Preparation and Evaluation of PDMS/Carbon Soot Particles Superhydrophobic Biomimetic Composite Coating with Self-Cleaning and Durability
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
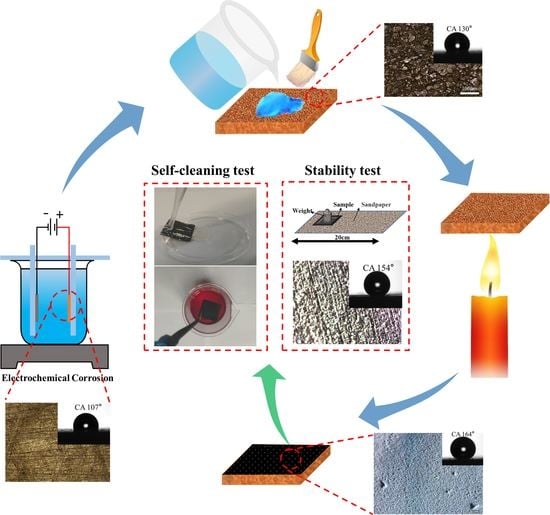
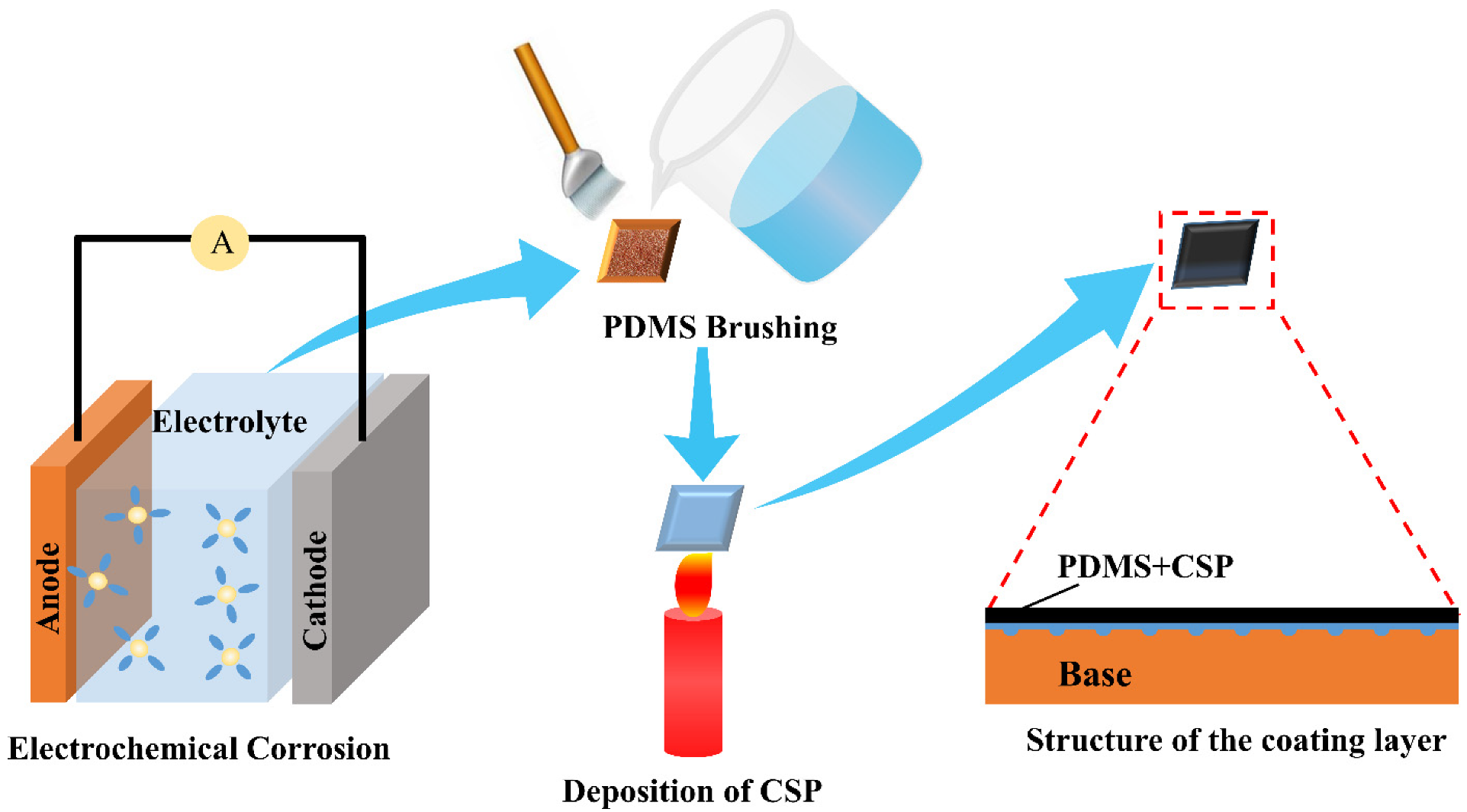
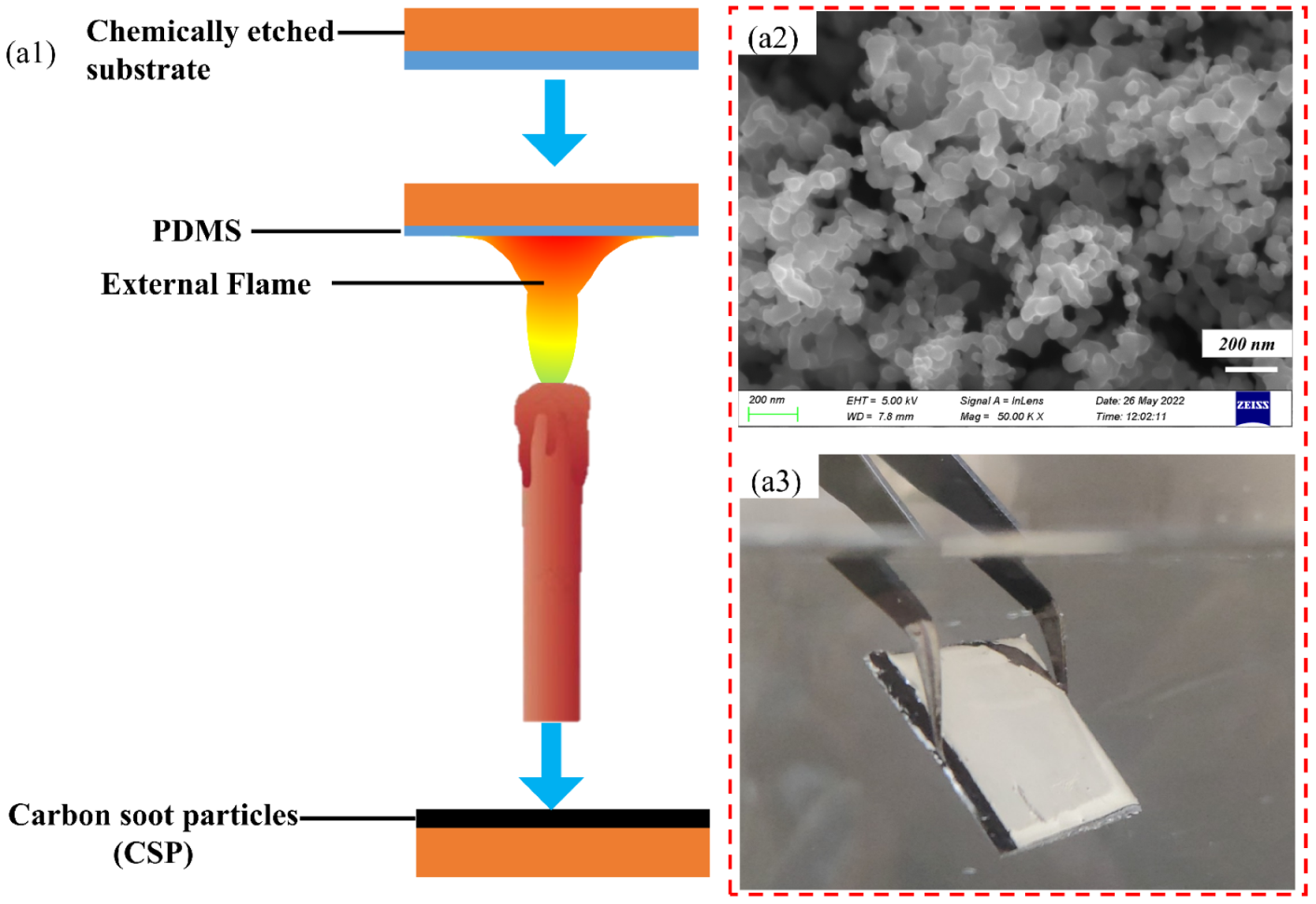
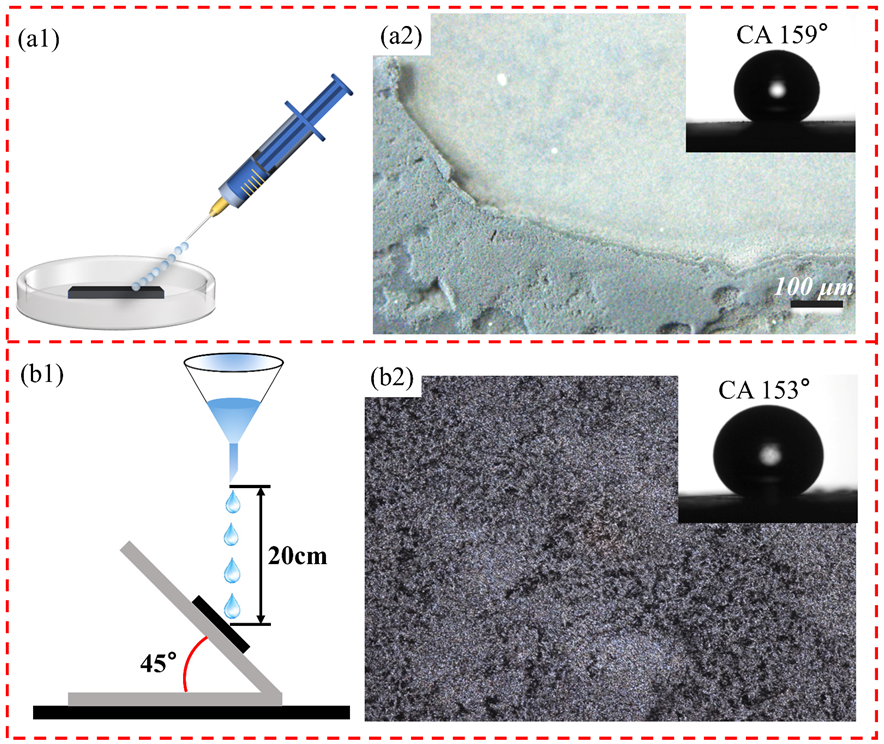
2.2. Preparation of Superhydrophobic Surfaces
2.3. Characterization Methods
2.3.1. Morphological Characterization
2.3.2. Contact Angle and Sliding Angle Measurement
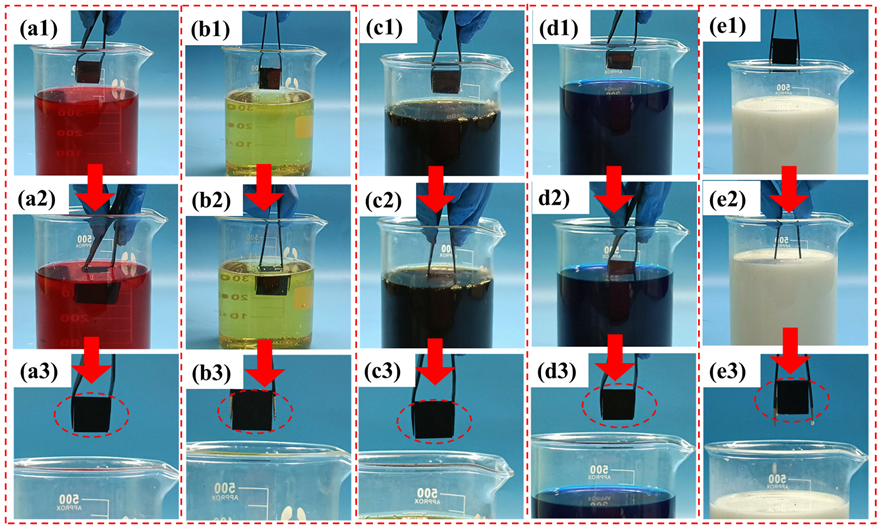
2.3.3. Self-Cleaning Test
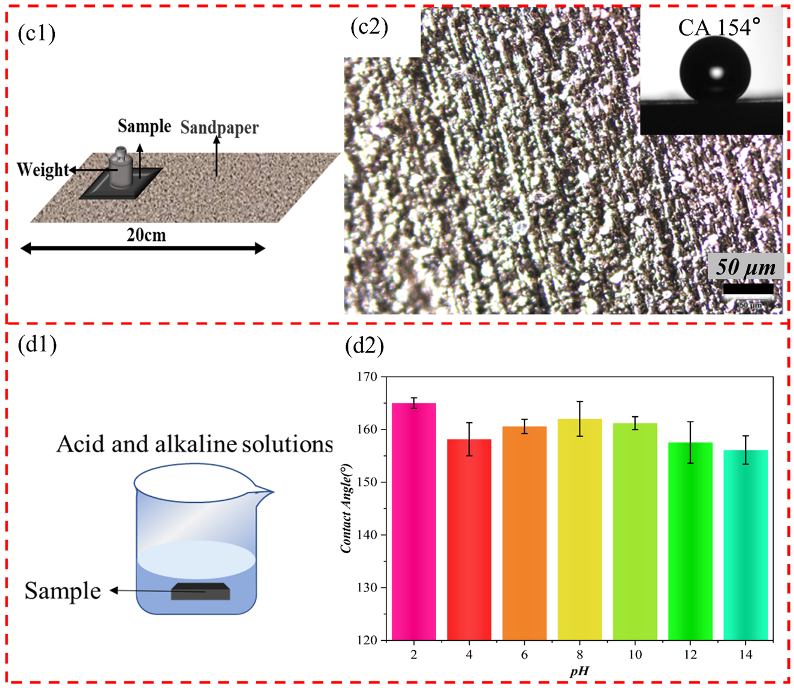
2.3.4. Physical and Chemical Damage Tests
3. Results and Discussion
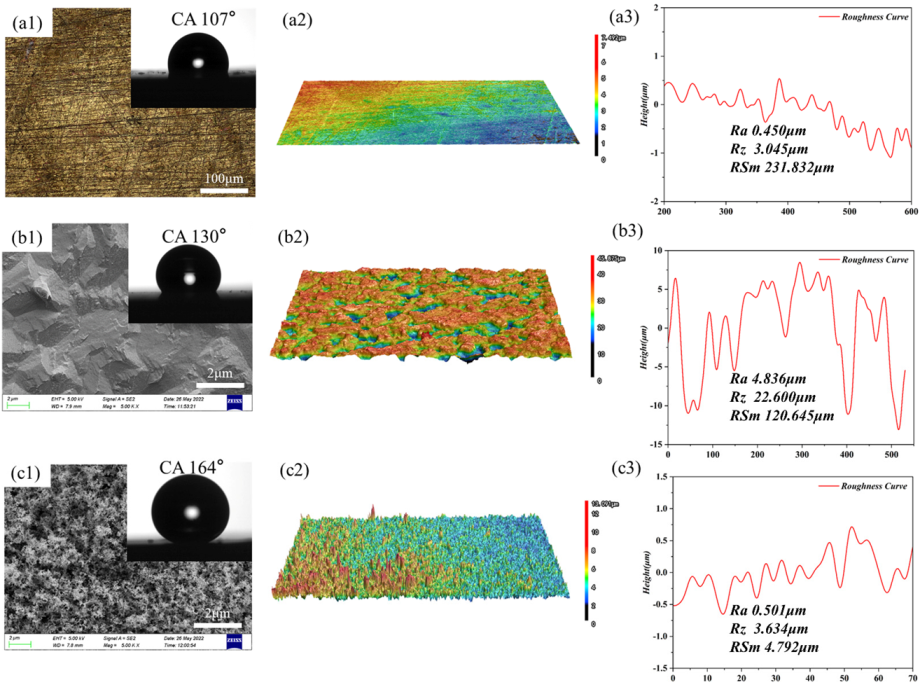
3.1. Surface Morphology and Wettability
3.2. Self-Cleaning Property
3.3. Durability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gustilana, A.; Puspitasari, P.; Pratama, M.M.A.; Sukarni, S.; Permanasari, A.A. Analysis of Corrosion Rate, Surface Roughness, and Microstructure of 48Cu-30Zn-18Nb Brass Alloy in Sea Water; AIP Publishing: Malang, Indonesia, 2021; p. 030003. [Google Scholar]
- Lu, X.; Liu, Y.; Liu, M.; Wang, Z. Corrosion Behavior of Copper T2 and Brass H62 in Simulated Nansha Marine Atmosphere. J. Mater. Sci. Technol. 2019, 35, 1831–1839. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, C.; Liu, R.; Zhang, Q.; Gong, J.; Tao, D.; Ji, Z. Fabrication of Superhydrophobic Surface with Enhanced Corrosion Resistance on H62 Brass Substrate. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124475. [Google Scholar] [CrossRef]
- Dominic, J.; Perumal, G.; Grewal, H.S.; Arora, H.S. Facile Fabrication of Superhydrophobic Brass Surface for Excellent Corrosion Resistance. Surf. Eng. 2020, 36, 660–664. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Tan, X.; Zhang, Z.; Jiang, Z.; Lian, J.; Wen, C.; Ren, L. Superhydrophobic Brass Surfaces with Tunable Water Adhesion Fabricated by Laser Texturing Followed by Heat Treatment and Their Anti-Corrosion Ability. Appl. Surf. Sci. 2022, 575, 151596. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.-Y.; Liu, Q.; Zhu, S.-P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of Robust Superhydrophobic Surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]
- Yang, F.; Guo, Z. Characterization of Micro-Morphology and Wettability of Lotus Leaf, Waterlily Leaf and Biomimetic ZnO Surface. J. Bionic Eng. 2015, 12, 88–97. [Google Scholar] [CrossRef]
- Shao, Y.; Zhao, J.; Fan, Y.; Wan, Z.; Lu, L.; Zhang, Z.; Ming, W.; Ren, L. Shape Memory Superhydrophobic Surface with Switchable Transition between “Lotus Effect” to “Rose Petal Effect”. Chem. Eng. J. 2020, 382, 122989. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Z.; Li, G. Programmable Droplet Manipulation by Combining a Superhydrophobic Magnetic Film and an Electromagnetic Pillar Array. Sens. Actuators B Chem. 2018, 262, 892–901. [Google Scholar] [CrossRef]
- Rius-Ayra, O.; Castellote-Alvarez, R.; Escobar, A.M.; Llorca-Isern, N. Robust and Superhydrophobic Coating Highly Resistant to Wear and Efficient in Water/Oil Separation. Surf. Coat. Technol. 2019, 364, 330–340. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Xu, J.; Yu, H. One-Step Method Using Laser for Large-Scale Preparation of Bionic Superhydrophobic & Drag-Reducing Fish-Scale Surface. Surf. Coat. Technol. 2021, 409, 126801. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, X.; Pang, G.; Zhang, F.; Han, Y.; Wang, X.; Liu, X.; Xue, L. Temperature-Based Adhesion Tuning and Superwettability Switching on Superhydrophobic Aluminum Surface for Droplet Manipulations. Surf. Coat. Technol. 2019, 375, 527–533. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, S.; Frank Cheng, Y. Stearic Acid Modified Zinc Nano-Coatings with Superhydrophobicity and Enhanced Antifouling Performance. Surf. Coat. Technol. 2018, 340, 55–65. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, G. Superhydrophobic Coatings on Iodine Doped Substrate with Photothermal Deicing and Passive Anti-Icing Properties. Surf. Coat. Technol. 2020, 402, 126342. [Google Scholar] [CrossRef]
- Cao, K.; Yu, Z.; Zhu, L.; Yin, D.; Chen, L.; Jiang, Y.; Wang, J. Fabrication of Superhydrophobic Layered Double Hydroxide Composites to Enhance the Corrosion-Resistant Performances of Epoxy Coatings on Mg Alloy. Surf. Coat. Technol. 2021, 407, 126763. [Google Scholar] [CrossRef]
- Chobaomsup, V.; Metzner, M.; Boonyongmaneerat, Y. Superhydrophobic Surface Modification for Corrosion Protection of Metals and Alloys. J. Coat. Technol. Res. 2020, 17, 583–595. [Google Scholar] [CrossRef]
- Cui, M.; Xu, C.; Shen, Y.; Tian, H.; Feng, H.; Li, J. Electrospinning Superhydrophobic Nanofibrous Poly(Vinylidene Fluoride)/Stearic Acid Coatings with Excellent Corrosion Resistance. Thin Solid Films 2018, 657, 88–94. [Google Scholar] [CrossRef]
- Siddiqui, A.R.; Maurya, R.; Katiyar, P.K.; Balani, K. Superhydrophobic, Self-Cleaning Carbon Nanofiber CVD Coating for Corrosion Protection of AISI 1020 Steel and AZ31 Magnesium Alloys. Surf. Coat. Technol. 2020, 404, 126421. [Google Scholar] [CrossRef]
- Wang, S.; Xue, Y.; Ban, C.; Xue, Y.; Taleb, A.; Jin, Y. Fabrication of Robust Tungsten Carbide Particles Reinforced Co Ni Super-Hydrophobic Composite Coating by Electrochemical Deposition. Surf. Coat. Technol. 2020, 385, 125390. [Google Scholar] [CrossRef]
- Vanithakumari, S.C.; Kumar, C.A.; Thinaharan, C.; Kishor, G.R.; George, R.P.; Kaul, R.; Bindra, K.S.; John, P. Laser Patterned Titanium Surfaces with Superior Antibiofouling, Superhydrophobicity, Self-Cleaning and Durability: Role of Line Spacing. Surf. Coat. Technol. 2021, 418, 127257. [Google Scholar] [CrossRef]
- Fihri, A.; Bovero, E.; Al-Shahrani, A.; Al-Ghamdi, A.; Alabedi, G. Recent Progress in Superhydrophobic Coatings Used for Steel Protection: A Review. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 378–390. [Google Scholar] [CrossRef]
- Wu, S.; Du, Y.; Alsaid, Y.; Wu, D.; Hua, M.; Yan, Y.; Yao, B.; Ma, Y.; Zhu, X.; He, X. Superhydrophobic Photothermal Icephobic Surfaces Based on Candle Soot. Proc. Natl. Acad. Sci. USA 2020, 117, 11240–11246. [Google Scholar] [CrossRef] [PubMed]
- Esmeryan, K.D.; Castano, C.E.; Chaushev, T.A.; Mohammadi, R.; Vladkova, T.G. Silver-Doped Superhydrophobic Carbon Soot Coatings with Enhanced Wear Resistance and Anti-Microbial Performance. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123880. [Google Scholar] [CrossRef]
- Seo, K.; Kim, M.; Kim, D.H. Candle-Based Process for Creating a Stable Superhydrophobic Surface. Carbon 2014, 68, 583–596. [Google Scholar] [CrossRef]
- Xiao, L.; Zeng, W.; Liao, G.; Yi, C.; Xu, Z. Thermally and Chemically Stable Candle Soot Superhydrophobic Surface with Excellent Self-Cleaning Properties in Air and Oil. ACS Appl. Nano Mater. 2018, 1, 1204–1211. [Google Scholar] [CrossRef]
- Sutar, R.S.; Latthe, S.S.; Nagappan, S.; Ha, C.; Sadasivuni, K.K.; Liu, S.; Xing, R.; Bhosale, A.K. Fabrication of Robust Self-cleaning Superhydrophobic Coating by Deposition of Polymer Layer on Candle Soot Surface. J. Appl. Polym. Sci. 2021, 138, 49943. [Google Scholar] [CrossRef]
- Samanta, A.; Wang, Q.; Shaw, S.K.; Ding, H. Roles of Chemistry Modification for Laser Textured Metal Alloys to Achieve Extreme Surface Wetting Behaviors. Mater. Des. 2020, 192, 108744. [Google Scholar] [CrossRef]
- Huang, R.C.; Anand, L. Non-Linear Mechanical Behavior of the Elastomer Polydimethylsiloxane (PDMS) Used in the Manufacture of Microfluidic Devices; Massachusetts Institute of Technology: Cambridge, MA, USA, 2005. [Google Scholar]
- Quan, Y.-Y.; Chen, Z.; Lai, Y.; Huang, Z.-S.; Li, H. Recent Advances in Fabricating Durable Superhydrophobic Surfaces: A Review in the Aspects of Structures and Materials. Mater. Chem. Front. 2021, 5, 1655–1682. [Google Scholar] [CrossRef]
- Liang, C.-J.; Liao, J.-D.; Li, A.-J.; Chen, C.; Lin, H.-Y.; Wang, X.-J.; Xu, Y.-H. Relationship between Wettabilities and Chemical Compositions of Candle Soots. Fuel 2014, 128, 422–427. [Google Scholar] [CrossRef]
- Karthik, N.; Sethuraman, M.G. Fabrication of Micro-Nanocomposite Coatings with Lotus Leaf like Texture by Combining Electroless and Candle Soot Depositions. New J. Chem. 2015, 39, 3337–3340. [Google Scholar] [CrossRef]
- Cao, H.; Fu, J.; Liu, Y.; Chen, S. Facile Design of Superhydrophobic and Superoleophilic Copper Mesh Assisted by Candle Soot for Oil Water Separation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 294–302. [Google Scholar] [CrossRef]
- Zhao, Y.; Huo, M.; Huo, J.; Zhang, P.; Shao, X.; Zhang, X. Preparation of Silica-Epoxy Superhydrophobic Coating with Mechanical Stability and Multifunctional Performance via One-Step Approach. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129957. [Google Scholar] [CrossRef]
- Wei, D.; Wang, J.; Liu, Y.; Wang, D.; Li, S.; Wang, H. Controllable Superhydrophobic Surfaces with Tunable Adhesion on Mg Alloys by a Simple Etching Method and Its Corrosion Inhibition Performance. Chem. Eng. J. 2021, 404, 126444. [Google Scholar] [CrossRef]
- Chang, Z.; Lu, Y. Fabrication of Superhydrophobic Surfaces with Cassie-Baxter State. J. Dispers. Sci. Technol. 2022, 43, 1099–1111. [Google Scholar] [CrossRef]
- Parkin, I.P.; Palgrave, R.G. Self-Cleaning Coatings. J. Mater. Chem. 2005, 15, 1689. [Google Scholar] [CrossRef]
- Yu, C.; Sasic, S.; Liu, K.; Salameh, S.; Ras, R.H.A.; van Ommen, J.R. Nature–Inspired Self–Cleaning Surfaces: Mechanisms, Modelling, and Manufacturing. Chem. Eng. Res. Des. 2020, 155, 48–65. [Google Scholar] [CrossRef]
- Deng, W.; Long, M.; Miao, X.; Wen, N.; Deng, W. Eco-Friendly Preparation of Robust Superhydrophobic Cu(OH)2 Coating for Self-Cleaning, Oil-Water Separation and Oil Sorption. Surf. Coat. Technol. 2017, 325, 14–21. [Google Scholar] [CrossRef]
- Geyer, F.; D’Acunzi, M.; Sharifi-Aghili, A.; Saal, A.; Gao, N.; Kaltbeitzel, A.; Sloot, T.-F.; Berger, R.; Butt, H.-J.; Vollmer, D. When and How Self-Cleaning of Superhydrophobic Surfaces Works. Sci. Adv. 2020, 6, eaaw9727. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sudhagar, P.; Devadoss, A.; Kumar, A.M.; Liu, S.; Terashima, C.; Nakata, K.; Fujishima, A. A Mechanically Bendable Superhydrophobic Steel Surface with Self-Cleaning and Corrosion-Resistant Properties. J. Mater. Chem. A 2015, 3, 14263–14271. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Liu, Y.; Zhou, H.; Tian, G. Preparation and Evaluation of PDMS/Carbon Soot Particles Superhydrophobic Biomimetic Composite Coating with Self-Cleaning and Durability. Biomimetics 2022, 7, 132. https://doi.org/10.3390/biomimetics7030132
Li F, Liu Y, Zhou H, Tian G. Preparation and Evaluation of PDMS/Carbon Soot Particles Superhydrophobic Biomimetic Composite Coating with Self-Cleaning and Durability. Biomimetics. 2022; 7(3):132. https://doi.org/10.3390/biomimetics7030132
Chicago/Turabian StyleLi, Fengqin, Yong Liu, Honggen Zhou, and Guizhong Tian. 2022. "Preparation and Evaluation of PDMS/Carbon Soot Particles Superhydrophobic Biomimetic Composite Coating with Self-Cleaning and Durability" Biomimetics 7, no. 3: 132. https://doi.org/10.3390/biomimetics7030132
APA StyleLi, F., Liu, Y., Zhou, H., & Tian, G. (2022). Preparation and Evaluation of PDMS/Carbon Soot Particles Superhydrophobic Biomimetic Composite Coating with Self-Cleaning and Durability. Biomimetics, 7(3), 132. https://doi.org/10.3390/biomimetics7030132