Effect of Treated Time of Hydrothermal Etching Process on Oxide Layer Formation and Its Antibacterial Properties
Abstract
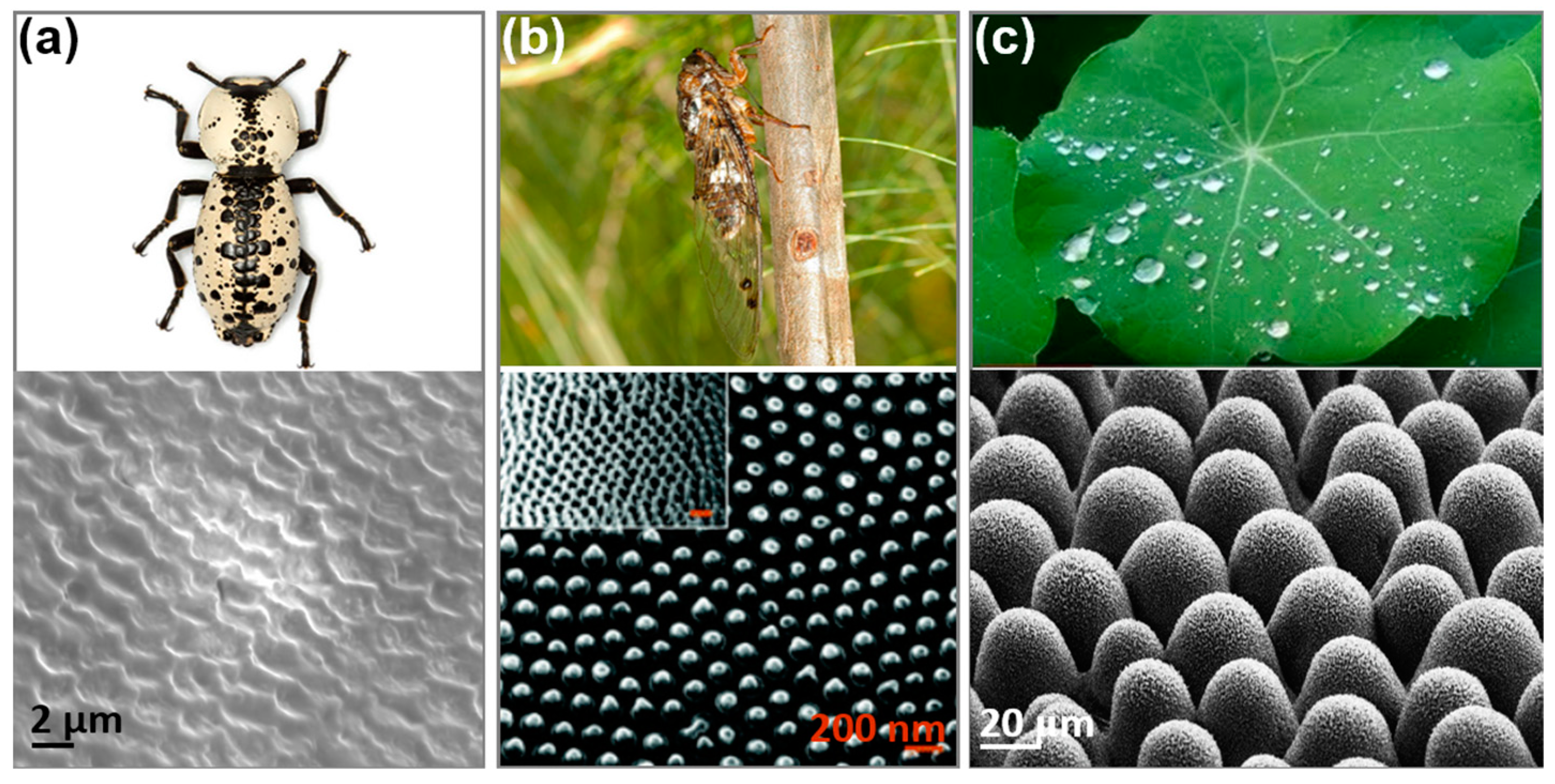
:1. Introduction
2. Materials and Methods
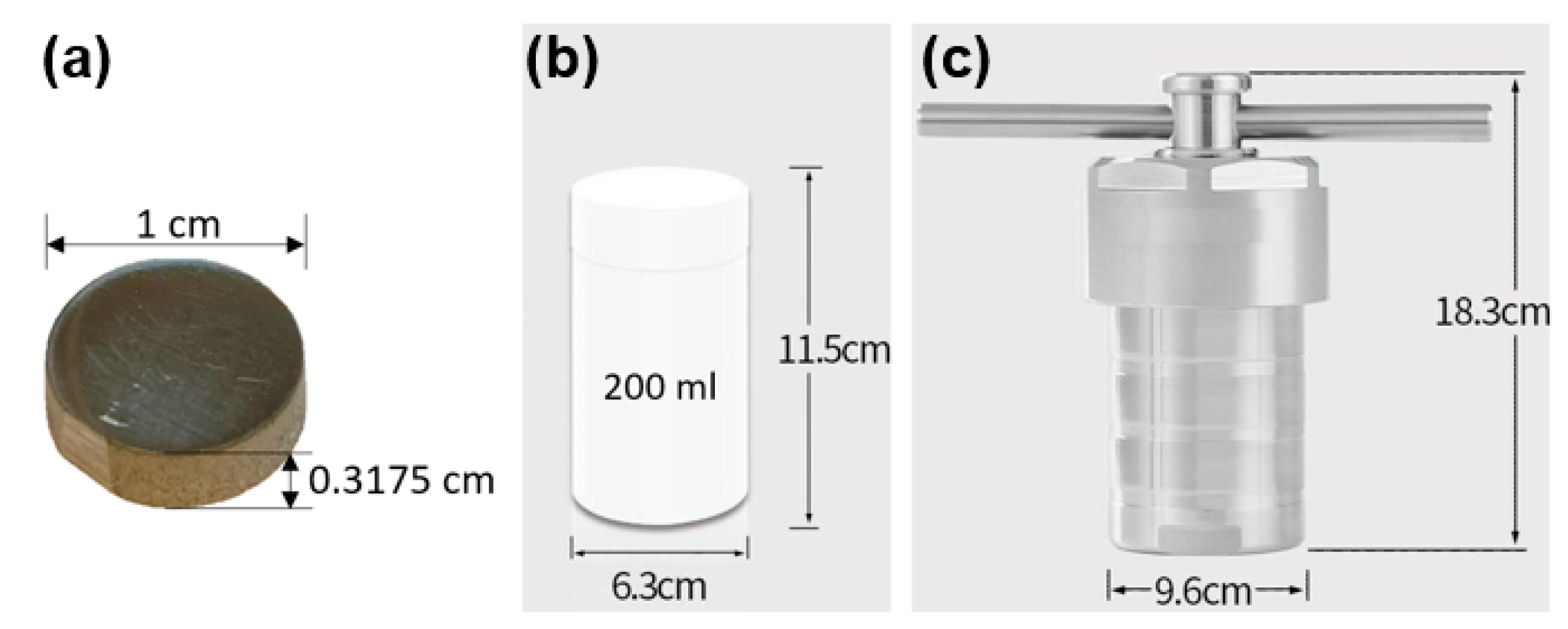
2.1. Hydrothermal Etching Procedure to Generate TiO2 Surface
2.2. Surface Characterization
2.3. Bacterial Adherence Assay
3. Results and Discussion
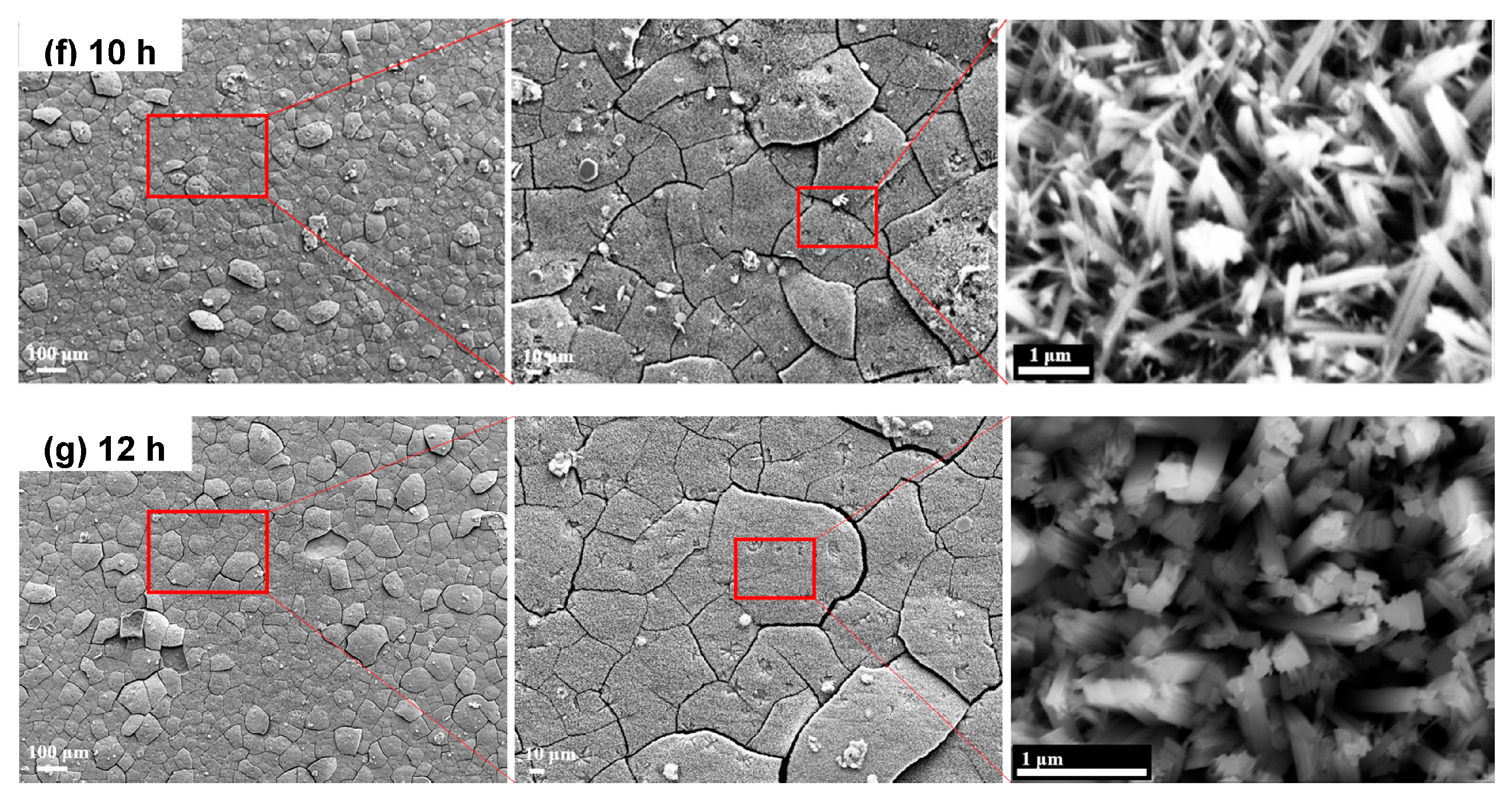
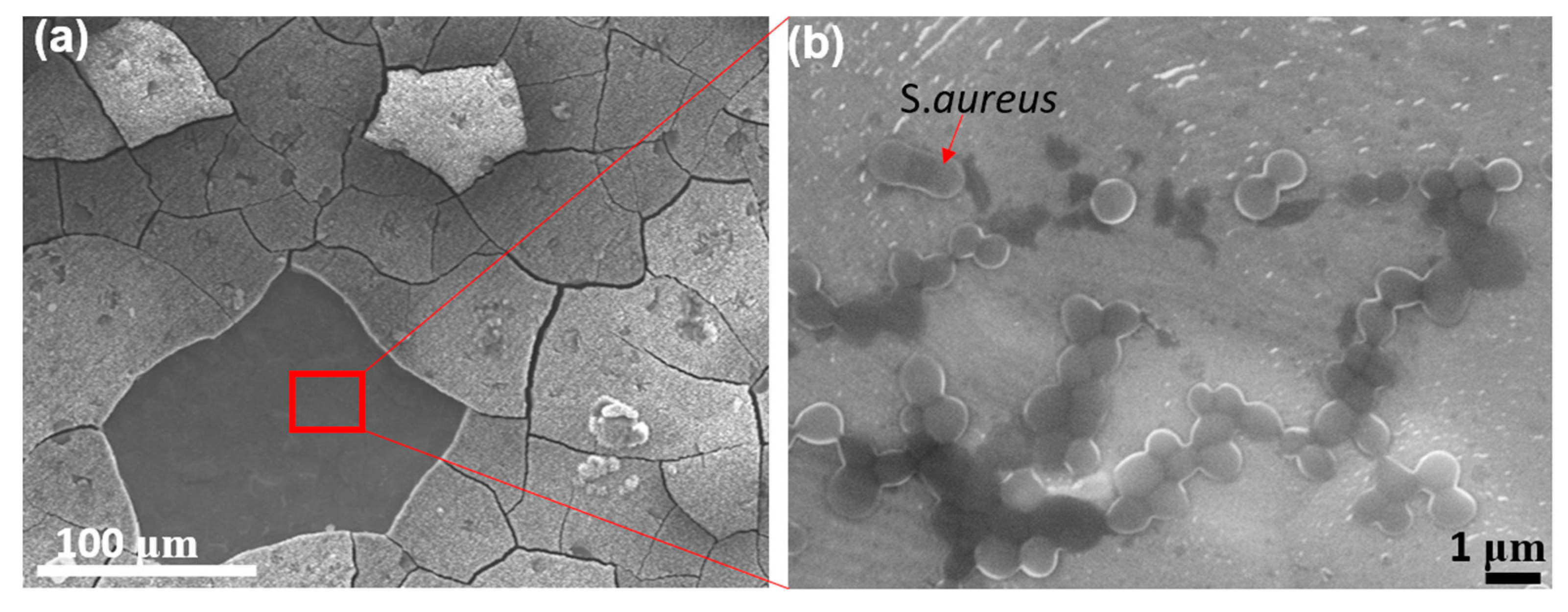
3.1. Characterization of Microstructure (SEM)
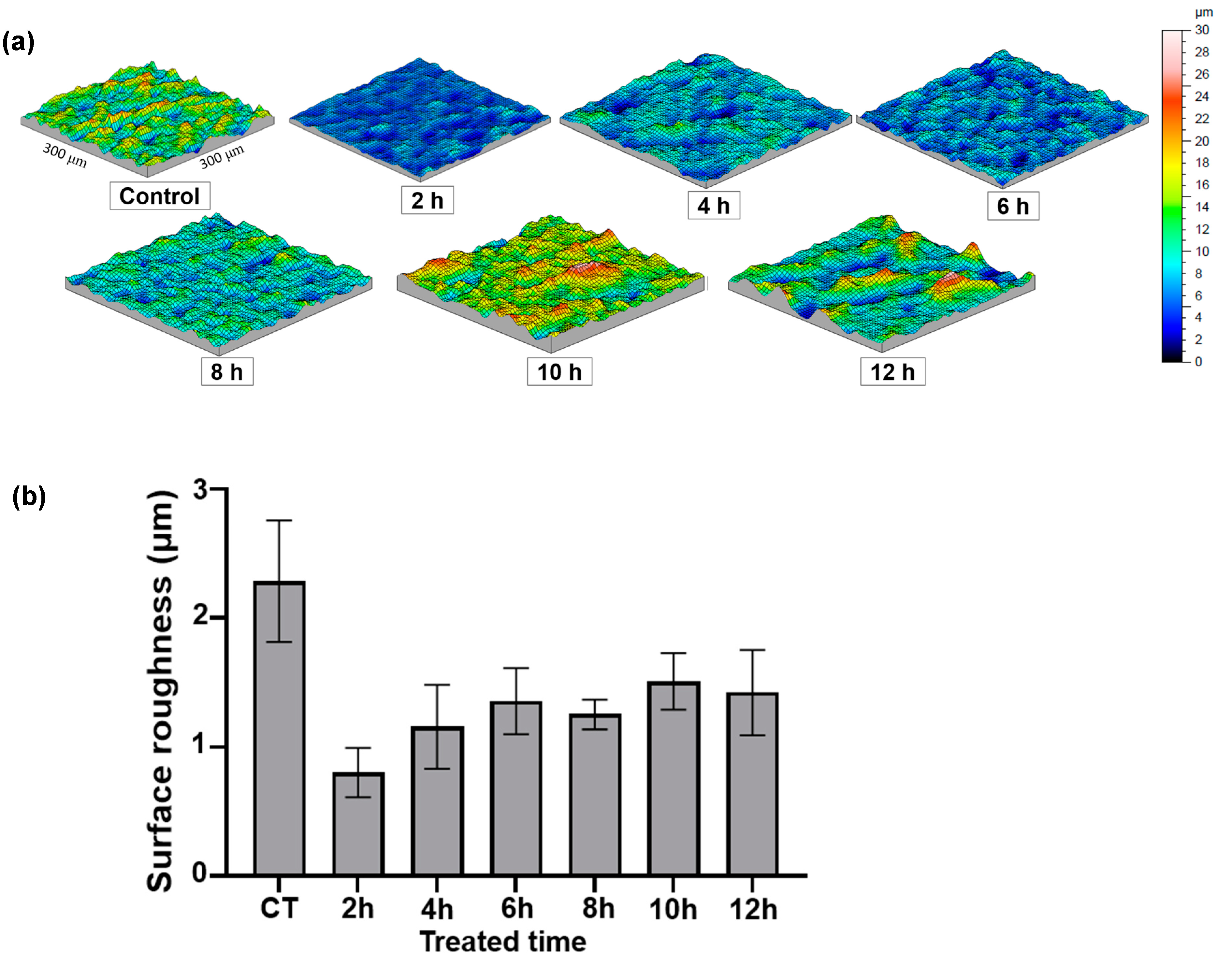
3.2. Surface Topography
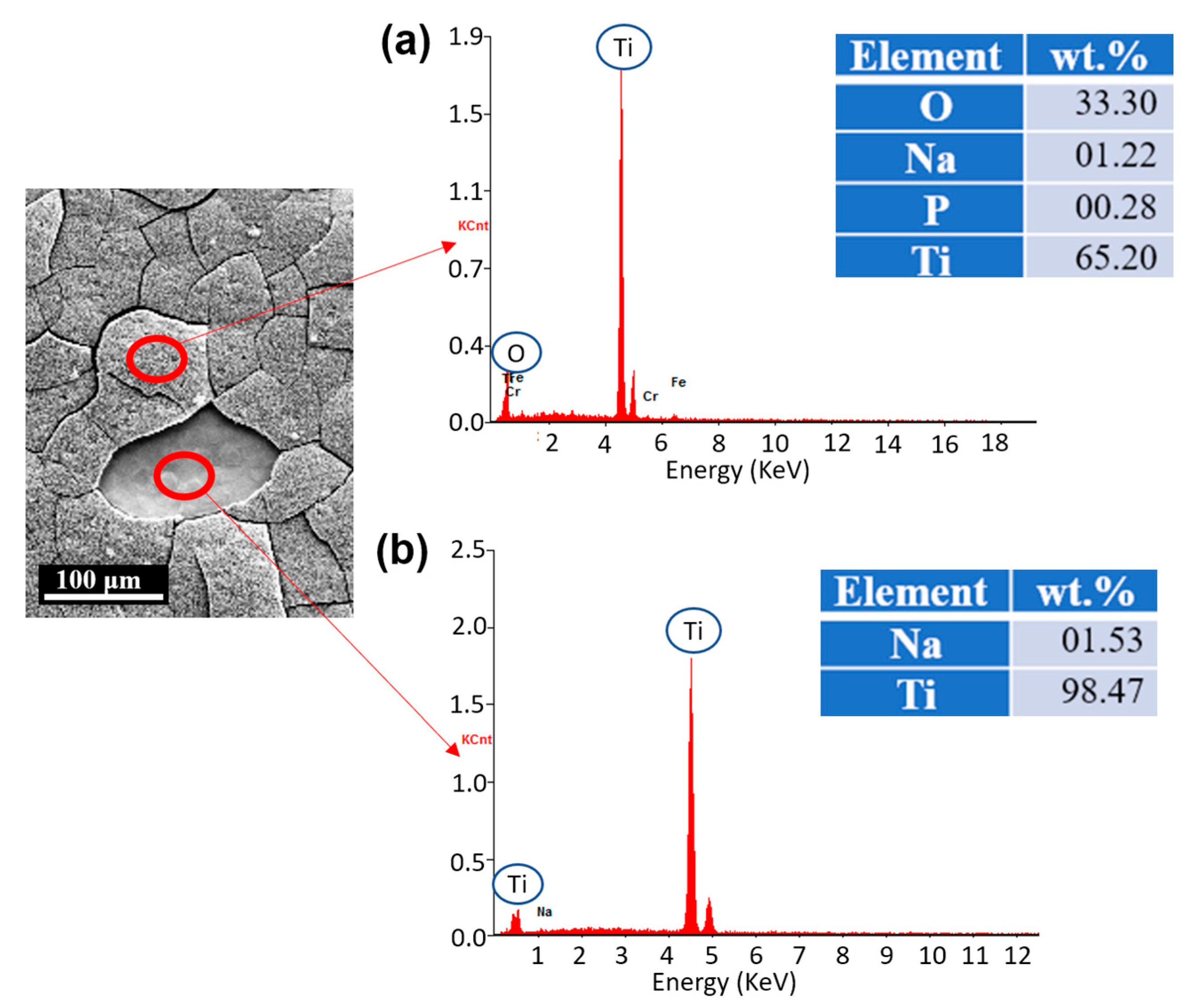
3.3. Chemical Compositions (EDS)
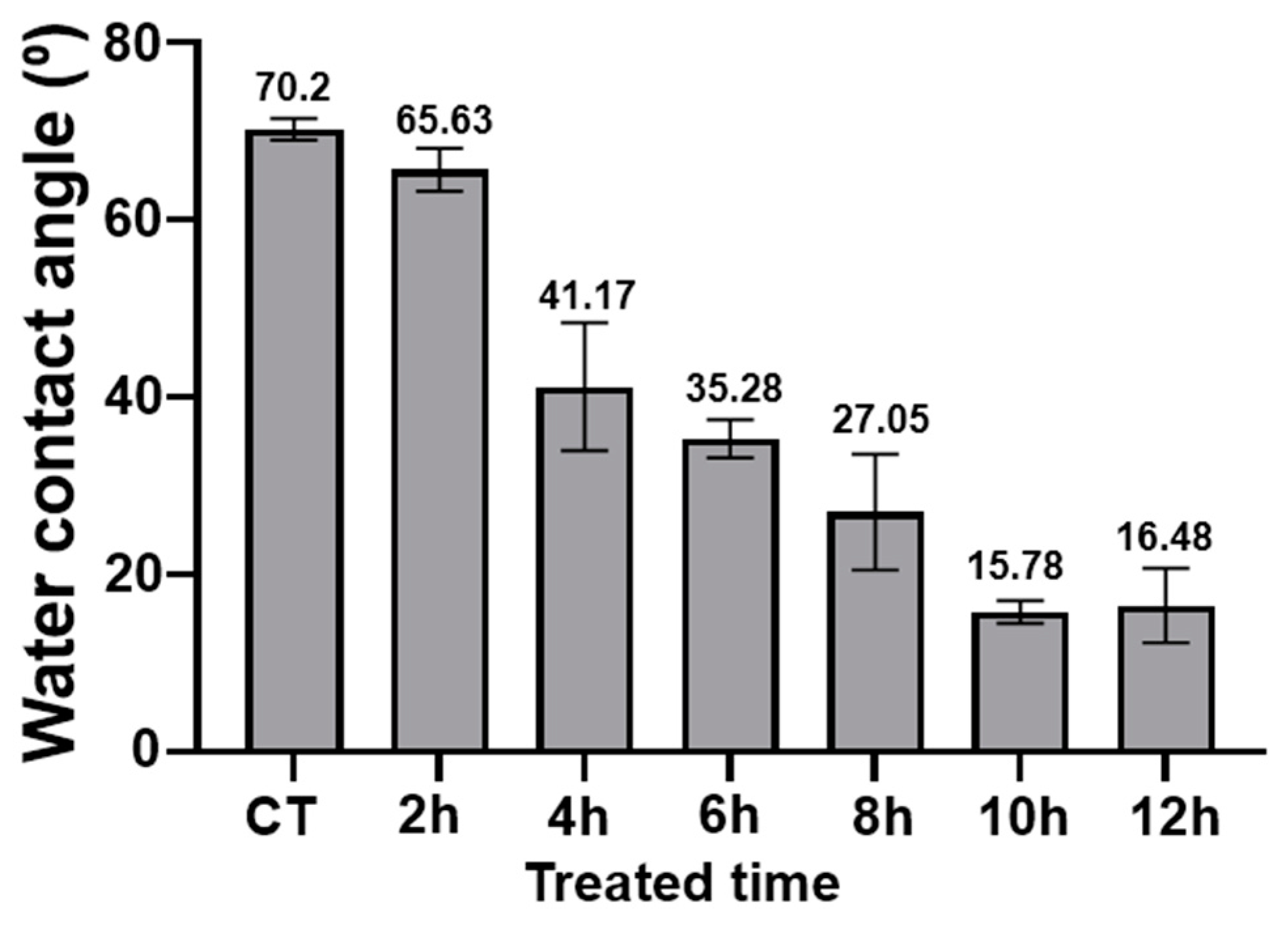
3.4. Wettability Test
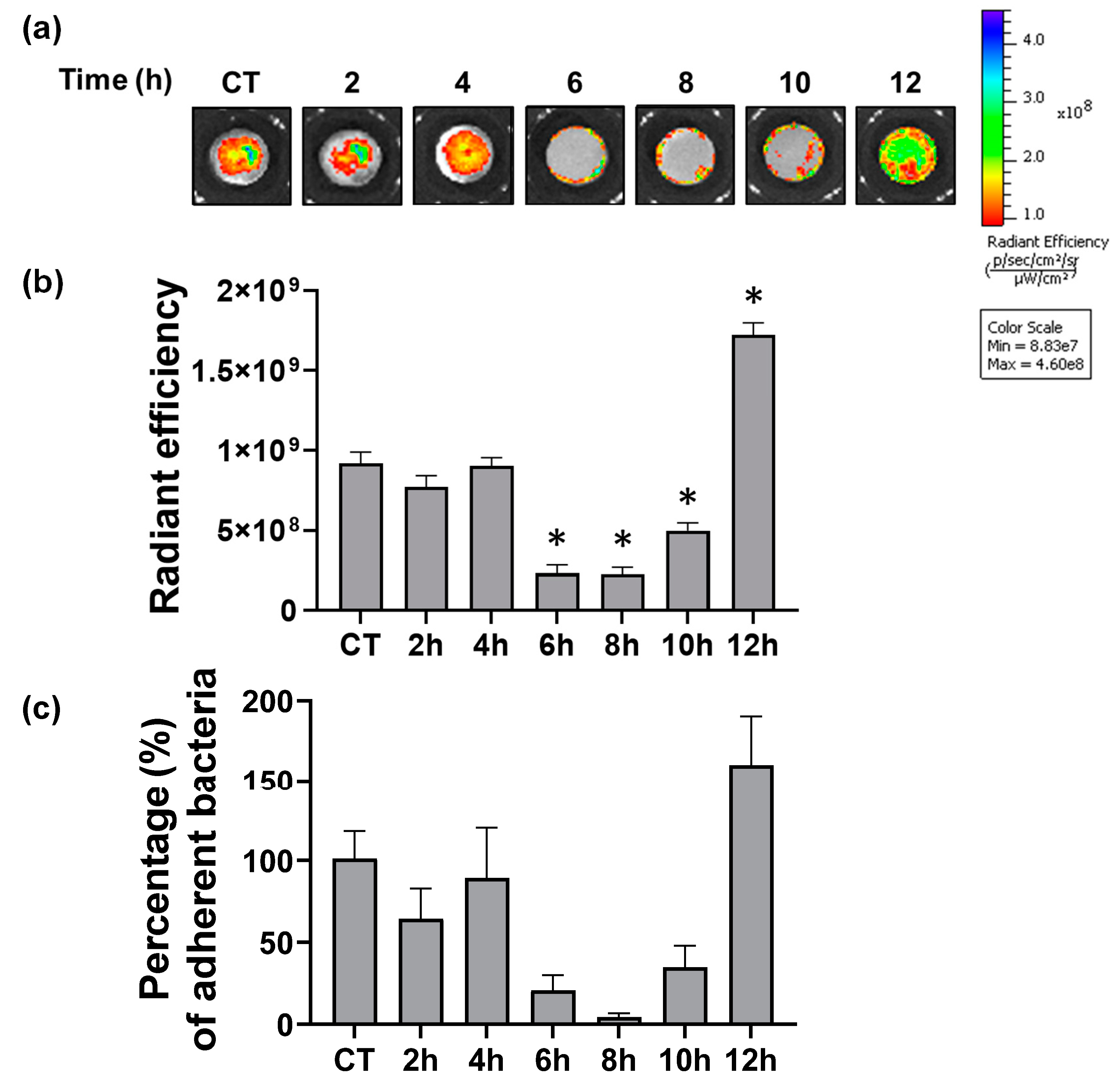
3.5. Antibacterial Effects
3.6. Problem of Cracking on the Oxide Layer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niinomi, M. Mechanical Biocompatibilities of Titanium Alloys for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Boehlert, C.J. Titanium Alloys for Biomedical Applications. In Advances in Metallic Biomaterials: Tissues, Materials and Biological Reactions; Niinomi, M., Narushima, T., Nakai, M., Eds.; Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2015; pp. 179–213. ISBN 978-3-662-46836-4. [Google Scholar]
- VanEpps, J.S.; Younger, J.G. Implantable Device Related Infection. Shock 2016, 46, 597–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, H.-C.; Neu, T.R.; Wozniak, D.J. The EPS Matrix: The “House of Biofilm Cells”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prevention and Control of Biofilm-Based Medical-Device-Related Infections|Pathogens and Disease|Oxford Academic. Available online: https://academic.oup.com/femspd/article/59/3/227/495267?login=true (accessed on 10 August 2021).
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K.D.V. Bio-Mimicking Nano and Micro-Structured Surface Fabrication for Antibacterial Properties in Medical Implants. J. Nanobiotechnol. 2017, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, J. Review on Antimicrobial Resistance: Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust: London, UK, 2016. [Google Scholar]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial Coatings on Titanium Implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef]
- Hori, K.; Matsumoto, S. Bacterial Adhesion: From Mechanism to Control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Hong, E.-J.; Na, K.-J.; Choi, I.-G.; Choi, K.-C.; Jeung, E.-B. Antibacterial and Antifungal Effects of Essential Oils from Coniferous Trees. Biol. Pharm. Bull. 2004, 27, 863–866. [Google Scholar] [CrossRef] [Green Version]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants with Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef]
- Gross, J.; Schumacher, K.; Schmidtberg, H.; Vilcinskas, A. Protected by Fumigants: Beetle Perfumes in Antimicrobial Defense. J. Chem. Ecol. 2008, 34, 179. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Lee, J.; Kim, Y.-J.; Bang, H.-S.; Yun, E.-Y.; Kim, S.-R.; Suh, H.-J.; Kang, B.-R.; Nam, S.-H.; Jeon, J.-P.; et al. Isolation and Characterization of a Defensin-Like Peptide (Coprisin) from the Dung Beetle, Copris Tripartitus. Int. J. Pept. 2009, 2009, 136284. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Sonnevend, A. Antimicrobial Peptides in Frog Skin Secretions. Methods Mol. Biol. 2010, 618, 3–14. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and Bioinspired Nanostructured Bactericidal Surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Bandara, C.D.; Singh, S.; Afara, I.O.; Wolff, A.; Tesfamichael, T.; Ostrikov, K.; Oloyede, A. Bactericidal Effects of Natural Nanotopography of Dragonfly Wing on Escherichia Coli. ACS Appl. Mater. Interfaces 2017, 9, 6746–6760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishnu, J.; Manivasagam, V.K.; Gopal, V.; Bartomeu Garcia, C.; Hameed, P.; Manivasagam, G.; Webster, T.J. Hydrothermal Treatment of Etched Titanium: A Potential Surface Nano-Modification Technique for Enhanced Biocompatibility. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102016. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial Effects of Nanopillar Surfaces Are Mediated by Cell Impedance, Penetration and Induction of Oxidative Stress. Nat. Commun. 2020, 11, 1626. [Google Scholar] [CrossRef]
- Lee, N.; Berthelson, P.R.; Nguyen, V.; Brinda, A.K.; Moser, R.D.; Horstemeyer, M.; Rhee, H.; Prabhu, R. Microstructure and Nanomechanical Properties of the Exoskeleton of an Ironclad Beetle (Zopherus Haldemani). Bioinspiration Biomim. 2021, 16, 036005. [Google Scholar] [CrossRef]
- Sun, J.; Tong, J.; Zhang, Z. Nanomechanical Properties and the Hierarchical Structure of Elytra Cuticle of Dung Beetle (Copris Ochus Motschulsky). In Proceedings of the 2009 International Conference on Mechatronics and Automation, Changchun, Beijing, 9–12 August 2009; pp. 4277–4282. [Google Scholar]
- Shahali, H.; Hasan, J.; Mathews, A.; Wang, H.; Yan, C.; Tesfamichael, T.; Yarlagadda, P.K.D.V. Multi-Biofunctional Properties of Three Species of Cicada Wings and Biomimetic Fabrication of Nanopatterned Titanium Pillars. J. Mater. Chem. B 2019, 7, 1300–1310. [Google Scholar] [CrossRef]
- Barthlott, W.; Mail, M.; Bhushan, B.; Koch, K. Plant Surfaces: Structures and Functions for Biomimetic Innovations. Nano-Micro Lett. 2017, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Davies, D. Understanding Biofilm Resistance to Antibacterial Agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of Antibiotic Penetration Limitation in Klebsiella Pneumoniae Biofilm Resistance to Ampicillin and Ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm Formation in Staphylococcus Implant Infections. A Review of Molecular Mechanisms and Implications for Biofilm-Resistant Materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef] [PubMed]
- Campanac, C.; Pineau, L.; Payard, A.; Baziard-Mouysset, G.; Roques, C. Interactions between Biocide Cationic Agents and Bacterial Biofilms. Antimicrob. Agents Chemother. 2002, 46, 1469–1474. [Google Scholar] [CrossRef] [Green Version]
- Hayles, A.; Hasan, J.; Bright, R.; Palms, D.; Brown, T.; Barker, D.; Vasilev, K. Hydrothermally Etched Titanium: A Review on a Promising Mechano-Bactericidal Surface for Implant Applications. Mater. Today Chem. 2021, 22, 100622. [Google Scholar] [CrossRef]
- López-Huerta, F.; Cervantes, B.; González, O.; Hernández-Torres, J.; García-González, L.; Vega, R.; Herrera-May, A.L.; Soto, E. Biocompatibility and Surface Properties of TiO2 Thin Films Deposited by DC Magnetron Sputtering. Materials 2014, 7, 4105–4117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishak, M.I.; Liu, X.; Jenkins, J.; Nobbs, A.H.; Su, B. Protruding Nanostructured Surfaces for Antimicrobial and Osteogenic Titanium Implants. Coatings 2020, 10, 756. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.; Lamb, R.N.; Baulin, V.A.; Watson, G.S. Bactericidal Activity of Black Silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Elliott, D.T.; Wiggins, R.J.; Dua, R. Bioinspired Antibacterial Surface for Orthopedic and Dental Implants. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2021, 109, 973–981. [Google Scholar] [CrossRef]
- Shah, F.A.; Trobos, M.; Thomsen, P.; Palmquist, A. Commercially Pure Titanium (Cp-Ti) versus Titanium Alloy (Ti6Al4V) Materials as Bone Anchored Implants—Is One Truly Better than the Other? Mater Sci. Eng. C Mater Biol. Appl. 2016, 62, 960–966. [Google Scholar] [CrossRef]
- Shen, X.; Shukla, P. A Review of Titanium Based Orthopaedic Implants (Part-I): Physical Characteristics, Problems and the Need for Surface Modification. Int. J. Peen. Sci. Technol. 2020, 1, 301–332. [Google Scholar]
- Leshuk, T.; Linley, S.; Baxter, G.; Gu, F. Mesoporous Hollow Sphere Titanium Dioxide Photocatalysts through Hydrothermal Silica Etching. ACS Appl. Mater. Interfaces 2012, 4, 6062–6070. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lee, S.; Bajpai, I.; Kim, S. Hydrothermal Treatment of Ti Surface to Enhance the Formation of Low Crystalline Hydroxyl Carbonate Apatite. Biomater. Res. 2015, 19, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, A.D.; Otto, M.; Braughton, K.R.; Whitney, A.R.; Chen, L.; Mathema, B.; Mediavilla, J.R.; Byrne, K.A.; Parkins, L.D.; Tenover, F.C.; et al. Epidemic Community-Associated Methicillin-Resistant Staphylococcus Aureus: Recent Clonal Expansion and Diversification. Proc. Natl. Acad. Sci. USA 2008, 105, 1327–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Webster, T.J. Bacteria Antibiotic Resistance: New Challenges and Opportunities for Implant-Associated Orthopedic Infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Minkiewicz-Zochniak, A.; Jarzynka, S.; Iwańska, A.; Strom, K.; Iwańczyk, B.; Bartel, M.; Mazur, M.; Pietruczuk-Padzik, A.; Konieczna, M.; Augustynowicz-Kopeć, E.; et al. Biofilm Formation on Dental Implant Biomaterials by Staphylococcus Aureus Strains Isolated from Patients with Cystic Fibrosis. Materials 2021, 14, 2030. [Google Scholar] [CrossRef]
- Paulitsch-Fuchs, A.H.; Wolrab, L.; Eck, N.; Dyer, N.P.; Bödendorfer, B.; Lohberger, B. TiAl6V4 Alloy Surface Modifications and Their Impact on Biofilm Development of S. Aureus and S. Epidermidis. J. Funct. Biomater. 2021, 12, 36. [Google Scholar] [CrossRef]
- De Jong, N.W.M.; Van Der Horst, T.; van Strijp, J.A.G.; Nijland, R. Fluorescent Reporters for Markerless Genomic Integration in Staphylococcus Aureus. Sci. Rep. 2017, 7, 43889. [Google Scholar] [CrossRef]
- Anitha, V.C.; Banerjee, A.N.; Joo, S.W.; Min, B.K. Morphology-Dependent Low Macroscopic Field Emission Properties of Titania/Titanate Nanorods Synthesized by Alkali-Controlled Hydrothermal Treatment of a Metallic Ti Surface. Nanotechnology 2015, 26, 355705. [Google Scholar] [CrossRef]
- López Zavala, M.Á.; Lozano Morales, S.A.; Ávila-Santos, M. Synthesis of Stable TiO2 Nanotubes: Effect of Hydrothermal Treatment, Acid Washing and Annealing Temperature. Heliyon 2017, 3, e00456. [Google Scholar] [CrossRef]
- Bhadra, C.M.; Khanh Truong, V.; Pham, V.T.H.; Al Kobaisi, M.; Seniutinas, G.; Wang, J.Y.; Juodkazis, S.; Crawford, R.J.; Ivanova, E.P. Antibacterial Titanium Nano-Patterned Arrays Inspired by Dragonfly Wings. Sci. Rep. 2015, 5, 16817. [Google Scholar] [CrossRef] [Green Version]
- Mohandas, A.; Krishnan, A.G.; Biswas, R.; Menon, D.; Nair, M.B. Antibacterial and Cytocompatible Nanotextured Ti Surface Incorporating Silver via Single Step Hydrothermal Processing. Mater. Sci. Eng. C 2017, 75, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Tsimbouri, P.M.; Fisher, L.; Holloway, N.; Sjostrom, T.; Nobbs, A.H.; Meek, R.M.D.; Su, B.; Dalby, M.J. Osteogenic and Bactericidal Surfaces from Hydrothermal Titania Nanowires on Titanium Substrates. Sci. Rep. 2016, 6, 36857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Sun, H.; Qin, Z.; Yin, S.; Tian, L.; Liu, Z. Bioinspired Photocatalytic ZnO/Au Nanopillar-Modified Surface for Enhanced Antibacterial and Antiadhesive Property. Chem. Eng. J. 2020, 398, 125575. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, C.H.; Jeong, S.W.; Song, Y.; Bae, N.H.; Lee, S.J.; Lee, K.G. Ultrasonic Fabrication of Flexible Antibacterial ZnO Nanopillar Array Film. Colloids Surf. B Biointerfaces 2018, 170, 172–178. [Google Scholar] [CrossRef]
- Sjöström, T.; Nobbs, A.H.; Su, B. Bactericidal Nanospike Surfaces via Thermal Oxidation of Ti Alloy Substrates. Mater. Lett. 2016, 167, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Lechner, J.; Noumbissi, S.; von Baehr, V. Titanium Implants and Silent Inflammation in Jawbone—a Critical Interplay of Dissolved Titanium Particles and Cytokines TNF-α and RANTES/CCL5 on Overall Health? EPMA J. 2018, 9, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Bavykin, D.V.; Carravetta, M.; Kulak, A.N.; Walsh, F.C. Application of Magic-Angle Spinning NMR to Examine the Nature of Protons in Titanate Nanotubes. Chem. Mater. 2010, 22, 2458–2465. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A Review on TiO2-Based Nanotubes Synthesized via Hydrothermal Method: Formation Mechanism, Structure Modification, and Photocatalytic Applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]

| Time (hours) | Molarity | Temperature | |
|---|---|---|---|
| Control | 0 | 5 Mol | 250 °C |
| Condition 1 | 2 | ||
| Condition 2 | 4 | ||
| Condition 3 | 6 | ||
| Condition 4 | 8 | ||
| Condition 5 | 10 | ||
| Condition 6 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, N.; Park, J.; Miralami, R.; Yu, F.; Skaines, N.; Armstrong, M.; McDonald, R.; Moore, E.; Viveros, A.; Borow, N.; et al. Effect of Treated Time of Hydrothermal Etching Process on Oxide Layer Formation and Its Antibacterial Properties. Biomimetics 2022, 7, 91. https://doi.org/10.3390/biomimetics7030091
Lee N, Park J, Miralami R, Yu F, Skaines N, Armstrong M, McDonald R, Moore E, Viveros A, Borow N, et al. Effect of Treated Time of Hydrothermal Etching Process on Oxide Layer Formation and Its Antibacterial Properties. Biomimetics. 2022; 7(3):91. https://doi.org/10.3390/biomimetics7030091
Chicago/Turabian StyleLee, Nayeon, Jooyoun Park, Raheleh Miralami, Fei Yu, Nikole Skaines, Megan Armstrong, Rachel McDonald, Emily Moore, Alicia Viveros, Nicholas Borow, and et al. 2022. "Effect of Treated Time of Hydrothermal Etching Process on Oxide Layer Formation and Its Antibacterial Properties" Biomimetics 7, no. 3: 91. https://doi.org/10.3390/biomimetics7030091






