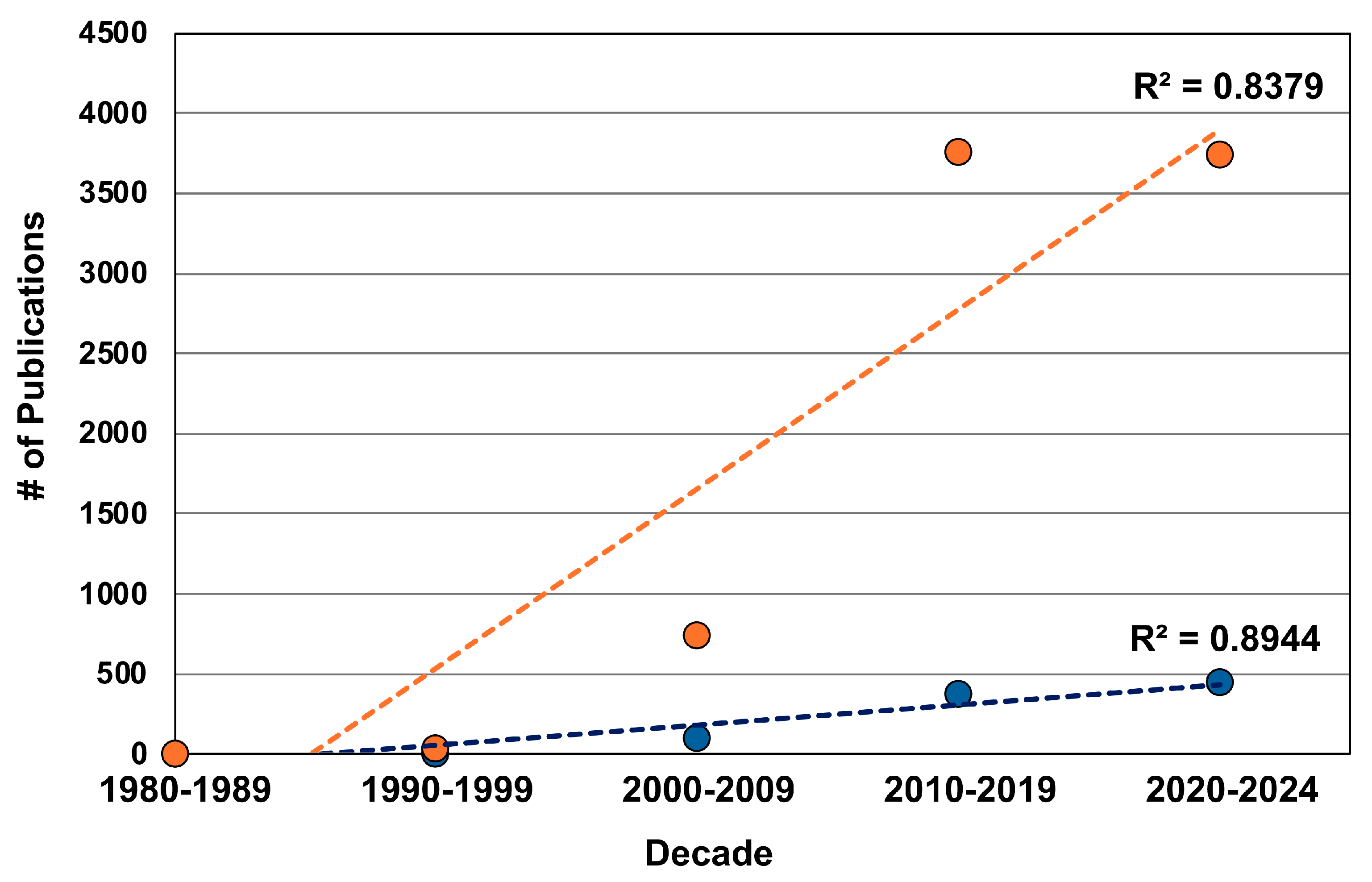
1. Introduction
2. Ancestral Mouthpart Condition and Structural Mouthpart Interactions
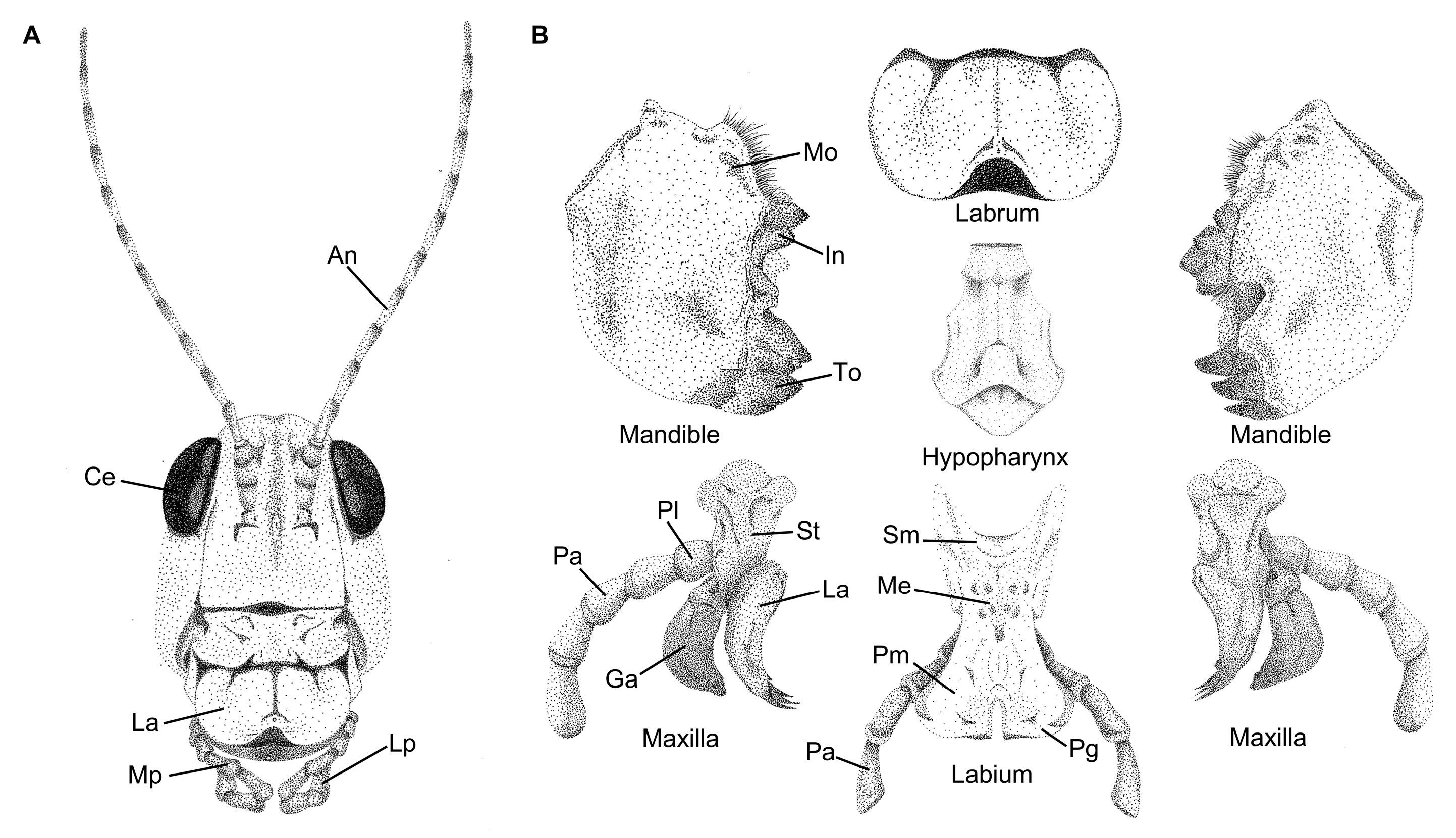
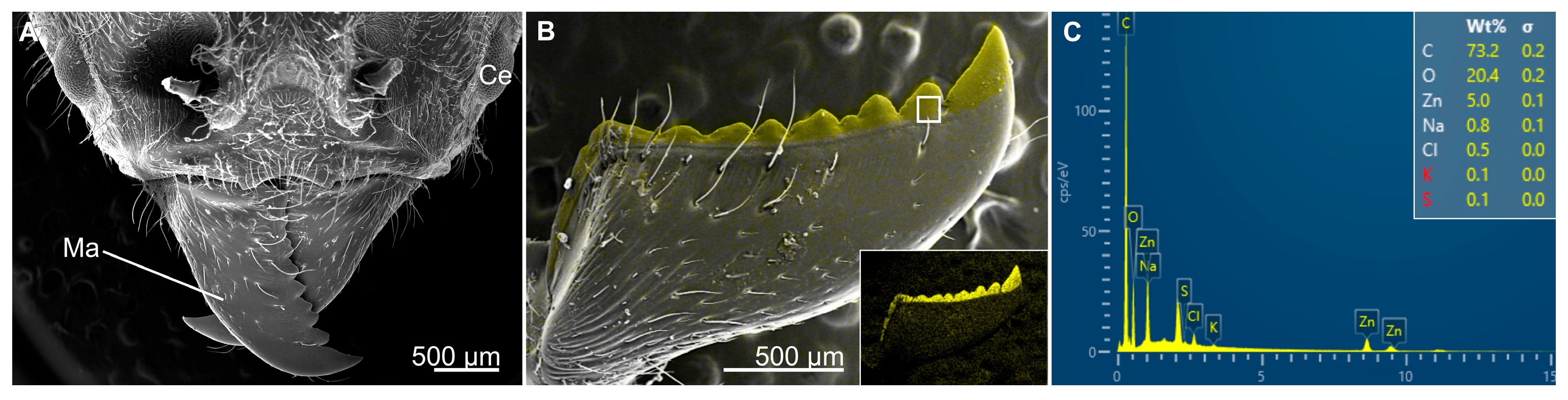
- Mandibles: Facilitates the biting, handling, and mastication of food. Mandibles might consist of a tooth region, an incisor region, and a molar region, depending on the feeding habit of the insect [19,52,53]. In addition, because the mandibles are dicondylic with two points of articulation, they tend to move along a medial-lateral axis.
- Maxillae: Provides several different functions, including assistance in handling food and gathering chemosensory information, and might aid in the mastication of food along with the mandibles [41]. The maxillae consist of several subcomponents, including a cardo and stipes that aid in articulation, and the lacinia and galea endites that extend from the maxillary palpus.
- Labium: Serves as the lower lip and provides a means to close the preoral cavity. Similar to the labrum, the labium assists in food handling and the determination of food suitability. The labium is a fused pair of appendages and consists of several subcomponents, including the gula, postmentum, and prementum. The labium also might have labial palpi that are similar in functionality to the maxillary palpi [34].
- Hypopharynx: Assists in moving food during the mastication process and functions similarly to a tongue, possessing a host of chemosensillae to determine food suitability [54].
3. Biomimicry of Insects with Mandibulate Mouthparts
3.1. Overview
3.2. Biomimicry with Biting-Chewing Mouthparts
3.2.1. Mandible-Inspired Cutting Tools
Cutters Inspired by Coleoptera
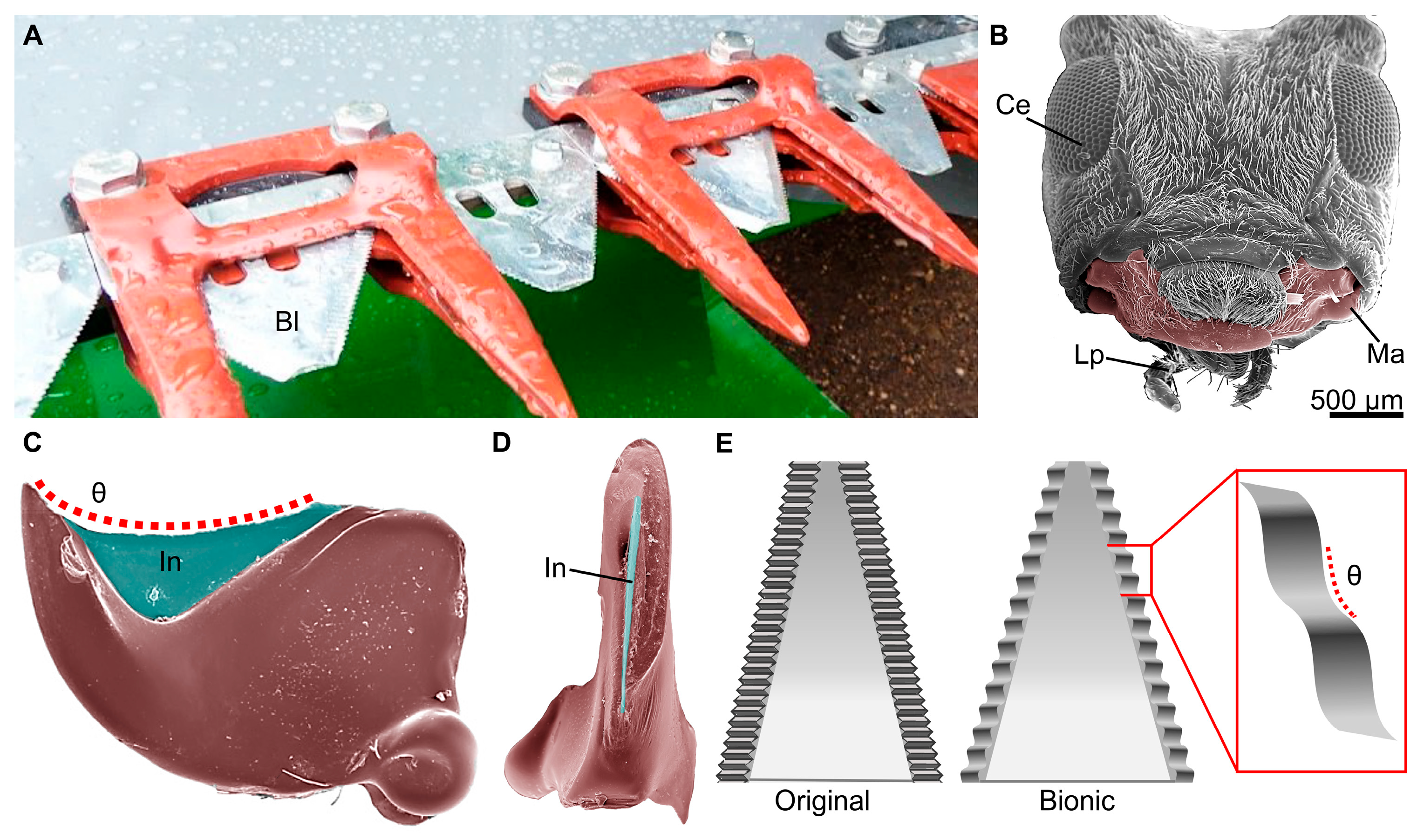
Cutters Inspired by Orthoptera
Cutters Inspired by Hymenoptera
3.2.2. Mandible-Inspired Clamps
3.2.3. Mandible-Inspired Robotics
3.2.4. Mandible-Inspired Materials
4. Biomimicry of Haustellate Mouthparts of Insects
4.1. Overview
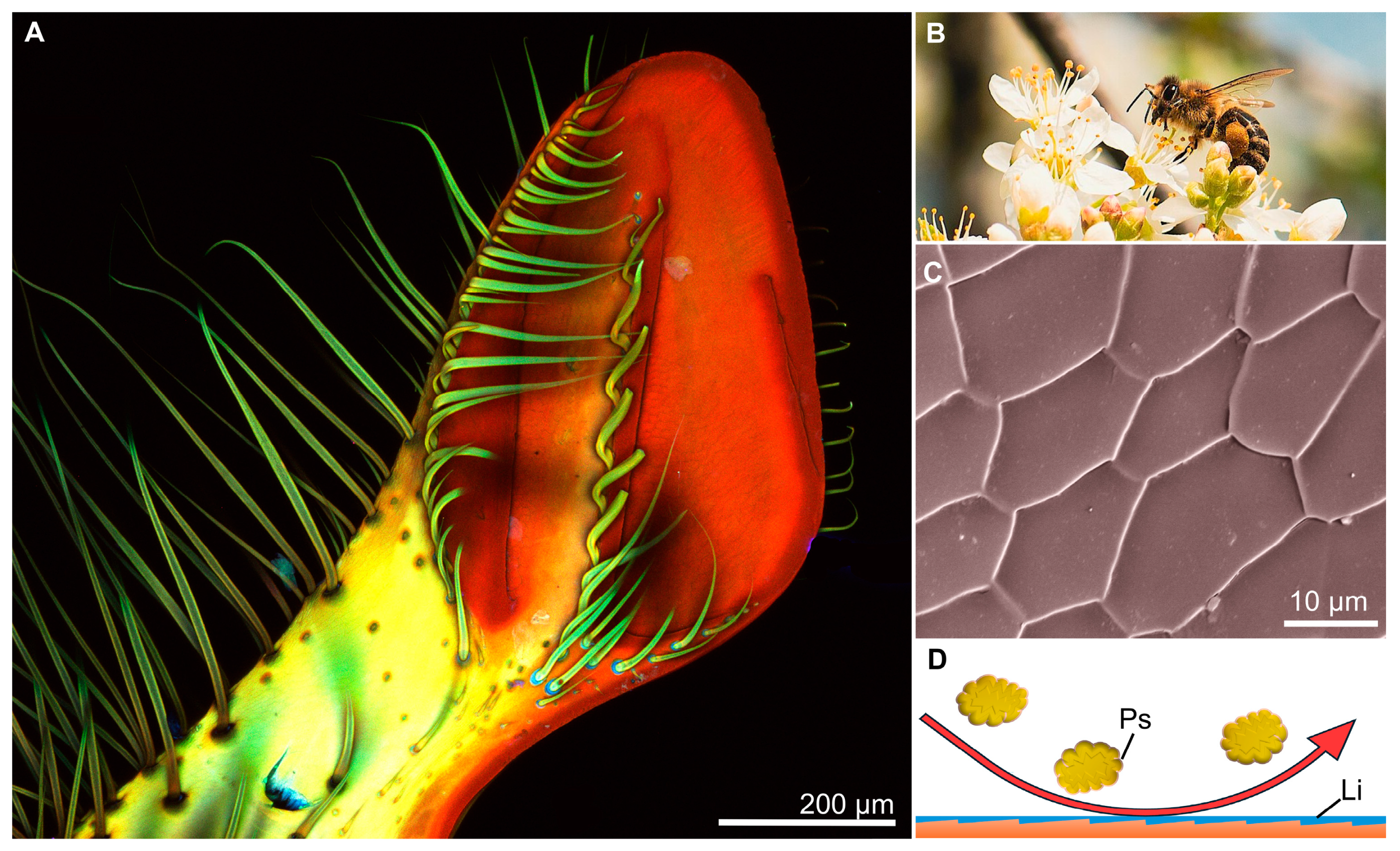
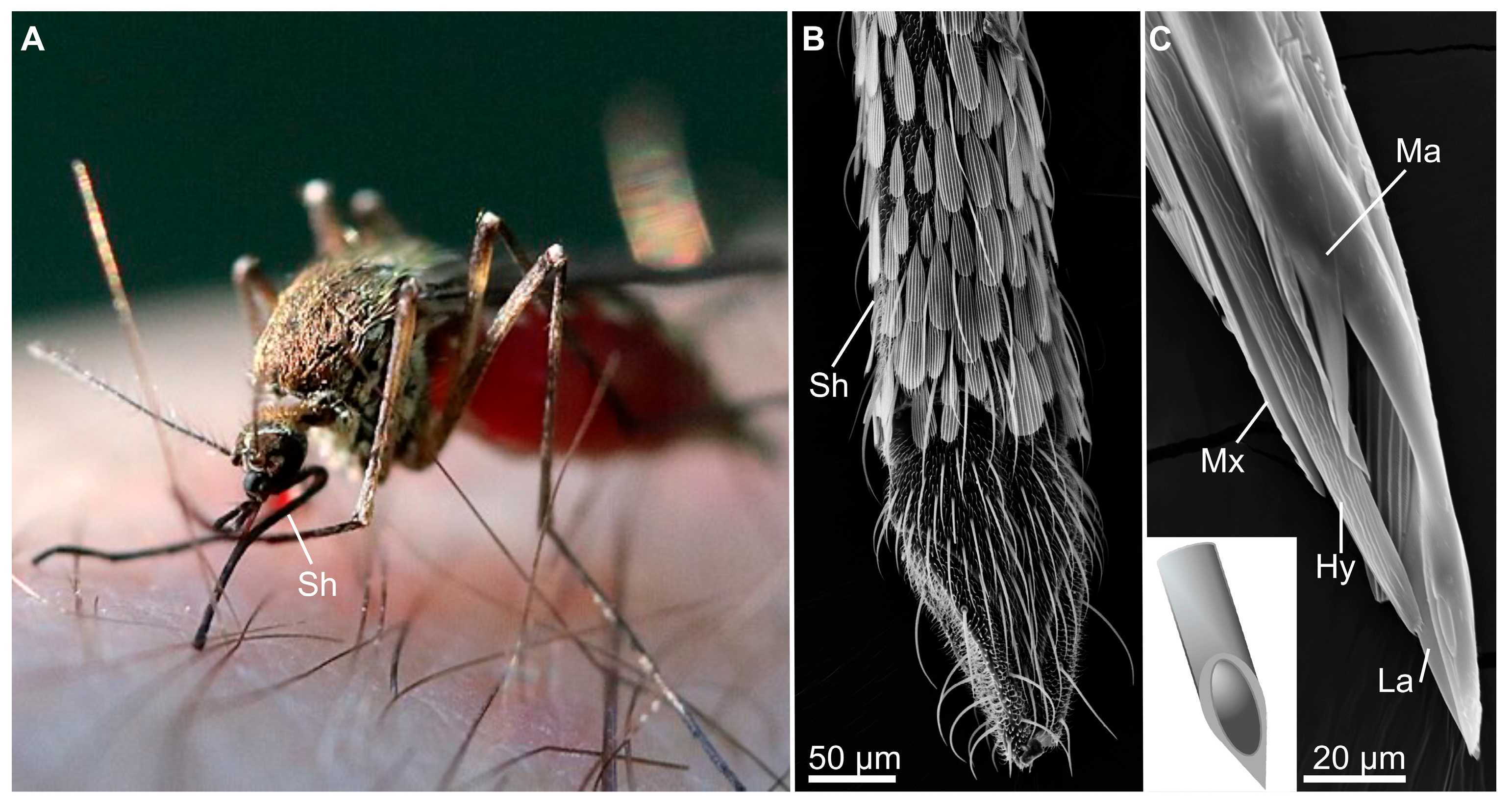
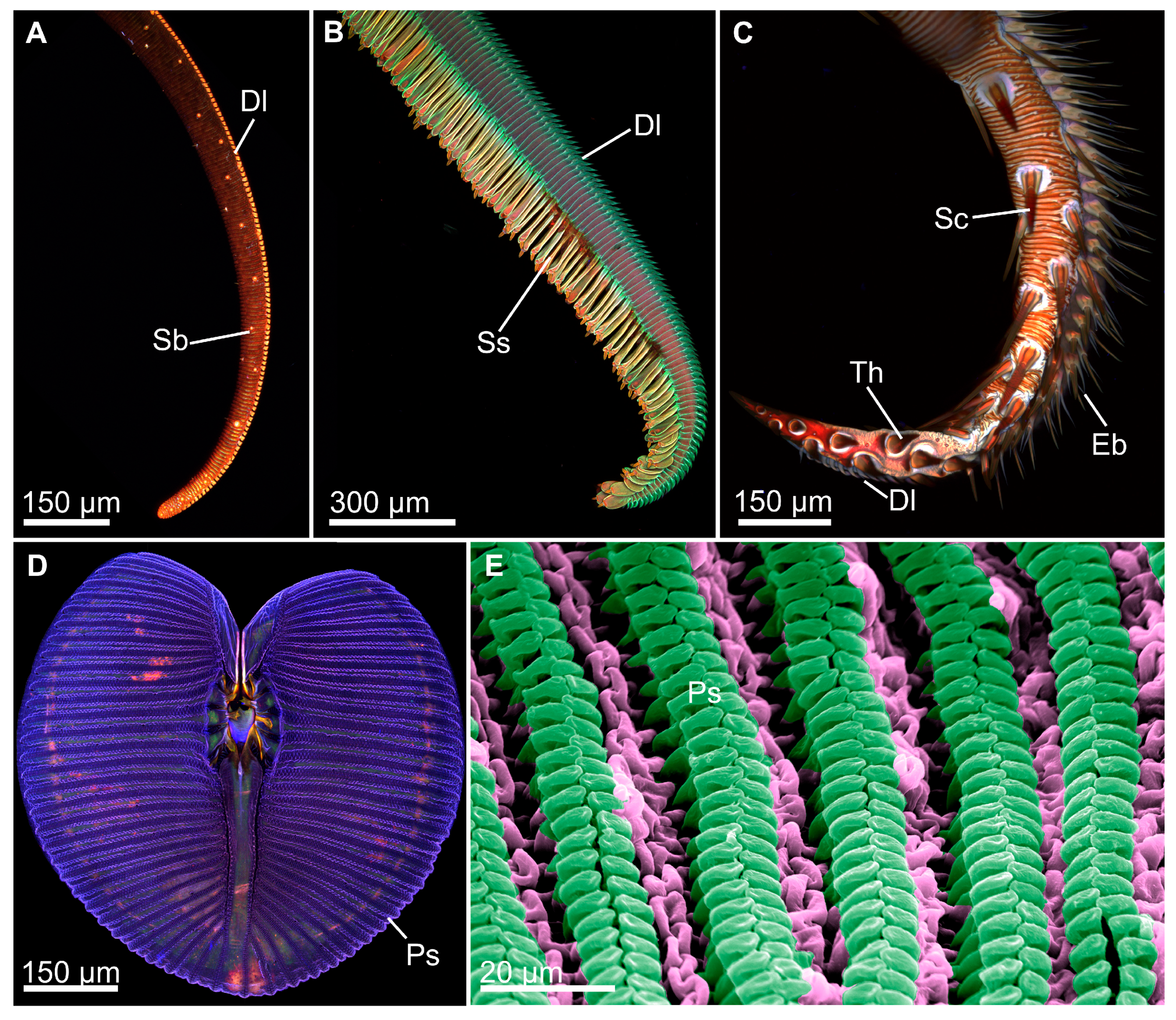
4.2. Biomimicry of Piercing-Sucking Mouthparts
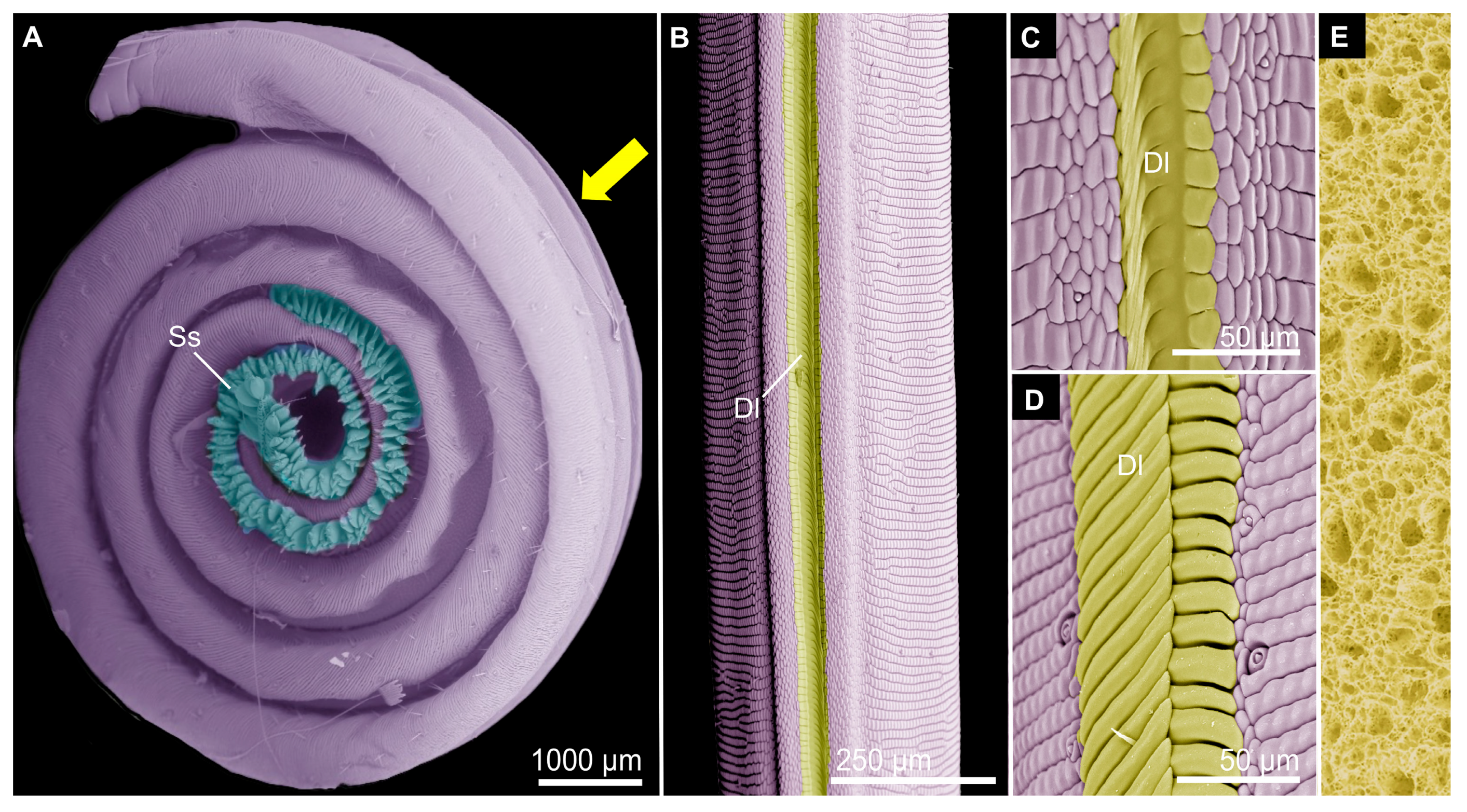
4.3. Biomimicry of Nonpiercing-Sucking Mouthparts
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CLSM | Confocal laser scanning microscopy |
| SEM | Scanning electron microscopy |
| EDS | Energy dispersive X-ray spectroscopy |
References
- Phan, H.V.; Park, H.C. Mimicking nature’s flyers: A review of insect-inspired flying robots. Curr. Opin. Insect Sci. 2020, 42, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Manoonpong, P.; Patanè, L.; Xiong, X.; Brodoline, I.; Dupeyroux, J.; Viollet, S.; Arena, P.; Serres, J.R. Insect-inspired robots: Bridging biological and artificial systems. Sensors 2021, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, H.; Shen, H.; Yu, Y.; He, H.; Feng, X.; Sun, Y.; Mao, Z.; Chen, G.; Tian, Z.; et al. An aerial-wall robotic insect that can land, climb, and take off from vertical surfaces. Research 2023, 6, 0144. [Google Scholar] [CrossRef]
- French, J.R.J.; Ahmed, B.M. The challenge of biomimetic design for carbon-neutral buildings using termite engineering. Insect Sci. 2010, 17, 154–162. [Google Scholar] [CrossRef]
- Claggett, N.; Surovek, A.; Streeter, B.; Nam, S.; Bardunias, P.; Cetin, B. Biomimicry and locally responsive construction: Lessons from termite mounds for structural sustainability. In Insights and Innovations in Structural Engineering, Mechanics and Computation; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Balila; Vahdati, M. Biomimicry in construction: Glycoprotein-stabilised adobe bricks for enhanced compressive strength inspired by termites mounds. Constr. Build. Mater. 2024, 438, 137077. [Google Scholar]
- Didari, A.; Mengüc, M.P. A biomimicry design for nanoscale radiative cooling applications inspired by Morpho didius butterfly. Sci. Rep. 2018, 8, 16891. [Google Scholar] [CrossRef]
- Jullien, A.; Neradovskiy, M.; Scarangella, A.; Mitov, M. Biomimicry of iridescent, patterned insect cuticles: Comparison of biological and synthetic, cholesteric microcells using hyperspectral imaging. J. R. Soc. Interface 2020, 17, 20200239. [Google Scholar] [CrossRef] [PubMed]
- Scarangella, A.; Soldan, V.; Mitov, M. Biomimetic design of iridescent insect cuticles with tailored, self-organized cholesteric patterns. Nat. Commun. 2020, 11, 4108. [Google Scholar] [CrossRef]
- Gorb, S.N. Insect-inspired technologies: Insects as a source for biomimetics. In Insect Biotechnology; Vilcinskas, A., Ed.; Biologically-Inspired System; Springer: Dordrecht, The Netherlands, 2011; Volume 2. [Google Scholar]
- Fowler, J.E.; Gorb, S.; Baio, J.E. Multi-technique investigation of a biomimetic insect tarsal adhesive fluid. Front. Mech. Eng. 2021, 7, 681120. [Google Scholar] [CrossRef]
- Li, W.; Zhou, R.; Ouyang, Y.; Guan, Q.; Shen, Y.; Saiz, E.; Li, M.; Hou, X. Harnessing biomimicry for controlled adhesion on material surfaces. Small 2024, 20, 2401859. [Google Scholar] [CrossRef]
- Franceschini, N.; Viollet, S.; Ruffier, F.; Serres, J. Neuromimetic Robots inspired by Insect Vision. In Proceedings of the 3rd International Conference “Smart, Materials, Structures and Systems” (CIMTEC), Sicily, Italy, 8–13 June 2008. [Google Scholar]
- Bogue, R. Developments in biomimetic vision. Sens. Rev. 2013, 33, 14–18. [Google Scholar]
- Serres, J.R.; Viollet, S. Insect-inspired vision for autonomous vehicles. Curr. Opin. Insect Sci. 2018, 30, 46–51. [Google Scholar] [PubMed]
- Miessner, M.; Peter, M.G.; Vincent, J.F.V. Preparation of insect-cuticle-like biomimetic materials. BOMAF6 2001, 2, 369–372. [Google Scholar] [PubMed]
- Chen, B.; Peng, X.; Wang, W.; Zhang, J.; Zhang, R. Research on the microstructure of insect cuticle and the strength of a biomimetic preformed hole composite. Micron 2002, 33, 571–574. [Google Scholar] [PubMed]
- Chen, B.; Peng, X.; Cai, C.; Niu, H.; Wu, X. Helicoidal microstructure of Scarabaei cuticle and biomimetic research. Mater. Sci. Eng. A 2006, 423, 237–242. [Google Scholar]
- Clissold, F.J. The biomechanics of chewing and plant fracture: Mechanisms and Implications. Adv. Insect Physiol. 2007, 34, 317–372. [Google Scholar]
- Hellemans, S.; Šobotnik, J.; Lepoint, G.; Mihaljevič, M.; Roisin, Y.; Bourguignon, T. Termite dispersal is influenced by their diet. Proc. R. Soc. B Biol. Sci. 2022, 289, 2022.0246. [Google Scholar]
- Wilson Rankin, E.E.; Knowlton, J.L.; Shmerling, A.J.; Hoey-Chamberlain, R. Diets of two non-native praying mantids (Tenodera sinensis and Mantis religiosa) show consumption of arthropods across all ecological roles. Food Webs. 2023, 35, e00280. [Google Scholar]
- Kitching, I.J. 2003. Phylogeny of the death’s head hawkmoths, Acherontia [Laspeyres], and related genera (Lepidoptera: Sphingidae: Sphinginae: Acherontiini). Syst. Entomol. 2003, 28, 71–88. [Google Scholar]
- Zaspel, J.; Scott, C.H.; Hill, S.; Ignell, R.; Kononenko, V.; Weller, S. Geographic distribution, phylogeny, and genetic diversity of the fruit- and blood-feeding moth Calyptra thalictra Borkhausen (Insecta: Lepidoptera: Erebidae). J. Parasitol. 2014, 10, 583–591. [Google Scholar]
- Lehnert, M.S.; Johnson, D.D.; Wu, J.; Sun, Y.; Fonseca, R.J.; Michels, J.; Shell, J.S.; Reiter, K.E. Physical adaptations of butterfly proboscises enable feeding from narrow floral tubes. Funct. Ecol. 2021, 35, 1925–1937. [Google Scholar]
- Barth, F.G. Insects and Flowers: The Biology of a Partnership; Princeton University Press: Princeton, NJ, USA, 1991. [Google Scholar]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Dicks, T.D.; Garibaldi, L.A.; Hill, R.; Settele, J.; Vanbergen, A.J. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar]
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; Alajmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of bee pollination and its economic value for crop production. Insects 2021, 12, 688. [Google Scholar] [CrossRef]
- Penn, H.J.; Crist, T.O. From dispersal to predation: A global synthesis of ant-seed interactions. Ecol. Evol. 2018, 8, 9122–9138. [Google Scholar]
- Putman, R.J. Carrion and Dung. The Decomposition of Animal Wastes; Edward Arnold: London, UK, 1983. [Google Scholar]
- French, B.W.; Chandler, L.D.; Ellsbury, M.M.; Fuller, B.W.; West, M. Ground beetle (Coleoptera: Carabidae) assemblages in a transgenic corn-soybean cropping system. Environ. Entomol. 2004, 33, 554–563. [Google Scholar]
- Kaunisto, K.M.; Roslin, T.; Forbes, M.R.; Morrill, A.; Sääksjärvi, I.E.; Puisto, A.I.E.; Lilley, T.M.; Vesterinen, E.J. Threats from the air: Damselfly predation on diverse prey taxa. J. Anim. Ecol. 2020, 89, 1365–1374. [Google Scholar]
- Pusceddu, M.; Mura, A.; Floris, I.; Satta, A. Feeding strategies and intraspecific competition in German yellowjackets (Vespula germanica). PLoS ONE 2018, 13, e0206301. [Google Scholar]
- Labandeira, C.C. Insect mouthparts: Ascertaining the paleobiology of insect feeding strategies. Annu. Rev. Ecol. Evol. Syst. 1997, 28, 153–193. [Google Scholar]
- Krenn, H.W. (Ed.) Insect Mouthparts-Form, Function, Development and Performance; Springer: Berlin/Heidelberg, Germany, 2019; p. 683. [Google Scholar]
- Kjer, K.; Carle, F.; Litman, J.; Ware, J.A. Molecular Phylogeny of Hexapoda. Arthropod. Syst. Phylogeny 2006, 64, 35–44. [Google Scholar] [CrossRef]
- Sasaki, G.; Ishiwata, K.; Machida, R.; Miyata, T.; and Zhi-Hui, S. Molecular phylogenetic analyses support the monophyly of Hexapoda and suggest the paraphyly of Entognatha. BMC Evol. Biol. 2013, 13, 236. [Google Scholar] [CrossRef]
- Blanke, A.; Rühr, P.T.; Mokso, R.; Villanueva, P.; Wilde, F.; Stampanoni, M.; Uesugi, K.; Machida, R.; Misof, B. Structural mouthpart interaction evolved already in the earliest lineages of insects. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151033. [Google Scholar] [CrossRef]
- Blanke, A.; Machida, R.; Szucsich, N.U.; Wilde, F.; Misof, B. Mandibles with two joints evolved much earlier in the history of insects: Dicondyly is a synapomorphy of bristletails, silverfish and winged insects. Syst. Entomol. 2015, 40, 357–364. [Google Scholar] [CrossRef]
- van de Kamp, T.; Mikó, I.; Staniczek, A.H.; Eggs, B.; Bajerlein, D.; Faragó, T.; Hagelstein, L.; Hamann, E.; Spiecker, R.; Baumbach, T.; et al. Evolution of flexible biting in hyperdiverse parasitoid wasps. Proc. R. Soc. B Biol. Sci. 2022, 289, 20212086. [Google Scholar] [CrossRef]
- Snodgrass, R.E. Principles of Insect Morphology; McGraw-Hill Book Company, Inc.: New York, NY, USA; London, UK, 1935; p. 667. [Google Scholar]
- Rogers, B.T.; Peterson, M.D.; Kaufman, T.C. The development and evolution of insect mouthparts as revealed by the expression patterns of gnathocephalic genes. Evol. Dev. 2002, 4, 96–110. [Google Scholar]
- Angelini, D.R.; Kaufman, T.C. Insect appendages and comparative ontogenetics. Dev. Biol. 2005, 286, 57–77. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: Cambridge, UK, 2005; p. 772. [Google Scholar]
- Labandeira, C.C. The fossil record of insect mouthparts: Innovation, functional convergence, and associations with other organisms. In Insect Mouthparts—Form, Function, Development and Performance; Krenn, H.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 567–671. [Google Scholar]
- Krenn, H.W.; Plant, J.D.; Szucsich, N.U. Mouthparts of flower-visiting insects. Arthropod Struct. Dev. 2005, 34, 1–40. [Google Scholar] [CrossRef]
- Krenn, H.W.; Aspock, H. Form, function and evolution of the mouthparts of blood-feeding Arthropoda. Arthropod Struct. Dev. 2012, 41, 101–118. [Google Scholar] [CrossRef]
- Lehnert, M.S.; Bennett, A.; Reiter, K.E.; Gerard, P.D.; Wei, Q.-H.; Byler, M.; Yan, H.; Lee, W.-K. Mouthpart conduit sizes of fluid feeding insects determine the ability to feed from pores. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162026. [Google Scholar] [CrossRef]
- Stern, D.L. The genetic causes of convergent evolution. Nat. Rev. Genet. 2013, 14, 751–764. [Google Scholar] [CrossRef]
- Olson, M.E. Linking xylem structure and function: The comparative method in from the cold. New Phytol. 2022, 235, 815–820. [Google Scholar] [CrossRef]
- Konieczny, L.; Roterman-Konieczna, I.; Spolnik, P. Chapter 1—The structure and function of living organisms. In Systems Biology: Functional Strategies of Living Organisms, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–52. [Google Scholar]
- Posnien, N.; Bashasab, F.; Bucher, G. The insect upper lip (labrum) is a nonsegmental appendage-like structure. Evol. Dev. 2009, 11, 480–488. [Google Scholar] [CrossRef]
- Bernays, E.A. Evolution of feeding behavior in insect herbivores. BioScience 1998, 48, 35–44. [Google Scholar] [CrossRef]
- Hoback, W.W.; Higley, L.G.; Stanley, D.W. Tigers eating tigers: Evidence of intraguild predation operating in an assemblage of tiger beetles. Ecol. Entomol. 2008, 26, 367–375. [Google Scholar] [CrossRef]
- Bueno da Silva, I.; Costa-Leonardo, A.M. Hypopharynx in termites: Morphological and functional aspects. Micron 2017, 101, 186–196. [Google Scholar] [CrossRef]
- Kornev, K.G.; Salamatin, A.A.; Adler, P.H.; Beard, C.E. Structure and physical determinants of the proboscis-sucking pump complex in the evolution of fluid-feeding insects. Sci. Rep. 2017, 7, 6582. [Google Scholar]
- Kikuchi, K.; Stremler, M.A.; Chatterjee, S.; Lee, W.; Mochizuki, O.; Socha, J.J. Burst mode pumping: A new mechanism of drinking in mosquitoes. Sci. Rep. 2018, 8, 4885. [Google Scholar] [CrossRef]
- Lehnert, M.S. (Ed.) Metals and Their Functional Role in the Structures of Invertebrates; Springer: Berlin/Heidelberg, Germany, 2024; p. 277. [Google Scholar]
- Merritt, R.W.; Wallace, J.B. Filter-feeding insects. Sci. Am. 1981, 244, 132–147. [Google Scholar] [CrossRef]
- Lehnert, M.S.; Lanba, A.; Reiter, K.E.; Fonseca, R.J.; Minninger, J.; Hall, B.; Huff, W. Mouthpart adaptations of antlion larvae facilitate prey handling and fluid feeding in sandy habitats. J. Exp. Biol. 2022, 225, 244220. [Google Scholar] [CrossRef]
- Schaefer, C.W.; Leschen, R.A.B. (Eds.) Functional Morphology of Insect Feeding; Entomological Society of America: Annapolis, MD, USA, 1993. [Google Scholar]
- Light, J.E.; Smith, V.S.; Allen, J.M.; Durden, L.A.; Reed, D.L. Evolutionary history of mammalian sucking lice (Phthiraptera: Anoplura). BMC Biol. 2010, 10, 292. [Google Scholar] [CrossRef]
- Lehnert, M.S. Chapter 7: Insect mouthparts. In Insect Anatomy; Moussian, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2025; p. 701. [Google Scholar]
- Cribb, B.W.; Stewart, A.; Huang, H.; Truss, R.; Noller, B.; Rasch, R.; Zalucki, M.P. Insect. mandibles—Comparative mechanical properties and links with metal incorporation. Sci. Nat. 2008, 95, 17–23. [Google Scholar] [CrossRef]
- Schofield, R.M.S.; Bailey, J.; Coon, J.J.; Devaraj, A.; Garrett, R.W.; Goggans, M.S.; Hebner, M.G.; Lee, B.S.; Lee, D.; Lovern, N.; et al. The homogenous alternative to biomineralization: Zn- and Mn-rich materials enable sharp organismal “tools” that reduce force requirements. Sci. Rep. 2021, 11, 17481. [Google Scholar]
- Smith, T.R.; Capinera, J.L. Mandibular morphology of some Floridian grasshoppers (Orthoptera: Acrididae). Fla. Entomol. 2005, 88, 204–207. [Google Scholar] [CrossRef]
- Angelini, D.R.; Smith, F.W.; Aspiras, A.C.; Kikuchi, M.; Jockusch, E.L. Patterning of the adult mandibulate mouthparts in the red flour beetle, Tribolium castaneum. Genetics 2012, 190, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Bianca, B.; Kenneth, K.; Bauder, J.A.-S.; Krenn, H.W. Mouthpart dimorphism in male and female wasps of Vespula vulgaris and Vespula germanica (Vespidae, Hymenoptera). Dtsch. Entomol. Z. 2018, 65, 65–74. [Google Scholar]
- Ball, G.E.; Acorn, J.H.; Shpeley, D. Mandibles and labrum-epipharynx of tiger beetles: Basic structure and evolution (Coleoptera, Carabidae, Cicindelitae). Zookeys 2011, 147, 39–83. [Google Scholar]
- Klunk, C.L.; Argenta, M.A.; Rosumek, F.B.; Schmelzle, S.; van de Kamp, T.; Hammel, J.U.; Pie, M.R.; Heethoff, M. Simulated biomechanical performance of morphologically disparate ant mandibles under bite loading. Sci. Rep. 2023, 13, 16833. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Economo, E.P. The feeding apparatus of ants: An overview of structure and function. Philos. Trans. R. Soc. B 2023, 378, 20220556. [Google Scholar] [CrossRef]
- Bernays, E.A. Evolution of insect morphology in relation to plants. Philos. Trans. R. Soc. B 1991, 333, 257–264. [Google Scholar]
- Bernays, E.A.; Janzen, D.H. Saturniid and sphingid caterpillars: Two ways to eat leaves. Ecology 1988, 69, 1153–1160. [Google Scholar] [CrossRef]
- Sasakawa, K. Utility of geometric morphometrics for inferring feeding habit from mouthpart morphology in insects: Tests with larval Carabidae (Insecta: Coleoptera). Biol. J. Linn. Soc. 2016, 118, 394–409. [Google Scholar] [CrossRef]
- Desai, K.A.; Rao, P.V.M. Effect of direction of parameterization on cutting forces and surface error in machining curved geometries. Int. J. Mach. Tools Manuf. 2008, 48, 249–259. [Google Scholar] [CrossRef]
- Confalonieri, F.; Ghisi, A.; Perego, U. Blade cutting of thin walled structure by explicit dynamics finite elements. Meccanica 2018, 53, 1271–1289. [Google Scholar]
- Haack, R.A.; Herard, F.; Sun, J.; Turgeon, J.J. Managing invasive populations of Asian longhorn beetle and citrus longhorn beetle: A worldwide perspective. Annu. Rev. Entomol. 2010, 55, 521–546. [Google Scholar]
- Kariyanna, B.; Mohan, M.; Gupta, R. Biology, ecology, and significance of longhorn beetles (Coleoptera: Cerambycidae). J. Entomol. Zool. Stud. 2017, 5, 1207–1212. [Google Scholar]
- Kunpeng, T.; Xianwang, L.; Cheng, S.; Bin, Z.; Jicheng, H.; Jinguo, W.; Yang, Z. Design and test of cutting blade of cannabis harvester based on longicorn bionic principle. Trans. Chin. Soc. Agric. Eng. 2017, 33, 56–61. [Google Scholar]
- Lucia, E. A Lesson from Nature: Joe Cox and his Revolutionary Saw Chain. J. For. Hist. 1981, 25, 4004486. [Google Scholar]
- Ma, J.; Wu, K.; Gao, A.; Du, Y.; Song, Y.; Ren, L. Study on the bionic design and cutting performance of alfalfa cutters based on the maxillary mouthparts of longicorn beetles. Agriculture 2024, 14, 1302. [Google Scholar] [CrossRef]
- McKenna, D.D.; Sequeira, A.S.; Marvaldi, A.E.; Farrell, B.D. Temporal lags in overlap in the diversification of weevils and flowering plants. Proc. Natl. Acad. Sci. USA 2009, 106, 7083–7088. [Google Scholar]
- Ren, J.; Zhang, R. Delimiting species, revealing cryptic diversity in Molytinae (Coleoptera: Curculionidae) weevil through DNA barcoding. J. Insect Sci. 2024, 24, ieae083. [Google Scholar]
- Tong, J.; Xu, S.; Chen, D.; Li, M. Design of a bionic blade for vegetable chopper J. J. Bionic Eng. 2017, 14, 163–171. [Google Scholar] [CrossRef]
- Wei, X.; Wang, G.; Miller Smith, L.; Chen, X.; Jiang, H. Effects of gradient distribution and aggregate structure of fibers on the flexibility and flexural toughness of natural moso bamboo (Phyllostachys edulis). J. Mater. Res. Technol. 2022, 16, 853–863. [Google Scholar]
- Santiago, D.R.; Castillo, A.G.; Arapan, R.S.; Navasero, N.V.; Eusebio, J.E. Efficacy of Metarhizium anisopliae (Metsch.) Sor. against the oriental migratory locust Locusta migratoria manilensis meyen. Philipp. Agric. Sci. 2001, 84, 26–34. [Google Scholar]
- Zhao, J.; Huo, M.; Dongyan, H.; Zhuang, J. Design of bionic locust mouthparts stubble cutting device. Int. J. Agric. Biol. Eng. 2021, 13, 20–28. [Google Scholar]
- Edel, C.; Ruhr, P.T.; Frenzel, M.; van de Kamp, T.; Farago, T.; Hammel, J.U.; Wilde, F.; Blanke, A. Bite force transmission and mandible shape in grasshoppers, crickets, and allies is not driven by dietary niches. Evolution 2024, 78, 1958–1968. [Google Scholar]
- Ginot, S.; Sommerfeld, S.; Blanke, A. Linking shape conspicuous asymmetry with shape covariation patterns and performance in the insect head and mandibles. Evolution 2024, 78, 1078–1091. [Google Scholar] [PubMed]
- Hu, J.; Xu, L.; Yu, Y.; Lu, J.; Han, D.; Chai, X.; Wu, Q.; Zhu, L. Design and Experiment of Bionic Straw-Cutting Blades Based on Locusta migratoria manilensis. Agriculture 2023, 13, 2231. [Google Scholar] [CrossRef]
- Changying, L.; Honglei, J.; Zhihong, Z.; Gang, W.; Hongchang, W. Grasshopper [Chondracris rosea rosea (De Geer)] incisors cutting edge contour data extraction and quantitative analysis. Appl. Mech. Mater. 2014, 461, 144–151. [Google Scholar]
- Sheehan, J.; Aden, A.; Paustian, K.; Killian, K.; Brenner, J.; Walsh, M.; Nelson, R. Energy and environmental aspects of using corn stover for fuel ethanol. J. Ind. Ecol. 2008, 7, 117–146. [Google Scholar]
- Jia, H.; Li, C.; Zhang, Z.; Wang, G. Design of bionic saw blade for corn stalk cutting. J. Bionic Eng. 2013, 10, 497–505. [Google Scholar]
- Du, Z.; Hu, Y.; Lu, Y.; Pang, J.; Li, X. Design of structural parameters of cutters for tea harvesters based on biomimetic methodology. Appl. Bionics Biomech. 2021, 2021, 8798299. [Google Scholar] [CrossRef]
- Hadizadeh, H.; Samiei, L.; Shakeri, A. Chrysanthemum, an ornamental genus with considerable medicinal value: A comprehensive review. S. Afr. J. Bot. 2022, 144, 23–43. [Google Scholar]
- Liu, Z.; Wang, T.; Liu, S.; Yan, X.; Zhao, H.; Wu, X.; Zhang, S. Design and Experimental Study of a Bionic Blade for Harvesting the Wild Chrysanthemum Stem. Agriculture 2023, 13, 190. [Google Scholar] [CrossRef]
- Dehghan-Hesar, H.; Kalantari, D. Design and Evaluation of Two New Biomimetic Blades for Reducing the Shear Energy Required for Cutting Herbal Plants. J. Agric. Mach. 2019, 9, 265–278. [Google Scholar] [CrossRef]
- Noonan, K.C. Recognition of queen larvae by worker honey bees (Apis mellifera). Ethology 1986, 73, 295–306. [Google Scholar] [CrossRef]
- Casadei-Ferreira, A.; Friedman, N.R.; Economo, E.P.; Pie, M.R.; Feitosa, R.M. Head and mandible shapes are highly integrated yet represent two distinct modules within and among worker subcastes of the ant genus Pheidole. Ecol. Evol. 2021, 11, 6104–6118. [Google Scholar] [PubMed]
- Puffel, F.; Walthaus, O.K.; Kang, V.; Labonte, D. Biomechanics of cutting: Sharpness, wear sensitivity and the scaling of cutting forces in leaf-cutter ant mandibles. Philos. Trans. R. Soc. B. 2023, 378, 20220547. [Google Scholar]
- Birkenfeld, V.; Gorb, S.N.; Krings, W. Mandible element composition and mechanical properties from distinct castes of the leafcutter ant Atta laevigata (Attini; Formicidae). Interface Focus 2024, 14, 20230048. [Google Scholar]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. A review of Ginseng species in different regions as a multipurpose herb in traditional Chinese medicine, modern herbology and pharmacological science. J. Med. Plants Res. 2019, 13, 213–226. [Google Scholar]
- Xiang, Y.; Shang, H.; Gao, X.; Zhang, B. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother. Res. 2008, 22, 851–858. [Google Scholar] [PubMed]
- Qi, H.; Ma, Z.; Xu, Z.; Wang, S.; Ma, Y.; Wu, S.; Guo, M. The Design and Experimental Validation of a Biomimetic Stubble-Cutting Device Inspired by a Leaf-Cutting Ant’s Mandibles. Biomimetics 2023, 8, 555. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, S.; Ma, L.; Yang, W.; Jin, Z.; Yan, Y.; Zhu, L. Design and experiment of bionic cutting blades for Panax notoginseng stem and leaf harvesting machine. Nongye Jixie Xuebao 2024, 55, 8. [Google Scholar]
- Davis, H.E. Leaf-Cutter Ants in Wound Closure. Wilderness Environ. Med. 2019, 30, 469–470. [Google Scholar] [CrossRef] [PubMed]
- Brito, T.O.; Elzubair, A.; Araújo, L.S.; de Souza Camargo, S.A.; Pereira Souza, J.L.; Almeida, L.H. Characterization of the Mandible Atta Laevigata and the Bioinspiration for the Development of a Biomimetic Surgical Clamp. Mater. Res. 2017, 20, 1525–1533. [Google Scholar] [CrossRef]
- Rodriguez, F.; Moreno, J.C.; Sanchez, J.A.; Berenguel, M. Grasping in agriculture: State-of-the-art and main characteristics. In Grasping in Robotics; Carbone, G., Ed.; Springer: London, UK, 2012; Volume 10. [Google Scholar]
- Zhang, B.; Xie, Y.; Zhou, J.; Wang, K.; Zhang, Z. State-of-the-art robotic grippers, grasping and control strategies, as well as their applications in agricultural robots: A review. Comput. Electron. Agric. 2020, 177, 105694. [Google Scholar] [CrossRef]
- Zhang, W.; Li, M.; Zheng, G.; Guan, Z.; Wu, J.; Wu, Z. Multifunctional mandibles of ants: Variation in gripping behavior facilitated by specific microstructures and kinematics. J. Insect Physiol. 2020, 120, 103993. [Google Scholar] [CrossRef]
- Zhang, W.; He, Z.; Sun, Y.; Wu, J.; Wu, Z. A mathematical modeling method elucidating the integrated gripping performance of ant mandibles and bio-inspired grippers. J. Bionic Eng. 2020, 17, 732–746. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Z.; Wang, Z.; Wang, Z.; Li, C.; Rajabi, H.; Wu, J. Double-rowed teeth: Design specialization of the H. venator ants for enhanced tribological stability. Bioinspiration Biomim. 2021, 16, 055003. [Google Scholar] [CrossRef] [PubMed]
- Büsse, S.; Koehnsen, A.; Rajabi, H.; and Gorb, S. A controllable dual-catapult system inspired by the biomechanics of the dragonfly larvae’s predatory strike. Sci. Robot. 2021, 6, eabc8170. [Google Scholar] [CrossRef]
- Sotiri, I.; Overton, J.C.; Waterhouse, A.; Howell, C. Immobilized liquid layers: A new approach to anti-adhesion surfaces for medical applications. Exp. Biol. Med. 2016, 241, 909–918. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Gomes, M.; Gomes, L.C.; Mergulhao, F.J. Antimicrobial and anti- adhesive properties of carbon nanotube-based surfaces for medical applications: A systematic review. iScience 2021, 24, 102001. [Google Scholar] [CrossRef]
- Uneputty, A.; Davila-Lezama, A.; Garibo, D.; Oknianska, A.; Bogdanchikova, N.; Hernandez-Sanches, J.F.; Susarrey-Arce, A. Strategies applied to modify structured and smooth surfaces: A step closer to reduce bacterial adhesion and biofilm formation. Colloid Interface. Sci. 2022, 46, 100560. [Google Scholar] [CrossRef]
- Sugihara, T.; Enomoto, T. Development of a cutting tool with a nano/micro-textured surface-improvement of anti-adhesive effect by considering the texture patterns. Precis. Eng. 2009, 33, 425–429. [Google Scholar]
- Saccardi, L.; Brümmer, F.; Schiebl, J.; Schwarz, O.; Kovalev, A.; Gorb, S. Interaction between honeybee mandibles and propolis. Beilstein J. Nanotechnol. 2022, 13, 958–974. [Google Scholar] [CrossRef]
- Tran, V.H.; Duke, R.K.; Abu-Mellal, A.; Duke, C.C. Propolis with high flavonoid content collected by honey bees from Acacia paradoxa. Phytochem. 2012, 81, 126–132. [Google Scholar]
- Saccardi, L.; Schiebl, J.; Balluff, F.; Christ, U.; Gorb, S.N.; Kovalev, A.; Schwarz, O. Anti-Adhesive Surfaces Inspired by Bee Mandible Surfaces. Biomimetics 2023, 8, 579. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, D.; Yan, Y.; Yin, X. Investigation of bionic composite laminates inspired by the natural impact-resistant helicoidal structure in the mandibles of trap-jaw ants. Bioinspiration Biomim. 2023, 18, 056005. [Google Scholar]
- Zhao, S.; Yin, X.; Zhang, D. A study of a bio-inspired impact resistant carbon fiber laminate with a sinusoidal helicoidal structure in the mandibles of trap-jaw ants. Acta Biomater. 2023, 169, 179–191. [Google Scholar] [PubMed]
- Kornev, K.G.; Adler, P.H. Chapter 8: Physical determinants of fluid-feeding in insects. In Insect Mouthparts-Form, Function, Development and Performance; Krenn, H.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 263–314. [Google Scholar]
- Araujo, R.N.; Gontijo, N.F.; Guarneri, A.A.; Gontijo, A.F.; Soares, A.C.; Pereira, M.H. Electromyogram of the cibarial pump and the feeding process in hematophagous Hemiptera. In Advances in Applied Electromyography; Mizrahi, J., Ed.; InTech: Nappanee, IN, USA, 2011; p. 228. [Google Scholar]
- Monaenkova, D.; Lehnert, M.S.; Andrukh, T.; Beard, C.E.; Rubin, B.; Tokarev, A.; Lee, W.K.; Adler, P.H.; Kornev, K.G. Butterfly proboscis: Combining a drinking straw with a nanosponge facilitated diversification of feeding habits. J. R. Soc. Interface 2012, 9, 720–726. [Google Scholar] [CrossRef]
- Ruschioni, S.; Ranieri, E.; Riolo, P.; Romani, R.; Almeida, R.P.P.; Isidoro, N. Functional anatomy of the precibarial valve in Philaenus spumarius (L.). PLoS ONE 2019, 14, e0213318. [Google Scholar]
- Lehnert, M.S.; Monaenkova, D.; Andrukh, T.; Beard, C.E.; Adler, P.H.; Kornev, K.G. Hydrophobic–hydrophilic dichotomy of the butterfly proboscis. J. R. Soc. Interface 2013, 10, 20130336. [Google Scholar] [CrossRef]
- Bast, E.M.; Marshall, N.T.; Myers, K.O.; Marsh, L.W.; Walschburger Hurtado, M.; Van Zandt, P.A.; Lehnert, M.S. Diverse material properties and morphology of moth proboscises relates to the feeding habits of some macromoth and other lepidopteran lineages. Interface Focus 2024, 14, 20230051. [Google Scholar]
- Zaspel, J.M.; Weller, S.J.; Branham, M.A. A comparative survey of proboscis morphology and associated structures in fruit-piercing, tear-feeding, and blood-feeding moths in Calpinae (Lepidoptera: Erebidae). Zoomorphology 2011, 130, 203–225. [Google Scholar]
- Karolyi, F.; Colville, J.F.; Handschuh, S.; Metscher, B.D.; Krenn, H.W. One proboscis, two tasks: Adaptations to blood-feeding and nectar-extracting in long-proboscid horse flies (Tabanidae, Philoliche). Arthropod Struct. Dev. 2014, 43, 403–413. [Google Scholar] [CrossRef]
- Reiter, K.E.; Perkovich, C.; Smith, K.; Feng, J.; Kritsky, G.; Lehnert, M.S. Comparative material and mechanical properties among cicada mouthparts: Cuticle enhanced with inorganic elements facilitates piercing through woody stems for feeding. Biology 2023, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Perkovich, C.P.; Haynes, B.R.; Reiter, K.E.; Kritsky, G.; Lehnert, M.S. Chapter 3: The presence and distribution of transition metals and other inorganic elements in the cuticle of cicadas (Hemiptera: Cicadidae). In Metals and Their Functional Role in the Structures of Invertebrates; Lehnert, M.S., Ed.; Springer Publishing: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Nalini, E.; Polidori, C. A comparison between sexes, castes, and life-histories supports the adaptive role of metal enrichment in the mandibles of Lasius ants. Eur. Zool. J. 2024, 91, 601–611. [Google Scholar] [CrossRef]
- Dixon, A.R.; Vondra, I. Biting innovations of mosquito-based biomaterials and medical devices. Materials 2022, 15, 4587. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, M.K.; Barham, O.M.; Swaminathan, V. Mechanics of a mosquito bite with applications to microneedle design. Bioinspiration Biomim. 2008, 3, 046001. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Q.; Wu, C.W. Mosquito proboscis: An elegant biomicroelectromechanical system. Phys. Rev. E-Stat. Nonlinear Soft Matter Phys. 2010, 82, 011910. [Google Scholar] [CrossRef]
- Lenau, T.A.; Hesselberg, T.; Drakes, A.; Silva, P.; Gomes, S. Mosquito inspired medical needles. In Proceedings of the SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring, Portland, OR, USA, 25–29 March 2017; Volume 10162, p. 1016208. [Google Scholar]
- Hara, Y.; Yamada, M.; Tatsukawa, C.; Takahashi, T.; Suzuki, M.; Aoyagi, S. Fabrication of stainless steel microneedle with laser-cut sharp tip and its penetration and blood sampling performance. Int. J. Autom. Technol. 2016, 10, 950–957. [Google Scholar] [CrossRef]
- Bhusan, B. Insects locomotion, piercing, sucking and stinging mechanisms. Microsyst. Technol. 2018, 24, 4703–4728. [Google Scholar] [CrossRef]
- Jiao, S.; Zhang, X.; Cai, H.; Wu, S.; Ou, X.; Han, G.; Zhao, J.; Li, Y.; Guo, W.; Liu, T.; et al. Recent advances in biomimetic hemostatic materials. Mater. Today Bio 2023, 19, 100592. [Google Scholar] [CrossRef]
- Rahamim, V.; Azagury, A. Bioengineered biomimetic and bioinspired noninvasive drug delivery systems. Adv. Funct. Mater. 2021, 31, 2102033. [Google Scholar] [CrossRef]
- Molleman, F.; Krenn, H.W.; Van Alphen, M.E.; Brakefield, P.M.; Devries, P.J.; Zwaan, B.J. Food intake of fruit-feeding butterflies: Evidence for adaptive variation in proboscis morphology Biol. J. Linn. Soc. 2005, 86, 333–343. [Google Scholar] [CrossRef]
- Krenn, H.W.; Zulka, K.P.; Gatschnegg, T. Proboscis morphology and food preferences in nymphalid butterflies (Lepidoptera: Nymphalidae). J. Zool. 2001, 254, 17–26. [Google Scholar] [CrossRef]
- Krenn, H.W. Feeding mechanisms of adult Lepidoptera: Structure, function, and evolution of the mouthparts. Annu. Rev. Entomol. 2010, 55, 307–327. [Google Scholar]
- Karolyi, F.; Morawetz, L.; Colville, J.F.; Handschuh, S.; Metscher, B.D.; Krenn, H.W. Time management and nectar flow: Flower handling and suction feeding in long-proboscid flies (Nemestrinidae: Prosoeca). Naturwissenschaften 2013, 100, 1083–1093. [Google Scholar]
- Ma, L.; Hu, K.; Li, P.; Liu, J.; Yuan, X. Ultrastructure of the proboscis sensilla of ten species of butterflies (Insecta: Lepidoptera). PLoS ONE 2019, 14, e0214658. [Google Scholar]
- Petr, D.; Stewart, K.W. Comparative morphology of sensilla styloconica on the proboscis of North American Nymphalidae and other selected taxa (Lepidoptera): Systematic and ecological considerations. Trans. Am. Entomol. Soc. 2004, 130, 293–409. [Google Scholar]
- Krenn, H.W. Proboscis sensilla in Vanessa cardui (Nymphalidae, Lepidoptera): Functional morphology and significance in flower-probing. Zoomorphology 1998, 118, 23–30. [Google Scholar]
- Eastham, L.E.S.; Eassa, Y.E.E. The feeding mechanism of the butterfly Pieris brassicae L. Philos. Trans. R. Soc. B. 1955, 239, 1–43. [Google Scholar]
- Lehnert, M.S.; Beard, C.E.; Gerard, P.D.; Kornev, K.G.; Adler, P.H. Structure of the lepidopteran proboscis in relation to feeding guild. J. Morphol. 2016, 277, 167–182. [Google Scholar]
- Tsai, C.-C.; Mikes, P.; Andrukh, T.; White, E.; Monaenkova, D.; Burtovyy, O.; Burtovyy, R.; Rubin, B.; Lukas, D.; Luzinov, I.; et al. Nanoporous artificial proboscis for probing minute amount of liquids. Nanoscale 2011, 3, 4685–4695. [Google Scholar]
- Kornev, K.G.; Monaenkova, D.; Adler, P.H.; Beard, C.E.; Lee, W.-K. The butterfly proboscis as a fiber-based, self-cleaning, micro-fluidic system. In Bioinspiration, Biomimetics, and Bioreplication; SPIE: Washington, CA, USA, 2016; Volume 9797. [Google Scholar] [CrossRef]
- Lehnert, M.S.; Wei, Q.-H. Chapter 9: Hierarchical Microstructures and Functions of the Lepidopteran Proboscis Cuticle. In Insect Mouthparts—Form, Function, Development and Performance; Krenn, H.W., Ed.; Springer Publishing: Berlin/Heidelberg, Germany, 2019; pp. 315–334. [Google Scholar]
- Li, L.; Guo, C.; Li, X.; Han, C. Morphology and nanoindentation properties of mouthparts in Cyrtotrachelus longimanus (Coleoptera: Curculionidae). Microsc. Res. Tech. 2017, 80, 704–711. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.K.; Lee, E. Anatomical observation of the mouthpart of Cyllorhynchites ursulus and comparison with other species. Korean J. Environ. Biol. 2019, 37, 343–350. [Google Scholar] [CrossRef]
- Goyens, J.; Dirckx, J.; Aerts, P. Built to fight: Variable loading conditions and stress distribution in stag beetle jaws. Bioinspiration Biomim. 2015, 10, 046006. [Google Scholar] [CrossRef]
- Martínez, R.D.; Basterra, L.-A.; Acuña, L.; Balmori, J.-A. Morphology and material properties of the mouthparts of Stromatium unicolor Olivier 1795 (Coleoptera: Cerambycidae) for Bionic Application. Forests 2020, 11, 715. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Cloetens, P.; Betz, O. Desert locusts (Schistocerca gregaria) feed with self-sharpening, scissor-like mandibles. Interface Focus 2024, 14, 20230069. [Google Scholar]
- Betz, O.; Dieterich, A.; Cloetens, P.; Koerner, L.; Lehnert, M.S. Chapter 5—Element sensitive synchrotron-based X-ray tomography as a technique for studying transition metals in the cuticle of insects. In Metals and Their Functional Role in the Structures of Invertebrates; Springer: Berlin/Heidelberg, Germany, 2024; pp. 115–171. [Google Scholar]
- Zimmermann, D.; Randolf, S.; Aspöck, U. From Chewing to Sucking via Phylogeny—From Sucking to Chewing via Ontogeny: Mouthparts of Neuroptera. In Insect Mouthparts—Form, Function, Development and Performance; Krenn, H.W., Ed. Springer Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Lambert, E.; Motta, P.; Lowry, D. Modulation in the Feeding Prey Capture of the Ant-lion, Myrmeleon crudelis. J. Exp. Zool. A Ecol. Genet. Physiol. 2011, 315, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Szwedo, J. The unity, diversity and conformity of bugs (Hemiptera) through time. Earth. Environ. Sci. Trans. R. Soc. Edinb. 2016, 107, 109–128. [Google Scholar]
- Wang, Y.; Li, L.; Dai, W. Fine Morphology of the Mouthparts in Cheilocapsus nigrescens. (Hemiptera: Heteroptera: Miridae) Reflects Adaptation for Phytophagous Habits. Insects 2019, 10, 143. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, W. How does the intricate mouthpart apparatus coordinate for feeding in the hemimetabolous insect pest Erthesina fullo? Insects 2020, 11, 503. [Google Scholar] [CrossRef]
- Gillett, J.D. Natural selection and feeding speed in a blood-sucking insect. Proc. R. Soc. Lond. B. 1967, 167, 316–329. [Google Scholar] [CrossRef]
- Lyimo, I.N.; Ferguson, H.M. Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends Parisitol. 2009, 25, 189–196. [Google Scholar] [CrossRef]
- Boerlijst, S.P.; Johnston, E.S.; Ummels, A.; Krol, L.; Boelee, E.; van Bodegom, P.M.; Schrama, M.J.J. Biting the hand that feeds: Anthropogenic drivers interactively make mosquitoes thrive. Sci. Total Environ. 2023, 858, 159716. [Google Scholar] [PubMed]
- Backus, E.A. Sensory systems and behaviours which mediate hemipteran plant-feeding: A taxonomic overview. J. Insect Physiol. 1988, 34, 151–165. [Google Scholar]
- Cohen, A.C. Feeding Adaptations of Some Predaceous Hemiptera. Ann. Entomol. Soc. Am. 1990, 83, 1215–1223. [Google Scholar] [CrossRef]
- Reinhardt, K.; Siva-Jothy, M.T. Biology of the Bed Bugs (Cimicidae). Annu. Rev. Entomol. 2007, 52, 351–374. [Google Scholar] [CrossRef]
- Krenn, H.W. Functional morphology and movements of the proboscis of Lepidoptera (Insecta). Zoomorphology 1990, 110, 105–114. [Google Scholar] [CrossRef]
- Lehnert, M.S.; Brown, E.; Lehnert, M.P.; Gerard, P.D.; Yan, H.; Kim, C. The golden ratio reveals geometric differences in proboscis coiling among butterflies of different feeding habits. Am. Entomol. 2015, 61, 18–26. [Google Scholar] [CrossRef][Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehnert, M.S.; Myers, K.O.; Reiter, K.E. The Right Tool for the Job: A Review of Insect Mouthparts as a Tool Kit for Biomimetic Studies. Biomimetics 2025, 10, 196. https://doi.org/10.3390/biomimetics10040196
Lehnert MS, Myers KO, Reiter KE. The Right Tool for the Job: A Review of Insect Mouthparts as a Tool Kit for Biomimetic Studies. Biomimetics. 2025; 10(4):196. https://doi.org/10.3390/biomimetics10040196
Chicago/Turabian StyleLehnert, Matthew S., Kendall O. Myers, and Kristen E. Reiter. 2025. "The Right Tool for the Job: A Review of Insect Mouthparts as a Tool Kit for Biomimetic Studies" Biomimetics 10, no. 4: 196. https://doi.org/10.3390/biomimetics10040196
APA StyleLehnert, M. S., Myers, K. O., & Reiter, K. E. (2025). The Right Tool for the Job: A Review of Insect Mouthparts as a Tool Kit for Biomimetic Studies. Biomimetics, 10(4), 196. https://doi.org/10.3390/biomimetics10040196






