Biomimetic Approaches in Clinical Endodontics
Abstract
:1. Introduction
2. Development of Regenerative Endodontic Procedures (REP)
3. Revascularization or Revitalization
3.1. Advantages of the Revascularization Approach
- Technically simple approach.
- There is no need of using expensive biotechnology due to currently available instruments and medicament techniques.
- There are almost negligible chances of immune rejection as this approach relies on the patient’s own blood.
- Bacterial microleakage can be eliminated through the induction of stem cells into the root canal space, followed by the intra-canal barrier, inducing a blood clot.
- The concerns of restoration retention need to be overcome.
- When this approach is applied to immature teeth, it reinforces their root walls.
- As the avulsed immature tooth has necrotic-pulp tissue along with an open apex, and short and intact roots; therefore, the newly formed tissue will easily reach the coronal-pulp horn because proliferation in a short distance is required. Therefore, the strategy behind the development of new tissue is to maintain the balance between the pulp-space infection and the proliferation of new tissue.
- Additional growth of open-apex root takes place due to minimum instrumentation that will preserve viable pulp tissue.
- The potential to regenerate more stem cells and the rapid capacity to heal the tissue in young patients needs to be recognised (Table 1).
3.2. Disadvantages of the Revascularization Approach
- The origin of where the tissue has been regenerated from is yet to be known.
- According to researchers, effective composition and concentration of cells are mandatory for tissue engineering. However, these cells are entombed in fibrin clots; therefore, researchers do not rely on blood-clot formation for tissue engineering function.
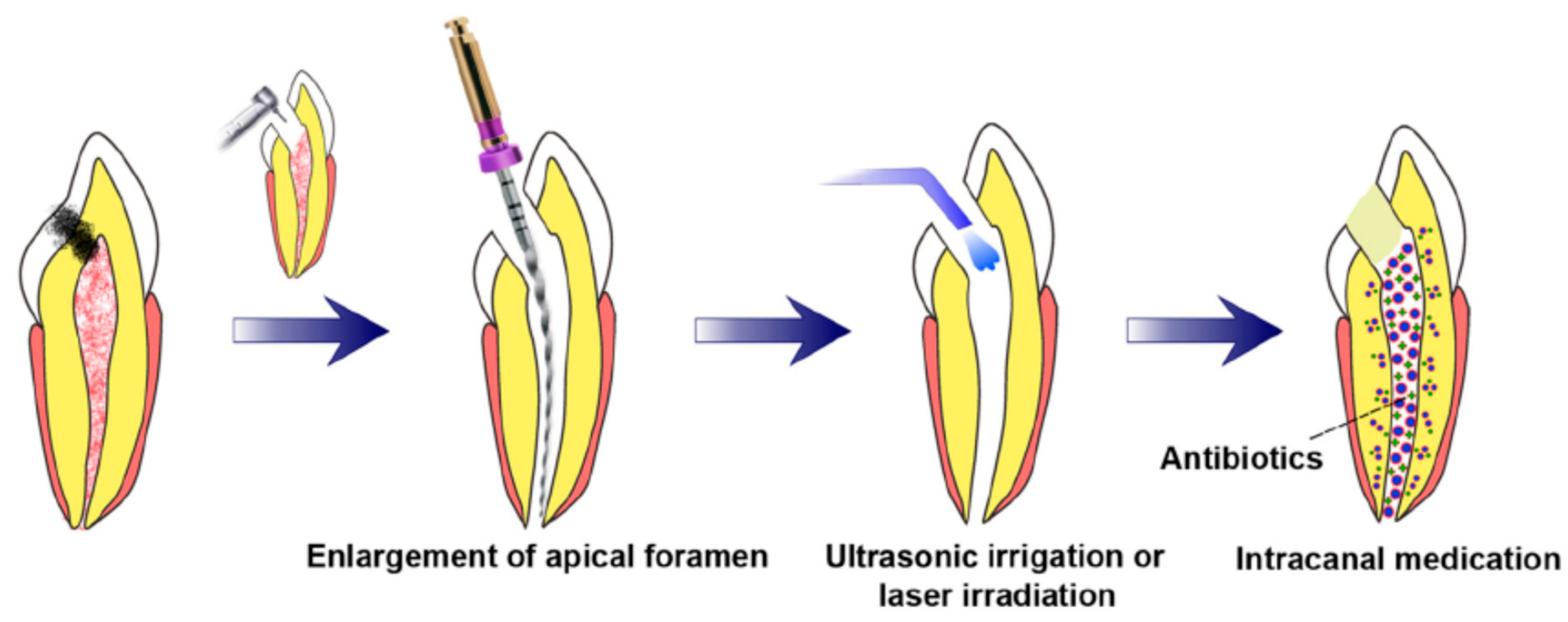
3.3. Prerequisites for Revascularization Approach (Figure 1)
- There should be open apices and necrotic pulp secondary to trauma.
- In addition, open apex should be less than 1.5 mm.
- The following agents can be incorporated to remove microorganisms from the canal.
- The coronal seal should be effective.
- There should be a matrix or the growth of new tissues.
- When trying to induce bleeding, anaesthesia should be used without a vasoconstrictor [70].
- Canals should not be instrumented.
- Sodium hypochlorite should be used as the irrigant.
- There should be blood-clot formation (Table 1).
4. Postnatal Stem Cell Therapy
4.1. Pulp Implantation
4.2. Scaffold Implantation
4.3. Three-Dimensional Cell Printing
4.4. Gene Therapy
4.5. Nitric Oxide
4.6. Platelet-Rich Plasma (PRP)
4.7. Cell Homing


5. Biomimetic Materials in Endodontics
5.1. Biointeractive Materials
5.1.1. Calcium Hydroxide
5.1.2. Calcium Sulfate
5.2. Bioactive Materials
5.2.1. Calcium Silicate Based-Cements
Mineral Trioxide Aggregate
Biodentine
Calcium Aluminate Cement
Theracal
5.2.2. Calcium Phosphate Based Cements
Hydroxyapatite
Bioactive Glass
5.2.3. Mixture of Calcium Silicate and Phosphate Based-Cements
Bioaggregate
Endosequence Root Repair Material
5.2.4. Sealer
Endosequence BC Sealer
5.2.5. Gutta-Percha
Bioceramic Coated Gutta-Percha
5.3. Remineralizing Agents
5.3.1. Enamel Matrix Derivative (Emdogain) Remineralizing Agent
5.3.2. Dentine Matrix Derivative/Demineralized Dentin Matrix
5.4. Miscellaneous
Calcium Phosphate Cements
6. Challenges in Regenerative Endodontics
- To induce bleeding, the clinicians should use anaesthesia without a vasoconstrictor.
- As discussed earlier, to place biomimetic-endodontic materials at the controlled and optimal level, the matrix of collagen could be helpful.
- Specifically, when treating anterior teeth and generally for all dentitions, patients/parents should be informed about the staining potential as this paste contains minocycline.
- Patients/parents should be informed about the multiple visits for proper case selection [70].
7. Clinical Outcomes of Regenerative Endodontics Procedure
- Primary goal: This is an essential goal. It consists of elimination of signs, symptoms, and bony healing.
- Secondary goal: This is a desirable goal. In this, there will be increased root length and root-wall thickness.
- Tertiary goal: Vitality testing is positive.
8. Future Perspective on the Regeneration of the Dental Pulp
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benyus, J.M. Biomimicry: Innovation Inspired by Nature; Morrow: New York, NY, USA, 1997. [Google Scholar]
- Vakili, V.; Shu, L.H. (Eds.) Towards biomimetic concept generation. In International Design Engineering Technical Conferences and Computers and Information in Engineering Conference; American Society of Mechanical Engineers: New York, NY, USA, 2001. [Google Scholar]
- Sonarkar, S.; Purba, R. Bioactive materials in conservative dentistry. Int. J. Contemp. Dent. Med. Rev. 2015, 2015, 340115. [Google Scholar]
- Harkness, J.M. An idea man (the life of Otto Herbert Schmitt). IEEE Eng. Med. Biol. Mag. 2004, 23, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic Aspects of Restorative Dentistry Biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Kottoor, J. Biomimetic endodontics: Barriers and strategies. Health Sci. 2013, 2, 7–12. [Google Scholar]
- About, I. Biodentine: From biochemical and bioactive properties to clinical applications. G. Ital. Endod. 2016, 30, 81–88. [Google Scholar]
- Sharma, V.; Srinivasan, A.; Nikolajeff, F.; Kumar, S. Biomineralization process in hard tissues: The interaction complexity within protein and inorganic counterparts. Acta Biomater. 2021, 120, 20–37. [Google Scholar] [CrossRef]
- Bazos, P.; Magne, P. Bio-emulation: Biomimetically emulating nature utilizing a histo-anatomic approach; structural analysis. Eur. J. Esthet. Dent. 2011, 6, 8–19. [Google Scholar]
- Hristov, M.; Erl, W.; Weber, P.C. Endothelial progenitor cells: Mobilization, differentiation, and homing. Arteriosclerosis, thrombosis, and vascular biology. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1185–1189. [Google Scholar]
- Zafar, M.S.; Fareed, M.A.; Riaz, S.; Latif, M.; Habib, S.R.; Khurshid, Z. Customized Therapeutic Surface Coatings for Dental Implants. Coatings 2020, 10, 568. [Google Scholar] [CrossRef]
- Dionysopoulos, D.; Gerasimidou, O. Biomimetic dentistry: Basic principles and protocols. ARC J. Dent. Sci. 2020, 5, 1–3. [Google Scholar]
- Tirlet, G.; Crescenzo, H.; Crescenzo, D.; Bazos, P. Ceramic adhesive restorations and biomimetic dentistry: Tissue preservation and adhesion. Int. J. Esthet. Dent. 2014, 9, 354–369. [Google Scholar] [PubMed]
- Dawood, A.E.; Parashos, P.; Wong, R.H.; Reynolds, E.C.; Manton, D.J. Calcium silicate-based cements: Composition, properties, and clinical applications. J. Investig. Clin. Dent. 2017, 8, 12195. [Google Scholar]
- Galler, K.M.; D’Souza, R.N.; Hartgerink, J.D. Biomaterials and their potential applications for dental tissue engineering. J. Mater. Chem. 2010, 20, 8730–8746. [Google Scholar] [CrossRef]
- Zafar, M.S.; Khurshid, Z.; Almas, K. Oral tissue engineering progress and challenges. Tissue Eng. Regen. Med. 2015, 12, 387–397. [Google Scholar] [CrossRef]
- Balla, R.; LoMonaco, C.J.; Skribner, J.; Lin, L.M. Histological study of furcation perforations treated with Tricalcium phosphate, hydroxylapatite, amalgam, and Life. J. Endod. 1991, 17, 234–238. [Google Scholar] [CrossRef]
- Soares, D.G.; Bordini, E.A.; Swanson, W.B.; de Souza Costa, C.A.; Bottino, M.C. Platform technologies for regenerative endodontics from multifunctional biomaterials to tooth-on-a-chip strategies. Clin.Oral Invest. 2021, 25(8), 4749–4779. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, J.O.; Farik, B.; Munksgaard, E.C. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent. Traumatol. 2002, 18, 134–137. [Google Scholar] [CrossRef]
- Hargreaves, K.M.; Diogenes, A.; Teixeira, F.B. Treatment options: Biological basis of regenerative endodontic procedures. Pediatr. Dent. 2013, 35, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.T. Pulp and dentin tissue engineering and regeneration: Current progress. Regen. Med. 2009, 4, 697–707. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.G.; Zheng, Y.; Zhou, J.; Chen, M.; Embree, M.C.; Song, K.; Jiang, N.; Mao, J.J. Dentin and dental pulp regeneration by the patient’s endogenous cells. Restor. Dent. Endod. 2013, 28, 106–117. [Google Scholar] [CrossRef]
- Mao, J.J.; Kim, S.G.; Zhou, J.; Ye, L.; Cho, S.; Suzuki, T.; Fu, S.Y.; Yang, R.; Zhou, X. Regenerative endodontics: Barriers and strategies for clinical translation. Dent. Clinic. 2012, 56, 639–649. [Google Scholar]
- Smith, J.; Smith, A.; Shelton, R.; Cooper, P. Recruitment of dental pulp cells by dentine and pulp extracellular matrix components. Exp. Cell Res. 2012, 318, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Turman, M. Revitalization of Tooth with Necrotic Pulp and Open Apex by Using Platelet-rich Plasma: A Case Report. J. Endod. 2011, 37, 265–268. [Google Scholar] [CrossRef]
- Srinivasan, K.; Chitra, S. Emerging trends in oral health profession: The biomimetic-a review. Arch. Dent. Med. Res. 2015, 1, 40–47. [Google Scholar]
- Galler, K.M.; Brandl, F.P.; Kirchhof, S.; Widbiller, M.; Eidt, A.; Buchalla, W.; Göpferich, A.; Schmalz, G. Suitability of Different Natural and Synthetic Biomaterials for Dental Pulp Tissue Engineering. Tissue Eng. Part A 2018, 24, 234–244. [Google Scholar] [CrossRef]
- Ostby, B.N. The role of the blood clot in endodontic therapy. An experimental histologic study. Acta Odontol. Scand. 1961, 19, 324–353. [Google Scholar] [PubMed]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef]
- Garcia-Godoy, F.; Murray, P. Recommendations for using regenerative endodontic procedures in permanent immature traumatized teeth. Dent. Traumatol. 2012, 28, 33–41. [Google Scholar] [CrossRef]
- Albuquerque, M.T.P.; Valera, M.C.; Nakashima, M.; Nör, J.E.; Bottino, M.C. Tissue-engineering-based Strategies for Regenerative Endodontics. J. Dent. Res. 2014, 93, 1222–1231. [Google Scholar] [CrossRef]
- Yuan, Z.; Nie, H.; Wang, S.; Lee, C.H.; Li, A.; Fu, S.Y.; Zhou, H.; Chen, L.; Mao, J.J. Biomaterial Selection for Tooth Regeneration. Tissue Eng. Part B Rev. 2011, 17, 373–388. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, K.M.; Giesler, T.; Henry, M.; Wang, Y. Regeneration potential of the young permanent tooth: What does the future hold? Pediatr. Dent. 2008, 30, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.-J.; Sonoyama, W.; Liu, Y.; Liu, H.; Wang, S.; Shi, S. The Hidden Treasure in Apical Papilla: The Potential Role in Pulp/Dentin Regeneration and BioRoot Engineering. J. Endod. 2008, 34, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Association of Endodontists. AAE Clinical Considerations for a Regenerative Procedure; American Association of Endodontists: Chicago, IL, USA, 2016. [Google Scholar]
- Sedgley, C.M.; Botero, T.M. Dental Stem Cells and Their Sources. Dent. Clin. N. Am. 2012, 56, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.; D’Souza, R.; Hartgerink, J.; Schmalz, G. Scaffolds for Dental Pulp Tissue Engineering. Adv. Dent. Res. 2011, 23, 333–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widbiller, M.; Driesen, R.B.; Eidt, A.; Lambrichts, I.; Hiller, K.-A.; Buchalla, W.; Schmalz, G.; Galler, K.M. Cell Homing for Pulp Tissue Engineering with Endogenous Dentin Matrix Proteins. J. Endod. 2018, 44, 956–962.e2. [Google Scholar] [CrossRef]
- Torabinejad, M.; Alexander, A.; Vahdati, S.A.; Grandhi, A.; Baylink, D.; Shabahang, S. Effect of Residual Dental Pulp Tissue on Regeneration of Dentin-pulp Complex: An In Vivo Investigation. J. Endod. 2018, 44, 1796–1801. [Google Scholar] [CrossRef]
- Iohara, K.; Zheng, L.; Ito, M.; Ishizaka, R.; Nakamura, H.; Into, T.; Matsushita, K.; Nakashima, M. Regeneration of dental pulp after pulpotomy by transplantation of CD31-/CD146-side population cells from a canine tooth. Regen Med. 2009, 4, 377–385. [Google Scholar]
- Souron, J.-B.; Petiet, A.; Decup, F.; Tran, X.V.; Lesieur, J.; Poliard, A.; Le Guludec, D.; Letourneur, D.; Chaussain, C.; Rouzet, F.; et al. Pulp Cell Tracking by Radionuclide Imaging for Dental Tissue Engineering. Tissue Eng. Part C Methods 2014, 20, 188–197. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Zhao, Y.; Yang, J.; Wang, W.; Wang, X.; Ling, L.; Ge, L. Simvastatin promotes dental pulp stem cell–induced coronal pulp regeneration in pulpotomized teeth. J. Endod. 2016, 42, 1049–1054. [Google Scholar] [CrossRef]
- Ito, T.; Kaneko, T.; Sueyama, Y.; Kaneko, R.; Okiji, T. Dental pulp tissue engineering of pulpotomized rat molars with bone marrow mesenchymal stem cells. Odontology 2017, 105, 392–397. [Google Scholar] [CrossRef]
- Endodontics, A.R. Endodontics Colleagues for Excellence; AAE: Chicago, IL, USA, 2013; pp. 1–8. [Google Scholar]
- Bose, R.; Nummikoski, P.; Hargreaves, K. A Retrospective Evaluation of Radiographic Outcomes in Immature Teeth with Necrotic Root Canal Systems Treated with Regenerative Endodontic Procedures. J. Endod. 2009, 35, 1343–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nygaard-Östby, B.; Hjortdal, O. Tissue formation in the root canal following pulp removal. Eur. J. Oral Sci. 1971, 79, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Banchs, F.; Trope, M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J. Endod. 2004, 30, 196–200. [Google Scholar] [CrossRef]
- Iwaya, S.; Ikawa, M.; Kubota, M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent. Traumat. 2001, 17, 185–187. [Google Scholar]
- Kubasad, G.C.; Ghivari, S.B. Apexification with apical plug of MTA-report of cases. Arch. Oral Sci. Res. 2011, 1, 104–107. [Google Scholar]
- Vojinović, J.; Čupić, S.; Dolić, O.; Mirjanić, Đ.; Sukara, S.; Obradović, M. Success rate of the endodontic treatment of young permanent teeth with calcium hydroxide. Growth Contemp. 2010, 4, 8. [Google Scholar]
- Nagaveni, N.; Umashankara, K.; Radhika, N.; Manjunath, S. Successful closure of the root apex in non-vital permanent incisors with wide open apices using single calcium hydroxide (caoh) dressing—Report of 2 cases. J. Clin. Exp. Dent. 2010, 1, 165–167. [Google Scholar] [CrossRef]
- Bucal, M.O. Apexification, Apexogenesis and Pulpal Revascularization: Literature Review and Clinical Applications. St. Vincent Char. Med. Cent. J. 2018, 40, 8. [Google Scholar]
- Ford, T.P.; Shabahang, S. Management of incompletely formed roots. In Principles and Practice of Endodontics; Saunders: Philadelphia, PA, USA, 2003; pp. 388–404. [Google Scholar]
- Lovelace, T.W.; Henry, M.A.; Hargreaves, K.M.; Diogenes, A. Evaluation of the Delivery of Mesenchymal Stem Cells into the Root Canal Space of Necrotic Immature Teeth after Clinical Regenerative Endodontic Procedure. J. Endod. 2011, 37, 133–138. [Google Scholar] [CrossRef]
- Thibodeau, B.; Teixeira, F.; Yamauchi, M.; Caplan, D.J.; Trope, M. Pulp Revascularization of Immature Dog Teeth with Apical Periodontitis. J. Endod. 2007, 33, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Thibodeau, B.; Trope, M.; Lin, L.M.; Huang, G.T.-J. Histologic Characterization of Regenerated Tissues in Canal Space after the Revitalization/Revascularization Procedure of Immature Dog Teeth with Apical Periodontitis. J. Endod. 2010, 36, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Jeeruphan, T.; Jantarat, J.; Yanpiset, K.; Suwannapan, L.; Khewsawai, P.; Hargreaves, K.M. Mahidol Study 1: Comparison of Radiographic and Survival Outcomes of Immature Teeth Treated with Either Regenerative Endodontic or Apexification Methods: A Retrospective Study. J. Endod. 2012, 38, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Wigler, R.; Kaufman, A.Y.; Lin, S.; Steinbock, N.; Hazan-Molina, H.; Torneck, C.D. Revascularization: A Treatment for Permanent Teeth with Necrotic Pulp and Incomplete Root Development. J. Endod. 2013, 39, 319–326. [Google Scholar] [CrossRef]
- Kahler, B.; Mistry, S.; Moule, A.; Ringsmuth, A.K.; Case, P.; Thomson, A.; Holcombe, T. Revascularization Outcomes: A Prospective Analysis of 16 Consecutive Cases. J. Endod. 2013, 40, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, J.E.; Duarte, P.; Ervolino, E.; Bomfim, S.R.M.; Abimussi, C.; Santos, L.M.D.S.; Lodi, C.S.; Oliveira, S.; Dezan, E.; Cintra, L. Histologic Characterization of Engineered Tissues in the Canal Space of Closed-apex Teeth with Apical Periodontitis. J. Endod. 2013, 39, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.M.; Tawfik, H.E.; Hashem, A.A.R.; Abu-Seida, A.M. Regenerative Potential of Immature Permanent Teeth with Necrotic Pulps after Different Regenerative Protocols. J. Endod. 2014, 40, 192–198. [Google Scholar] [CrossRef]
- Iwaya, S.; Ikawa, M.; Kubota, M. Revascularization of an immature permanent tooth with periradicular abscess after luxation. Dent. Traumat. 2011, 27, 55–58. [Google Scholar]
- Huang, G.-J.; Garcia-Godoy, F. Missing Concepts in de novo Pulp Regeneration. J. Dent. Res. 2014, 93, 717–724. [Google Scholar] [CrossRef] [Green Version]
- Llames, S.G.; Del Rio, M.; Larcher, F.; García, E.; García, M.; Escámez, M.J.; Jorcano, J.L.; Holguín, P.; Meana, A. Human plasma as a dermal scaffold for the generation of a completely autologous bioengineered skin. Transplantation 2004, 77, 350–355. [Google Scholar] [CrossRef]
- Frye, C.A.; Wu, X.; Patrick, C.W. Microvascular endothelial cells sustain preadipocyte viability under hypoxic conditions. Cell. Dev. Biol. Anim. 2005, 41, 160–164. [Google Scholar] [CrossRef]
- Hofer, S.; Mitchell, G.; Penington, A.; Morrison, W.; RomeoMeeuw, R.; Keramidaris, E.; Palmer, J.; Knight, K. The use of pimonidazole to characterise hypoxia in the internal environment of an in vivo tissue engineering chamber. Br. J. Plast. Surg. 2005, 58, 1104–1114. [Google Scholar] [CrossRef]
- Lembert, N.; Wesche, J.; Petersen, P.; Doser, M.; Zschocke, P.; Becker, H.D.; Ammon, H.P.T. Encapsulation of Islets in Rough Surface, Hydroxymethylated Polysulfone Capillaries Stimulates VEGF Release and Promotes Vascularization after Transplantation. Cell Transplant. 2005, 14, 97–108. [Google Scholar] [CrossRef]
- Chueh, L.-H.; Huang, G.T.-J. Immature Teeth with Periradicular Periodontitis or Abscess Undergoing Apexogenesis: A Paradigm Shift. J. Endod. 2006, 32, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Logani, A.; Bhaskar, U.; Aggarwal, V. Efficacy of Revascularization to Induce Apexification/Apexogensis in Infected, Nonvital, Immature Teeth: A Pilot Clinical Study. J. Endod. 2008, 34, 919–925. [Google Scholar] [CrossRef]
- Petrino, J.A.; Boda, K.; Shambarger, S.; Bowles, W.R.; McClanahan, S.B. Challenges in Regenerative Endodontics: A Case Series. J. Endod. 2010, 36, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yuan, G.; Chen, Z. Pulp Regeneration: Current Approaches and Future Challenges. Front. Physiol. 2016, 7, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kindler, V. Postnatal stem cell survival: Does the niche, a rare harbor where to resist the ebb tide of differentiation, also provide lineage-specific instructions? J. Leukol. Biol. 2005, 78, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Akamine, A. The Application of Tissue Engineering to Regeneration of Pulp and Dentin in Endodontics. J. Endod. 2005, 31, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Brazelton, T.R.; Blau, H.M. Optimizing Techniques for Tracking Transplanted Stem Cells In Vivo. Stem Cells 2005, 23, 1251–1265. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, M. Bone morphogenetic proteins in dentin regeneration for potential use in endodontic therapy. Cytokine Growth Factor Rev. 2005, 16, 369–376. [Google Scholar] [CrossRef]
- Fukuda, J.; Khademhosseini, A.; Yeh, J.; Eng, G.; Cheng, J.; Farokhzad, O.C.; Langer, R. Micropatterned cell co-cultures using layer-by-layer deposition of extracellular matrix components. Biomaterials 2006, 27, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.-J.; Sonoyama, W.; Chen, J.; Park, S.H. In vitro characterization of human dental pulp cells: Various isolation methods and culturing environments. Cell Tissue Res. 2006, 324, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Helmlinger, G.; Yuan, F.; Dellian, M.; Jain, R.K. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat. Med. 1997, 3, 177–182. [Google Scholar] [CrossRef]
- Nakashima, M. Tissue Engineering in Endodontics. Aust. Dent. J. 2005, 31, 111–113. [Google Scholar] [CrossRef]
- Tabata, Y. Nanomaterials of Drug Delivery Systems for Tissue Regeneration. Methods Mol. Biol. 2005, 300, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Blaker, J. Bioactive composite materials for tissue engineering scaffolds. Expert Rev. Med. Devices 2005, 2, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Kitasako, Y.; Shibata, S.; Pereira, P.N.; Tagami, J. Short-term dentin bridging of mechanically-exposed pulps capped with adhesive resin systems. Oper. Dent. 2000, 25, 155–162. [Google Scholar]
- Mjor, I.A.; Dah, E.; Cox, C.F. Healing of pulp exposures: An ultrastructural study. J. Oral Pathol. Med. 1991, 20, 496–501. [Google Scholar] [CrossRef]
- Silva, T.; Rosa, A.; Lara, V. Dentin matrix proteins and soluble factors: Intrinsic regulatory signals for healing and resorption of dental and periodontal tissues? Oral Dis. 2004, 10, 63–74. [Google Scholar] [CrossRef]
- Elisseeff, J.; Puleo, C.; Yang, F.; Sharma, B. Advances in skeletal tissue engineering with hydrogels. Orthod. Craniofac. Res. 2005, 8, 150–161. [Google Scholar] [CrossRef]
- Trojani, C.; Weiss, P.; Michiels, J.-F.; Vinatier, C.; Guicheux, J.; Daculsi, G.; Gaudray, P.; Carle, G.F.; Rochet, N. Three-dimensional culture and differentiation of human osteogenic cells in an injectable hydroxypropylmethylcellulose hydrogel. Biomaterials 2005, 26, 5509–5517. [Google Scholar] [CrossRef] [PubMed]
- Alhadlaq, A.; Mao, J.J. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J. Bone Jt. Surg. Am. 2005, 87, 936–944. [Google Scholar] [CrossRef] [Green Version]
- Desgrandchamps, F. Biomaterials in functional reconstruction. Curr. Opin. Urol. 2000, 10, 201–206. [Google Scholar] [CrossRef]
- Luo, Y.; Shoichet, M.S. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat. Mater. 2004, 3, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Dusseiller, M.R.; Schlaepfer, D.; Koch, M.; Kroschewski, R.; Textor, M. An inverted microcontact printing method on topographically structured polystyrene chips for arrayed micro-3-D culturing of single cells. Biomaterials 2005, 26, 5917–5925. [Google Scholar] [CrossRef] [PubMed]
- Barron, J.A.; Krizman, D.B.; Ringeisen, B.R. Laser Printing of Single Cells: Statistical Analysis, Cell Viability, and Stress. Ann. Biomed. Eng. 2005, 33, 121–130. [Google Scholar] [CrossRef]
- Barron, J.; Wu, P.; Ladouceur, H.; Ringeisen, B. Biological Laser Printing: A Novel Technique for Creating Heterogeneous 3-dimensional Cell Patterns. Biomed. Microdevices 2004, 6, 139–147. [Google Scholar] [CrossRef]
- Rutherford, R.B. BMP-7 gene transfer to inflamed ferret dental pulps. Eur. J. Oral Sci. 2001, 109, 422–424. [Google Scholar] [CrossRef] [Green Version]
- Jüllig, M.; Zhang, W.V.; Stott, N.S. Gene therapy in orthopaedic surgery: The current status. ANZ J. Surg. 2004, 74, 46–54. [Google Scholar] [CrossRef]
- Stolberg, S.G. Trials are halted on a gene therapy. The New York Times, 2002; p. 4. [Google Scholar]
- Nakashima, M.; Reddi, A.H. The application of bone morphogenetic proteins to dental tissue engineering. Nat. Biotechnol. 2003, 21, 1025–1032. [Google Scholar] [CrossRef]
- Kimura, H.; Esumi, H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim. Pol. 2003, 50, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Slomberg, D.L.; Chudasama, S.L.; Lu, Y.; Schoenfisch, M.H. Nitric Oxide-Releasing Dendrimers as Antibacterial Agents. Biomacromolecules 2012, 13, 3343–3354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backlund, C.J.; Worley, B.V.; Schoenfisch, M.H. Anti-biofilm action of nitric oxide-releasing alkyl-modified poly(amidoamine) dendrimers against Streptococcus mutans. Acta Biomater. 2016, 29, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Moon, C.-Y.; Nam, O.H.; Kim, M.; Lee, H.-S.; Kaushik, S.N.; Walma, D.A.C.; Jun, H.-W.; Cheon, K.; Choi, S.C. Effects of the nitric oxide releasing biomimetic nanomatrix gel on pulp-dentin regeneration: Pilot study. PLoS ONE 2018, 13, e0205534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, R.Y.; Cheung, G.S.-P.; Chen, J.; Yin, X.; Wang, Q.Q.; Zhang, C. Pulp Revascularization of Immature Teeth with Apical Periodontitis: A Clinical Study. J. Endod. 2009, 35, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Sánchez, M.; Nurden, A.T.; Nurden, P.; Orive, G.; Andía, I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006, 24, 227–234. [Google Scholar] [CrossRef]
- Ogino, Y.; Ayukawa, Y.; Kukita, T.; Koyano, K. The contribution of platelet-derived growth factor, transforming growth factor-β1, and insulin-like growth factor-I in platelet-rich plasma to the proliferation of osteoblast-like cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, 724–729. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Samadi, P.; Sheykhhasan, M.; Khoshinani, H.M. The Use of Platelet-Rich Plasma in Aesthetic and Regenerative Medicine: A Comprehensive Review. Aesthetic Plast. Surg. 2019, 43, 803–814. [Google Scholar] [CrossRef]
- Whitman, D.H.; Berry, R.L.; Green, D.M. Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J. Oral Maxillofac. Surg. 1997, 55, 1294–1299. [Google Scholar] [CrossRef]
- Lee, U.-L.; Jeon, S.H.; Park, J.-Y.; Choung, P.-H. Effect of platelet-rich plasma on dental stem cells derived from human impacted third molars. Regen. Med. 2011, 6, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Faras, H. A Clinical and Histological Report of a Tooth with an Open Apex Treated with Regenerative Endodontics Using Platelet-rich Plasma. J. Endod. 2012, 38, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhu, X.; Huang, G.T.-J.; Cheung, G.S.P.; Dissanayaka, W.; Zhang, C. Regeneration of dental pulp tissue in immature teeth with apical periodontitis using platelet-rich plasma and dental pulp cells. Int. Endod. J. 2013, 46, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Milan, M.; Shabahang, S.; Wright, K.R.; Faras, H. Histologic Examination of Teeth with Necrotic Pulps and Periapical Lesions Treated with 2 Scaffolds: An Animal Investigation. J. Endod. 2015, 41, 846–852. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Zhang, C. Pulp Revascularization on Permanent Teeth with Open Apices in a Middle-aged Patient. J. Endod. 2015, 41, 1571–1575. [Google Scholar] [CrossRef]
- DeLong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-Rich Plasma: The PAW Classification System. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 998–1009. [Google Scholar] [CrossRef]
- Zhang, D.-D.; Chen, X.; Bao, Z.-F.; Chen, M.; Ding, Z.-J.; Zhong, M. Histologic Comparison between Platelet-rich Plasma and Blood Clot in Regenerative Endodontic Treatment: An Animal Study. J. Endod. 2014, 40, 1388–1393. [Google Scholar] [CrossRef]
- Lee, C.H.; Cook, J.L.; Mendelson, A.; Moioli, E.K.; Yao, H.; Mao, J.J. Regeneration of the articular surface of the rabbit synovial joint by cell homing: A proof of concept study. Lancet 2010, 376, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Xin, X.; Moioli, E.K.; Chung, J.; Lee, C.H.; Chen, M.; Fu, S.Y.; Koch, P.D.; Mao, J.J. Regeneration of Dental-Pulp-like Tissue by Chemotaxis-Induced Cell Homing. Tissue Eng. Part A 2010, 16, 3023–3031. [Google Scholar] [CrossRef] [Green Version]
- Ishizaka, R.; Iohara, K.; Murakami, M.; Fukuta, O.; Nakashima, M. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials 2012, 33, 2109–2118. [Google Scholar] [CrossRef]
- Rosa, V.; Botero, T.M.; Nör, J.E. Regenerative endodontics in light of the stem cell paradigm. Int. Dent. J. 2011, 61, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casagrande, L.; Cordeiro, M.M.; Nör, S.A.; Nör, J.E. Dental pulp stem cells in regenerative dentistry. Odontology 2011, 99, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Eidt, A.; Schmalz, G. Cell-free Approaches for Dental Pulp Tissue Engineering. J. Endod. 2014, 40, S41–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Society of Endodontology. Quality guidelines for endodontic treatment: Consensus report of the European Society of Endodontology. Int. Endod. J. 2006, 39, 921–930. [Google Scholar] [CrossRef]
- Qadiri, S.Y.; Mustafa, S. Role of calcium hydroxide in root canal therapy: A comprehensive review. J. Adv. Med. Dent. Sci. Res. 2019, 7, 1–3. [Google Scholar]
- Mohammadi, Z.; Shalavi, S.; Yazdizadeh, M. Antimicrobial Activity of Calcium Hydroxide in Endodontics: A Review. Chonnam Med. J. 2012, 48, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, M.; Saujanya, K.P.; Jain, D.; Sajjanshetty, S.; Arun, A.; Uppin, L.; Kadri, M. Role of Calcium Hydroxide in Endodontics: A Review. Glob. J. Health Sci. 2012, 1, 66–70. [Google Scholar]
- Shuping, G.B.; Orstavik, D.; Sigurdsson, A.; Trope, M. Reduction of Intracanal Bacteria Using Nickel-Titanium Rotary Instrumentation and Various Medications. J. Endod. 2000, 26, 751–755. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Lopes, H.P. Mechanisms of antimicrobial activity of calcium hydroxide: A critical review. Int. Endod. J. 1999, 32, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, F.B.; Levin, L.G.; Trope, M. Investigation of pH at different dentinal sites after placement of calcium hydroxide dressing by two methods. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 99, 511–516. [Google Scholar] [CrossRef]
- Al-Hiyasat, A.S.; El-Farraj, H.S.; Alebrahim, M.A. The effect of calcium hydroxide on dentine composition and root fracture resistance of human teeth: An in vitro study. Eur. J. Oral Sci. 2021, 129, e12798. [Google Scholar] [PubMed]
- Ruparel, N.B.; Teixeira, F.B.; Ferraz, C.C.; Diogenes, A. Direct Effect of Intracanal Medicaments on Survival of Stem Cells of the Apical Papilla. J. Endod. 2012, 38, 1372–1375. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Chugal, N.; Lin, L.M. Alkaline materials and regenerative endodontics: A review. Materials 2017, 10, 1389. [Google Scholar]
- Galler, K.M.; Buchalla, W.; Hiller, K.-A.; Federlin, M.; Eidt, A.; Schiefersteiner, M.; Schmalz, G. Influence of Root Canal Disinfectants on Growth Factor Release from Dentin. J. Endod. 2015, 41, 363–368. [Google Scholar] [CrossRef]
- Galler, K.M.; Widbiller, M.; Buchalla, W.; Eidt, A.; Hiller, K.-A.; Hoffer, P.C.; Schmalz, G. EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int. Endod. J. 2016, 49, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Althumairy, R.I.; Teixeira, F.B.; Diogenes, A. Effect of Dentin Conditioning with Intracanal Medicaments on Survival of Stem Cells of Apical Papilla. J. Endod. 2014, 40, 521–525. [Google Scholar] [CrossRef]
- Alhadainy, H.A.; Himel, V.T. Evaluation of the sealing ability of amalgam, cavit and glass ionomer cement in the repair of furcation perforations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 1993, 75, 362–366. [Google Scholar] [CrossRef]
- Zaen El-Din, A.M.; Hamama, H.H.; Abo El-Elaa, M.A.; Grawish, M.E.; Mahmoud, S.H.; Neelakantan, P. The effect of four materials on direct pulp capping: An animal study. Aus. Endont. J. 2020, 46, 249–256. [Google Scholar]
- Fridland, M.; Rosado, R. Mineral trioxide aggregate solubility and porosity with different water to powder ratios. J. Endod. 2003, 29, 814–817. [Google Scholar] [CrossRef] [Green Version]
- Moazami, F.; Sahebi, S.; Jamshidi, D.; Alavi, A. The long-term effect of calcium hydroxide, calcium-enriched mixture cement and mineral trioxide aggregate on dentin strength. Iran. Endod. J. 2014, 9, 185–189. [Google Scholar]
- Revathi, N.; Chandra, S.S. Merits and demerits of calcium hydroxide as a therapeutic agent: A review. Int. J. Dent. Res. 2014, 2, 1–4. [Google Scholar]
- Bagoff, R.; Mamidwar, S.; Chesnoiu-Matei, I.; Ricci, J.L.; Alexander, H.; Tovar, N.M. Socket Preservation and Sinus Augmentation Using a Medical Grade Calcium Sulfate Hemihydrate and Mineralized Irradiated Cancellous Bone Allograft Composite. J. Oral Implant. 2013, 39, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.; Yanni, T.; Jamshidi, P.; Grover, L.M. Inorganic cements for biomedical application: Calcium phosphate, calcium sulphate and calcium silicate. Adv. Appl. Ceram. 2015, 114, 65–76. [Google Scholar] [CrossRef]
- Coetzee, A.S. Regeneration of bone in the presence of calcium sulphate. Arch. Otolaryngol. 1980, 106, 405–409. [Google Scholar] [PubMed]
- Gutmann, J.L.; Sinjab, Y.H.; Sinjab, K.H.; Navarrete-Bedoya, C. Calcium sulfate applications in dentistry: A literature review. Endodontology 2020, 32, 167. [Google Scholar] [CrossRef]
- Kameda, T.; Mano, H.; Yamada, Y.; Takai, H.; Amizuka, H.; Kobori, M.; Izumi, N.; Kawashima, H.; Ozawa, H.; Ikeda, K.; et al. Calcium-sensing receptor in mature osteoclasts, which are bone-resorbing cells. Biochem. Biphys. Res. Commun. 1998, 245, 419–422. [Google Scholar] [CrossRef]
- Chen, J.; Gao, J.; Yin, H.; Liu, F.; Wang, A.; Zhu, Y.; Wu, Z.; Jiang, T.; Qin, D.; Chen, B.; et al. Size-controlled preparation of a-calcium sulphate hemihydrate starting from calcium sulphate dihydrate in the presence of modifiers and the dissolution rate in simulated body fluid. Mater. Sci. Eng. C-Mater. Biol. Appl. 2013, 33, 3256–3262. [Google Scholar] [CrossRef]
- Peltier, L.F.; Jones, R.F. Treatment of unicameral bone cysts by curettage and packing with plaster-of-Paris pellets. J. Bone Jt. Surg. Am. 1978, 60, 820–822. [Google Scholar] [CrossRef]
- Yoshikawa, G.; Murashima, Y.; Wadachi, R.; Sawada, N.; Suda, H. Guided bone regeneration (GBR) using membranes and calcium sulphate after apicectomy: A comparative histomorphometrical study. Int. Endod. J. 2002, 35, 255–263. [Google Scholar] [CrossRef]
- Pecora, G.; De Leonardis, D.; Ibrahim, N.; Bovi, M.; Cornelini, R. The use of calcium sulphate in the surgical treatment of a ‘through and through’periradicular lesion. Int. Endod. J. 2001, 34, 189–197. [Google Scholar] [CrossRef]
- Van der Stok, J.; Van Lieshout, E.M.; El-Massoudi, Y.; Van Kralingen, G.H.; Patka, P. Bone substitutes in the Netherlands–a systematic literature review. Acta Biomater. 2011, 7, 739–750. [Google Scholar] [PubMed] [Green Version]
- Yahav, A.; Kurtzman, G.M.; Katzap, M.; Dudek, D.; Baranes, D. Bone regeneration: Properties and clinical applications of biphasic calcium sulfate. Dent. Clin. 2020, 64, 453–472. [Google Scholar]
- Podaropoulos, L.; Veis, A.A.; Papadimitriou, S.; Alexandridis, C.; Kalyvas, D. Bone Regeneration Using B-Tricalcium Phosphate in a Calcium Sulfate Matrix. J. Oral Implant. 2009, 35, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-Y.; Yang, D.-A.; Zhang, N.; Ji, C.-G.; Zhu, L.; Zhang, T. Poly (propylene fumarate)/(calcium sulphate/β-tricalcium phosphate) composites: Preparation, characterization and in vitro degradation. Acta Biomater. 2009, 5, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1972, 5, 117–141. [Google Scholar] [CrossRef]
- Lee, S.-J.; Monsef, M.; Torabinejad, M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J. Endod. 1993, 19, 541–544. [Google Scholar] [CrossRef]
- Asgary, S.; Parirokh, M.; Eghbal, M.J.; Brink, F. Chemical Differences between White and Gray Mineral Trioxide Aggregate. J. Endod. 2005, 31, 101–103. [Google Scholar] [CrossRef]
- Rajasekharan, S.; Martens, L.C.; Cauwels, R.G.E.C.; Verbeeck, R.M.H. Biodentine™ material characteristics and clinical applications: A review of the literature. Eur. Arch. Paediatr. Dent. 2014, 15, 147–158. [Google Scholar] [CrossRef]
- Camilleri, J. Hydration mechanisms of mineral trioxide aggregate. Int. Endod. J. 2007, 40, 462–470. [Google Scholar]
- Kadali, N.; Alla, R.K.; Guduri, V.; Ramaraju, A.V.; Sajjan, S.; Rudraraju, V.R. Mineral Trioxide Aggregate: An overview of composition, properties and clinical applications. Int. J. Dent. Mater. 2020, 2, 11-8. [Google Scholar] [CrossRef]
- Aeinehchi, M.; Eslami, B.; Ghanbariha, M.; Saffar, A.S. Mineral trioxide aggregate (mta) and calcium hydroxide as pulp-capping agents in human teeth: A preliminary report. Int. Endod. J. 2003, 36, 225–235. [Google Scholar] [CrossRef] [PubMed]
- White, C., Jr.; Bryant, N.N. Combined therapy of mineral trioxide aggregate and guided tissue regeneration in the treatment of external root resorption and an associated osseous defect. J. Periodontol. 2002, 73, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Tawil, P.Z.; Duggan, D.J.; Galicia, J.C. MTA: A clinical review. Compend. Contin. Educ. 2015, 36, 247. [Google Scholar]
- Cervino, G.; Laino, L.; D’Amico, C.; Russo, D.; Nucci, L.; Amoroso, G.; Gorassini, F.; Tepedino, M.; Terranova, A.; Gambino, D.; et al. Mineral Trioxide Aggregate Applications in Endodontics: A Review. Eur. J. Dent. 2020, 14, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Faraco, I.M., Jr.; Holland, R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent. Traumatol. 2001, 17, 163–166. [Google Scholar] [CrossRef]
- Dominguez, M.S.; E Witherspoon, D.; Gutmann, J.L.; Opperman, L. Histological and Scanning Electron Microscopy Assessment of Various Vital Pulp-Therapy Materials. J. Endod. 2003, 29, 324–333. [Google Scholar] [CrossRef]
- Tziafas, D.; Pantelidou, O.; Alvanou, A.; Belibasakis, G.; Papadimitriou, S. The dentinogenic effect of mineral trioxide aggregate (MTA) in short-term capping experiments. Int. Endod. J. 2002, 35, 245–254. [Google Scholar] [CrossRef]
- Torabinejad, M.; Hong, C.U.; McDonald, F.; Ford, T.P. Physical and chemical properties of a new root-end filling material. J. Endod. 1995, 21, 349–353. [Google Scholar] [CrossRef]
- Hilton, T.J.; Ferracane, J.L.; Mancl, L. Northwest Practice-based Research Collaborative in Evidence-based Dentistry (NWP). Comparison of CaOH with MTA for direct pulp capping: A PBRN randomized clinical trial. J. Dent. Res. 2013, 92 (Suppl. S7), 16S–22S. [Google Scholar]
- Torabinejad, M.; Hong, C.U.; Lee, S.J.; Monsef, M.; Ford, T.R. Investigationof mineral trioxide aggregate for root-endfillingindogs. J. Endod. 1995, 21, 603–608. [Google Scholar] [CrossRef]
- Wattanapakkavong, K.; Srisuwan, T. Release of Transforming Growth Factor Beta 1 from Human Tooth Dentin after Application of Either ProRoot MTA or Biodentine as a Coronal Barrier. J. Endod. 2019, 45, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.; Iezzi, G.; Piattelli, A.; Prati, C.; Scarano, A. Osteoinductive potential and bone-bonding ability of ProRoot MTA, MTA Plus and Biodentine in rabbit intramedullary model: Microchemical characterization and histological analysis. Dent. Mater. 2017, 33, e221–e238. [Google Scholar] [CrossRef] [PubMed]
- Torreira, M.G.; Santos, A.D.; Cobos, M.R.; Boquete, I.F.; Abelleira, A.C. The osteoinductive potential of mta (mineral trioxide aggregate): A histologic study in rabbits. Eur. J. Anat. 2004, 8, 101–105. [Google Scholar]
- Jacobovitz, M.; Vianna, M.E.; Pandolfelli, V.C.; Oliveira, I.R.; Rossetto, H.L.; Gomes, B.P. Root canal filling with cements based on mineral aggregates: An in vitro analysis of bacterial microleakage. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 140–144. [Google Scholar] [CrossRef]
- Arandi, N.Z.; Thabet, M. Minimal intervention in dentistry: A literature review on Biodentine as a bioactive pulp capping material. BioMed Res. Int. 2021. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlińska, J. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J. Endont. 2013, 39, 743–747. [Google Scholar] [CrossRef]
- Malkondu, O.; Kazandag, M.K.; Kazazoglu, E. A review on biodentine, a contemoprary dentin replacement and repair material. BioMed. Res. Int. 2014, 2014, 160951. [Google Scholar] [CrossRef]
- Luo, Z.; Li, D.; Kohli, M.R.; Yu, Q.; Kim, S.; He, W.-X. Effect of Biodentine™ on the proliferation, migration and adhesion of human dental pulp stem cells. J. Dent. 2014, 42, 490–497. [Google Scholar] [CrossRef]
- Chang, S.W.; Lee, S.Y.; Ann, H.J.; Kum, K.Y.; Kim, E.C. Efects of calcium silicate endodontic cements on biocompatibility and mineralization-inducing potentials in human dental pulp cells. J. Endod. 2014, 40, 1194–1200. [Google Scholar] [CrossRef]
- Dawood, A.; Manton, D.; Parashos, P.; Wong, R.; Palamara, J.; Stanton, D.; Reynolds, E. The physical properties and ion release of CPP-ACP-modified calcium silicate-based cements. Aust. Dent. J. 2015, 60, 434–444. [Google Scholar] [CrossRef] [Green Version]
- Gandolfi, M.G.; Siboni, F.; Botero, T.; Bossù, M.; Riccitiello, F.; Prati, C. Calcium silicate and calcium hydroxide materials for pulp capping: Biointeractivity, porosity, solubility and bioactivity of current formulations. J. Appl. Biomater. Funct. Mater. 2015, 13, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Grech, L.; Mallia, B.; Camilleri, J. Investigation of the physical properties of tricalcium silicate cement-based root-end filling materials. Dent. Mater. 2013, 29, e20–e28. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Grech, L.; Galea, K.; Keir, D.; Fenech, M.; Formosa, L.; Damidot, D.; Mallia, B. Porosity and root dentine to material interface assessment of calcium silicate-based root-end flling materials. Clin. Oral Investig. 2014, 18, 1437–1446. [Google Scholar] [CrossRef]
- Caron, G.; Azérad, J.; Faure, M.-O.; Machtou, P.; Boucher, Y. Use of a new retrograde filling material (Biodentine) for endodontic surgery: Two case reports. Int. J. Oral Sci. 2014, 6, 250–253. [Google Scholar] [CrossRef]
- Bhavana, V.; Chaitanya, K.P.; Dola, B.; Gandi, P.; Patil, J.; Reddy, R.B. Evaluation of antibacterial and antifungal activity of new calcium-based cement (Biodentine) compared to MTA and glass ionomer cement. J. Conserv. Dent. 2015, 18, 44–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; Onate-Sánchez, R.E.; Forner, L.; Llena, C.; Lozano, A.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Biocompatibility of new pulp-capping materials NeoMTA Plus, MTA Repair HP, and Biodentine on human dental pulp stem cells. J. Endod. 2018, 44, 126–132. [Google Scholar] [PubMed] [Green Version]
- Cuadros-Fernández, C.; Rodríguez, A.I.L.; Sáez-Martínez, S.; García-Binimelis, J.; About, I.; Mercade, M. Short-term treatment outcome of pulpotomies in primary molars using mineral trioxide aggregate and Biodentine: A randomized clinical trial. Clin. Oral Investig. 2016, 20, 1639–1645. [Google Scholar] [CrossRef]
- Rajasekharan, S.; Martens, L.; Vandenbulcke, J.; Jacquet, W.; Bottenberg, P.; Cauwels, R.G.E.C. Efcacy of three diferent pulpotomy agents in primary molars—A randomised control trial. Int. Endod. J. 2016, 50, 215–228. [Google Scholar]
- Topçuoğlu, G.; Topçuoğlu, H.S. Regenerative endodontic therapy in a single visit using platelet-rich plasma and Biodentine in necrotic and asymptomatic immature molar teeth: A report of 3 cases. J. Endod. 2016, 42, 1344–1346. [Google Scholar] [CrossRef]
- Aguilar, F.G.; Garcia, L.F.R.; Pires-De-Souza, F.C.P. Biocompatibility of New Calcium Aluminate Cement (EndoBinder). J. Endod. 2012, 38, 367–371. [Google Scholar] [CrossRef]
- Parker, K.; Sharp, J. Refractory calcium aluminate cements. Rev. Pap. 1982. [Google Scholar]
- Pandolfelli, V.C.; Oliveira, I.R.; Rosseto, H.L.; Jacobovitz, M. A Composition Based on Aluminate Cement for Application in Endodontics and the Obtained Cement Product. Patent Registration. INPI 0704502-6, 2007. [Google Scholar]
- Garcia, L.F.R.; Aguilar, F.G.; Sabino, M.G.; Rossetto, H.L.; Pires-De-Souza, F. Mechanical and microstructural characterisation of new calcium aluminate cement (EndoBinder). Adv. Appl. Ceram. 2011, 110, 469–475. [Google Scholar] [CrossRef]
- Oliveira, I.R.; Pandolfelli, V.C.; Jacobovitz, M. Chemical, physical and mechanical properties of a novel calcium aluminate endodontic cement. Int. Endod. J. 2010, 43, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Castro-Raucci, L.M.; Oliveira, I.R.; Teixeira, L.N.; Rosa, A.L.; Oliveira, P.T.; Jacobovitz, M. Effects of a novel calcium aluminate cement on the early events of the progression of osteogenic cell cultures. Braz. Dent. J. 2011, 22, 99–104. [Google Scholar] [PubMed] [Green Version]
- Garcia, L.d.F.R.; Huck, C.; Scardueli, C.R.; de Souza Costa, C.A. Repair of bone defects filled with new calcium aluminate cement (EndoBinder). J. Endod. 2015, 41, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Borkar, S.A.; Ataide, I. Biodentine pulpotomy several days after pulp exposure: Four case reports. J. Conserv. Dent. 2015, 18, 73–78. [Google Scholar] [PubMed] [Green Version]
- Gandolfi, M.; Siboni, F.; Prati, C. Chemical–physical properties of theracal, a novel light-curable mta-like material for pulp capping. Int. Endod. J. 2012, 45, 571–579. [Google Scholar]
- Lee, B.-N.; Chang, H.-S.; Hwang, Y.-C.; Hwang, I.-N.; Oh, W.-M. Effects of a novel light-curable material on odontoblastic differentiation of human dental pulp cells. Int. Endod. J. 2016, 50, 464–471. [Google Scholar] [CrossRef]
- Camilleri, J. Hydration characteristics of Biodentine and Theracal used as pulp capping materials. Dent. Mater. 2014, 30, 709–715. [Google Scholar] [CrossRef]
- Camilleri, J.; Laurent, P.; About, I. Hydration of biodentine, theracal LC, and a prototype tricalcium silicate-based dentin replacement material after pulp capping in entire tooth cultures. J. Endod. 2014, 40, 1846–1854. [Google Scholar] [CrossRef]
- Yamamoto, S.; Han, L.; Noiri, Y.; Okiji, T. Evaluation of the Ca ion release, pH and surface apatite formation of International Journal of Dentistry 5 a prototype tricalcium silicate cement. Int. Endod. J. 2017, 50, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Shin, Y.; Kim, S.-O.; Lee, H.-S.; Choi, H.-J.; Song, J.S. Comparative study of pulpal responses to pulpotomy with ProRoot MTA, RetroMTA, and +eraCal in Dogs’ teeth. J. Endod. 2015, 41, 1317–1324. [Google Scholar] [PubMed]
- Hebling, J.; Lessa, F.; Nogueira, I.; Carvalho, R.M.; Costa, C.A.D.S. Cytotoxicity of resin-based light-cured liners. Am. J. Dent. 2009, 22, 137–142. [Google Scholar]
- Jeanneau, C.; Laurent, P.; Rombouts, C.; Giraud, T.; About, I. Light-cured Tricalcium Silicate Toxicity to the Dental Pulp. J. Endod. 2017, 43, 2074–2080. [Google Scholar] [CrossRef]
- Bakhtiar, H.; Nekoofar, M.H.; Aminishakib, P.; Abedi, F.; Naghi Moosavi, F.; Esnaashari, E.; Azizi, A.; Esmailian, S.; Ellini, M.R.; Mesgarzadeh, V.; et al. Human Pulp Responses to Partial Pulpotomy Treatment with TheraCal as Compared with Biodentine and ProRoot MTA: A Clinical Trial. J. Endod. 2017, 43, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Petrolo, F.; Comba, A.; Scansetti, M.; Alovisi, M.; Pasqualini, D.; Berutti, E.; Scotti, N. Effects of lightcured MTA like material on direct pulp capping. Dent. Mater. 2014, 30, e15. [Google Scholar] [CrossRef]
- Alazrag, M.A.; Abu-Seida, A.M.; El-Batouty, K.M.; El Ashry, S.H. Marginal adaptation, solubility and biocompatibility of TheraCal LC compared with MTA-angelus and biodentine as a furcation perforation repair material. BMC Oral Health 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, W.L.; Scott, D.F. Current Concepts Review—Total Hip Arthroplasty with Hydroxyapatite-Coated Prostheses*. J. Bone Jt. Surg. 1996, 78, 1918–1934. [Google Scholar] [CrossRef]
- Moursi, A.M.; Winnard, A.V.; Winnard, P.L.; Lannutti, J.J.; Seghi, R.R. Enhanced osteoblast response to a polymethylmethacrylate–hydroxyapatite composite. Biomaterials 2002, 23, 133–144. [Google Scholar] [CrossRef]
- Yang, J.Z.; Sultana, R.; Hu, X.Z.; Ichim, P. Novel Layered Hydroxyapatite/Tri-Calcium Phosphate–Zirconia Scaffold Composite with High Bending Strength for Load-Bearing Bone Implant Application. Int. J. Appl. Ceram. 2014, 11, 22–30. [Google Scholar]
- Sakkers, R.J.B.; Dalmeyer, R.A.J.; Brand, R.; Rozing, P.M.; van Blitterswijk, C. Assessment of bioactivity for orthopedic coatings in a gap-healing model. J. Biomed. Mater. Res. 1997, 36, 265–273. [Google Scholar] [CrossRef]
- Roeder, R.K.; Converse, G.; Kane, R.J.; Yue, W. Hydroxyapatite-reinforced polymer biocomposites for synthetic bone substitutes. Jom 2008, 60, 38–45. [Google Scholar] [CrossRef]
- Mendelson, B.C.; Jacobson, S.R.; Lavoipierre, A.M.; Huggins, R.J. The fate of porous hydroxyapatite granules used in facial skeletal augmentation. Aesthetic Plast. Surg. 2010, 34, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorozhkin, S.V. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. Engl. 2002, 41, 3130–3146. [Google Scholar]
- Martz, E.O.; Goel, V.K.; Pope, M.H.; Park, J.B. Materials and design of spinal implants—A review. J. Biomed. Mater. Res. 1997, 38, 267–288. [Google Scholar] [CrossRef]
- Choi, J.-W.; Kong, Y.-M.; Kim, H.-E.; Lee, I.-S. Reinforcement of Hydroxyapatite Bioceramic by Addition of Ni3Al and Al2O3. J. Am. Ceram. Soc. 1998, 81, 1743–1748. [Google Scholar] [CrossRef]
- Jean, A.; Kerebel, B.; Kerebel, L.-M.; Legeros, R.Z.; Hamel, H. Effects of various calcium phosphate biomaterials on reparative dentin bridge formation. J. Endod. 1988, 14, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Pissiotis, E.; Spngberg, L.S. Biological evaluation of collagen gels containing calcium hydroxide and hydroxyapatite. J. Endod. 1990, 16, 468–473. [Google Scholar] [CrossRef]
- Chohayeb, A.; Adrian, J.; Salamat, K. Pulpal response to tricalcium phosphate as a capping agent. Oral Surg. Oral Med. Oral Pathol. 1991, 71, 343–345. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Shen, W.; Chen, T.; Hu, L.; Chen, Z. Evaluation of the biocompatibility of a nonceramic hydroxyapatite. J. Endod. 1997, 23, 490–493. [Google Scholar] [CrossRef]
- Kitikuson, P.; Srisuwan, T. Attachment Ability of Human Apical Papilla Cells to Root Dentin Surfaces Treated with Either 3Mix or Calcium Hydroxide. J. Endod. 2016, 42, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Osorio, E.; Cabello, I.; Toledano, M. Zinc Induces Apatite and Scholzite Formation during Dentin Remineralization. Caries Res. 2014, 48, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Func. Biomater. 2018, 9, 25. [Google Scholar]
- Zhang, D.; Leppäranta, O.; Munukka, E.; Ylänen, H.; Viljanen, M.K.; Eerola, E.; Hupa, M.; Hupa, L. Antibacterial effects and dissolution behavior of six bioactive glasses. J. Biomed. Mater. Res. Part A 2010, 93, 475–483. [Google Scholar]
- Gong, W.; Huang, Z.; Dong, Y.; Gan, Y.; Li, S.; Gao, X.; Chen, X. Ionic Extraction of a Novel Nano-sized Bioactive Glass Enhances Differentiation and Mineralization of Human Dental Pulp Cells. J. Endod. 2014, 40, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, X.; Gong, W.; Zhang, Z.; Chen, X.; Dong, Y. Odontogenic differentiation and dentin formation of dental pulp cells under nanobioactive glass induction. Acta Biomater. 2014, 10, 2792–2803. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Ducheyne, P.; Barbier, L. Long term clinical evaluation of bioactive glass particles of narrow size range. Bioceramics 1996, 9, 99–102. [Google Scholar]
- Hench, L.L. Bioactive materials: The potential for tissue regeneration. J. Biomed. Mater. Res. 1998, 41, 511–518. [Google Scholar] [CrossRef]
- Hilton, T.J. Keys to Clinical Success with Pulp Capping: A Review of the Literature. Oper. Dent. 2009, 34, 615–625. [Google Scholar] [CrossRef] [Green Version]
- Macwan, C.S.; Deshpande, A. Mineral trioxide aggregate (MTA) in dentistry: A review of literature. J. Oral Res. Rev. 2014, 6, 71. [Google Scholar] [CrossRef]
- Gholami, S.; Labbaf, S.; Houreh, A.B.; Ting, H.-K.; Jones, J.R.; Esfahani, M.-H.N. Long term effects of bioactive glass particulates on dental pulp stem cells in vitro. Biomed. Glas. 2017, 3, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.; Liu, S.; Zhu, L.; Liang, Q.; Chen, X.; Dong, Y. Evaluation of Pulp Response to Novel Bioactive Glass Pulp Capping Materials. J. Endod. 2017, 43, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H. Monoblocks in Root Canals: A Hypothetical or a Tangible Goal. J. Endod. 2007, 33, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandke, L. Importance of coronal seal: Preventing coronal leakage in endodontics. J. Restor. Dent. 2016, 4, 71. [Google Scholar] [CrossRef]
- Elzubair, A.; Elias, C.N.; Suarez, J.C.M.; Lopes, H.P.; Vieira, M.V.B. The physical characterization of a thermoplastic polymer for endodontic obturation. J. Dent. 2006, 34, 784–789. [Google Scholar] [CrossRef]
- Mehrvarzfar, P.; Dahi-Taleghani, A.; Saghiri, M.A.; Karamifar, K.; Shababi, B.; Behnia, A. The comparison of MTA, Geristore® and Amalgam with or without Bioglass as a matrix in sealing the furcal perforations (in vitro study). Saudi Dent. J. 2010, 22, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Belladonna, F.G.; Calasans-Maia, M.D.; Alves, A.T.N.N.; de Brito Resende, R.F.; Souza, E.M.; Silva, E.J.N.L.; Fidel, S.R.; De-Deus, G. Biocompatibility of a Self-adhesive Gutta-percha–based Material in Subcutaneous Tissue of Mice. J. Endod. 2014, 40, 1869–1873. [Google Scholar]
- Wu, M.K.; Fan, B.; Wesselink, P. Diminished leakage along root canals filled with gutta-percha without sealer over time: A laboratory study. Int. Endod. J. 2000, 33, 121–125. [Google Scholar] [CrossRef]
- Marending, M.; Bubenhofer, S.B.; Sener, B.; De-Deus, G. Primary assessment of a self-adhesive gutta-percha material. Int. Endod. J. 2013, 46, 317–322. [Google Scholar] [CrossRef]
- Mohn, D.; Bruhin, C.; Luechinger, N.; Stark, W.J.; Imfeld, T.; Zehnder, M. Composites made of flame-sprayed bioactive glass 45S5 and polymers: Bioactivity and immediate sealing properties. Int. Endod. J. 2010, 43, 1037–1046. [Google Scholar]
- Mukhtar-Fayyad, D. Cytocompatibility of new bioceramic-based materials on human fibroblast cells (MRC-5). Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, e137–e142. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Hong, S.-H.; Kim, J.-H.; Lee, S.-J.; Shin, S.-J. X-ray diffraction analysis of White ProRoot MTA and Diadent BioAggregate. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Fan, M. BioAggregate and iRoot BP Plus optimize the proliferation and mineralization ability of human dental pulp cells. Int. Endod. J. 2013, 46, 923–929. [Google Scholar] [PubMed]
- Yan, P.; Yuan, Z.; Jiang, H.; Peng, B.; Bian, Z. Effect of bioaggregate on differentiation of human periodontal ligament fibroblasts. Int. Endod. J. 2010, 43, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Peng, B.; Jiang, H.; Bian, Z.; Yan, P. Effect of Bioaggregate on Mineral-associated Gene Expression in Osteoblast Cells. J. Endod. 2010, 36, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- De-Deus, G.; Canabarro, A.; Alves, G.; Linhares, A.; Senne, M.I.; Granjeiro, J.M. Optimal Cytocompatibility of a Bioceramic Nanoparticulate Cement in Primary Human Mesenchymal Cells. J. Endod. 2009, 35, 1387–1390. [Google Scholar] [CrossRef]
- Memiş Özgül, B.; Bezgin, T.; Şahin, C.; Sarı, Ş. Resistance to leakage of various thicknesses of apical plugs of Bioaggregate using liquid filtration model. Dent. Traumatol. 2015, 31, 250–254. [Google Scholar]
- Leal, F.; De-Deus, G.; Brandão, C.; Luna, A.; Fidel, S.R.; Souza, E.M. Comparison of the root-end seal provided by bioceramic repair cements and White MTA. Int. Endod. J. 2011, 44, 662–668. [Google Scholar] [CrossRef]
- Madfa, A.A.; Al-Sanabani, F.A.; Al-Kudami, N.H.A.-Q. Endodontic Repair Filling Materials: A Review Article. Br. J. Med. Med Res. 2014, 4, 3059–3079. [Google Scholar] [CrossRef] [Green Version]
- Tuloglu, N.; Bayrak, S. Comparative evaluation of mineral trioxide aggregate and bioaggregate as apical barrier material in traumatized nonvital, immature teeth: A clinical pilot study. Niger. J. Clin. Pract. 2016, 19, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.-W.; Lee, S.-Y.; Kum, K.-Y.; Kim, E.-C. Effects of ProRoot MTA, Bioaggregate, and Micromega MTA on Odontoblastic Differentiation in Human Dental Pulp Cells. J. Endod. 2014, 40, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-E.; Lee, B.-N.; Koh, J.-T.; Park, Y.-J.; Joo, N.-E.; Chang, H.-S.; Hwang, I.-N.; Oh, W.-M.; Hwang, Y.-C. Cytotoxicity and physical properties of tricalcium silicate-based endodontic materials. Restor. Dent. Endod. 2014, 39, 89–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saghiri, M.A.; Nazari, A.; Garcia-Godoy, F.; Asatourian, A.; Malekzadeh, M.; Elyasi, M. Mechanical response of dental cements as determined by nanoindentation and scanning electron microscopy. Microsc. Microanal. 2013, 19, 1458–1464. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Garcia-Godoy, F.; Asatourian, A.; Lotfi, M.; Banava, S.; Khezri-Boukani, K. Effect of pH on compressive strength of some modification of mineral trioxide aggregate. Med. Oral Patol. Oral Circ. Buca. 2013, 18, e714–e720. [Google Scholar] [CrossRef]
- American Association of Endodontists. Glossary of Endodontic Terms; American Association of Endodontists: New York, NY, USA, 2003. [Google Scholar]
- Jew, R.C.; Weine, F.S.; Keene, J.J., Jr.; Smulson, M.H. A histologic evaluation of periodontal tissues adjacent to root perforations filled with Cavit. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 1982, 54, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Sinai, I.H. Endodontic perforations: Their prognosis and treatment. J. Am. Dent. Assoc. 1977, 95, 90–95. [Google Scholar] [CrossRef]
- Fuss, Z.; Tsesis, I.; Lin, S. Root resorption—diagnosis, classification and treatment choices based on stimulation factors. Dent. Traumatol. 2003, 19, 175–182. [Google Scholar] [CrossRef]
- Ingle, J.I. A standardized endodontic technique utilizing newly designed instruments and filling materials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 1961, 14, 83–91. [Google Scholar] [CrossRef]
- Kerekes, K.S.; Tronstad, L. Long-term results of endodontic treatment performed with a standardized technique. J. Endod. 1979, 5, 83–90. [Google Scholar] [CrossRef]
- Farzaneh, M.; Abitbol, S.; Friedman, S. Treatment Outcome in Endodontics: The Toronto Study. Phases I and II: Orthograde Retreatment. J. Endod. 2004, 30, 627–633. [Google Scholar] [CrossRef]
- Kakani, A.K.; Veeramachaneni, C. Sealing ability of three different root repair materials for furcation perforation repair: An in vitro study. J. Conserv. Dent. 2020, 23, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Banu, K.; Swathi, M. A Comparison of Solubility of Endosequence Root Repair Material Fast Set Putty and Mineral Trioxide Aggregate: An in Vitro Study. Saudi J. Oral Dent. Res. 2019. [Google Scholar] [CrossRef]
- Sharma, V.; Nawal, R.R.; Augustine, J.; Urs, A.B.; Talwar, S. Evaluation of Endosequence Root Repair Material and Endocem MTA as direct pulp capping agents: An in vivo study. Aust. Endod. J. 2021, 48, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, C.S.; Patel, N.S.; Patel, L.M.; E Kadouri, D.; Hartwell, G.R. Comparison of sealing ability of MTA and EndoSequence Bioceramic Root Repair Material: A bacterial leakage study. Quintessence Int. 2013, 44, e157–e162. [Google Scholar]
- Hansen, S.W.; Marshall, J.G.; Sedgley, C.M. Comparison of Intracanal EndoSequence Root Repair Material and ProRoot MTA to Induce pH Changes in Simulated Root Resorption Defects over 4 Weeks in Matched Pairs of Human Teeth. J. Endod. 2011, 37, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Fagogeni, I.; Metlerska, J.; Lipski, M.; Falgowski, T.; Maciej, G.; Nowicka, A. Materials used in regenerative endodontic procedures and their impact on tooth discoloration. J. Oral Sci. 2019, 61, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Giacomino, C.M.; Wealleans, J.A.; Kuhn, N.; Diogenes, A. The antimicrobial effect of bioceramic sealer on an 8-week matured Enterococcus faecalis biofilm attached to root canal dentinal surface. J. Endod. 2019, 45, 51–56. [Google Scholar] [CrossRef]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Forner, L.; Llena, C.; Lozano, A.; Castelo-Baz, P.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Comparative analysis of the biological effects of the endodontic bioactive cements MTA-Angelus, MTA Repair HP and NeoMTA Plus on human dental pulp stem cells. Int. Endod. J. 2017, 50, e63–e72. [Google Scholar]
- Donnermeyer, D.; Bürklein, S.; Dammaschke, T.; Schäfer, E. Endodontic sealers based on calcium silicates: A systematic review. Odontology 2019, 107, 421–436. [Google Scholar] [CrossRef]
- Linu, S.; Lekshmi, M.; Varunkumar, V.; Joseph, V.S. Treatment Outcome Following Direct Pulp Capping Using Bioceramic Materials in Mature Permanent Teeth with Carious Exposure: A Pilot Retrospective Study. J. Endod. 2017, 43, 1635–1639. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Dong, Y. Evaluation of a Bioceramic as a Pulp Capping Agent In Vitro and In Vivo. J. Endod. 2015, 41, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Al–Saudi, K.W.; Nabih, S.M.; Farghaly, A.M.; AboHager, E.A.-A. Pulpal repair after direct pulp capping with new bioceramic materials: A comparative histological study. Saudi Dent. J. 2019, 31, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef]
- Hess, D.; Solomon, E.; Spears, R.; He, J. Retreatability of a Bioceramic Root Canal Sealing Material. J. Endod. 2011, 37, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- de Miranda Candeiro, G.T.; Correia, F.C.; Duarte, M.A.H.; Ribeiro-Siqueira, D.C.; Gavini, G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J. Endod. 2012, 38, 842–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Lozano, F.J.; López-García, S.; García-Bernal, D.; Tomás-Catalá, C.J.; Santos, J.M.; Llena, C.; Lozano, A.; Murcia, L.; Forner, L. Chemical composition and bioactivity potential of the new Endosequence BC Sealer formulation HiFlow. Int. Endod. J. 2020, 53, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Haapasalo, M.; Mobuchon, C.; Li, X.; Ma, J.; Shen, Y. Cytotoxicity and the Effect of Temperature on Physical Properties and Chemical Composition of a New Calcium Silicate–based Root Canal Sealer. J. Endod. 2020, 46, 531–538. [Google Scholar] [CrossRef]
- Giacomino, C.M.; Wealleans, J.A.; Kuhn, N.; Diogenes, A. Comparative Biocompatibility and Osteogenic Potential of Two Bioceramic Sealers. J. Endod. 2019, 45, 51–56. [Google Scholar] [CrossRef]
- Carvalho, C.N.; Wang, Z.; Shen, Y.; Gavini, G.; Martinelli, J.R.; Manso, A.; Haapasalo, M. Comparative analyses of ion release, pH and multispecies biofilm formation between conventional and bioactive gutta-percha. Int. Endod. J. 2016, 49, 1048–1056. [Google Scholar] [PubMed]
- Wolfson, E.M.; Seltzer, S. Reaction of rat connective tissue to some gutta-percha formulations. J. Endod. 1975, 1, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, U.; Sundqvist, G.; Nair, P.R. Tissue reaction to gutta-percha particles of various sizes when implanted subcutaneously in guinea pigs. Eur. J. Oral Sci. 1995, 103, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Jitaru, S.; Hodisan, I.; Timis, L.; Lucian, A.; Bud, M. the use of bioceramics in endodontics—literature review. Med. Pharm. Rep. 2016, 89, 470–473. [Google Scholar] [CrossRef] [Green Version]
- Meneses, C.B.; Gambini, A.F.; Olivi, L.T.; Dos Santos, M.; Sipert, C.R. Effect of CPoint, EndoSequence BC, and gutta-percha points on viability and gene expression of periodontal ligament fibroblasts. Eur. Endont. J. 2019, 4, 57. [Google Scholar]
- Al-Haddad, A.Y.; Kutty, M.G.; Ab Aziz, Z.A.C. Push-Out Bond Strength of Experimental Apatite Calcium Phosphate Based Coated Gutta-Percha. Int. J. Biomater. 2018, 2018, 1731857. [Google Scholar] [CrossRef] [Green Version]
- Cochrane, N.; Cai, F.; Huq, N.L.; Burrow, M.; Reynolds, E. New Approaches to Enhanced Remineralization of Tooth Enamel. J. Dent. Res. 2010, 89, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Zeichner-David, M. Is there more to enamel matrix proteins than biomineralization? Matrix Biol. 2001, 20, 307–316. [Google Scholar] [CrossRef]
- Hoang, A.; Oates, T.; Cochran, D. In Vitro Wound Healing Responses to Enamel Matrix Derivative. J. Periodontol. 2000, 71, 1270–1277. [Google Scholar] [CrossRef]
- Schwartz, Z.; Carnes, D.L., Jr.; Pulliam, R.; Lohmann, C.H.; Sylvia, V.L.; Liu, Y.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Porcine fetal enamel matrix derivative stimulates proliferation but not differentiation of pre-osteoblastic 2T9 cells, inhibits proliferation and stimulates differentiation of osteoblast-like MG63 cells, and increases proliferation and differentiation of normal human osteoblast NHOst cells. J. Periodontol. 2000, 71, 1287–1296. [Google Scholar]
- Sculean, A.; Kiss, A.; Miliauskaite, A.; Schwarz, F.; Arweiler, N.B.; Hannig, M. Ten-year results following treatment of intra-bony defects with enamel matrix proteins and guided tissue regeneration. J. Clin. Periodontol. 2008, 35, 817–824. [Google Scholar] [CrossRef]
- Van der Pauw, M.T.; Van den Bos, T.; Everts, V.; Beertsen, W. Enamel matrix-derived protein stimulates attachment of periodontal ligament fibroblasts and enhances alkaline phosphatase activity and transforming growth factor β1 release of periodontal ligament and gingival fibroblasts. J. Periodontol. 2000, 71, 31–43. [Google Scholar] [CrossRef]
- Al-Hezaimi, K.; Naghshbandi, J.; Simon, J.H.S.; Oglesby, S.; Rotstein, I. Successful treatment of a radicular groove by intentional replantation and Emdogain® therapy. Dent. Traumatol. 2004, 20, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Azim, A.A.; Lloyd, A.; Huang, G.T.-J. Management of Longstanding Furcation Perforation Using a Novel Approach. J. Endod. 2014, 40, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Kerezoudis, N.P.; Siskos, G.J.; Tsatsas, V. Bilateral buccal radicular groove in maxillary incisors: Case report. Int. Endod. J. 2003, 36, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Ge, L. Effects of the enamel matrix derivative on the proliferation and odontogenic differentiation of human dental pulp cells. J. Dent. 2014, 42, 53–59. [Google Scholar] [CrossRef]
- Alobaid, A.S.; Cortes, L.M.; Lo, J.; Nguyen, T.T.; Albert, J.; Abu-Melha, A.S.; Lin, L.M.; Gibbs, J.L. Radiographic and Clinical Outcomes of the Treatment of Immature Permanent Teeth by Revascularization or Apexification: A Pilot Retrospective Cohort Study. J. Endod. 2014, 40, 1063–1070. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Hammarström, L.; Lundberg, E.; Ekdahl, H.; Matsumoto, K.; Gestrelius, S.; Lyngstadaas, S. Enamel Matrix Derivative Promotes Reparative Processes in the Dental Pulp. Adv. Dent. Res. 2001, 15, 105–107. [Google Scholar] [CrossRef]
- Barbizam, J.V.; Massarwa, R.; da Silva, L.A.B.; da Silva, R.A.B.; Nelson-Filho, P.; Consolaro, A.; Cohenca, N. Histopathological evaluation of the effects of variable extraoral dry times and enamel matrix proteins (enamel matrix derivatives) application on replanted dogs’ teeth. Dent. Traumatol. 2015, 31, 29–34. [Google Scholar] [CrossRef]
- de Oliveira, M.T.; Bentregani, L.G.; Pasternak, B.; Cancelier, F.D.; de Jesus, D.R.; Molina, G.O. Histometric study of resorption on replanted teeth with enamel matrix-derived protein. J. Contemp. Dent. Pract. 2013, 14, 468. [Google Scholar]
- Matsumoto, N.; Minakami, M.; Hatakeyama, J.; Haruna, C.; Morotomi, T.; Izumi, T.; Anan, H. Histologic Evaluation of the Effects of Emdogain Gel on Injured Root Apex in Rats. J. Endod. 2014, 40, 1989–1994. [Google Scholar] [CrossRef]
- Panzarini, S.R.; Gulinelli, J.L.; Poi, W.R.; Sonoda, C.K.; Pedrini, D.; Brandini, D.A. Treatment of root surface in delayed tooth replantation: A review of literature. Dent. Traumatol. 2008, 24, 277–282. [Google Scholar]
- Sugaya, T.; Tomita, M.; Motoki, Y.; Miyaji, H.; Kawamami, M. Influence of enamel matrix derivative on healing of root surfaces after bonding treatment and intentional replantation of vertically fractured roots. Dent. Traumatol. 2016, 32, 397–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Xu, G.; Gao, Z.; Liu, Z.; Xu, J.; Wang, J.; Zhang, C.; Wang, S. Demineralized Dentin Matrix Induces Odontoblastic Differentiation of Dental Pulp Stem Cells. Cells Tissues Organs 2016, 201, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Smith, A.J. Cells and Extracellular Matrices of Dentin and Pulp: A Biological Basis for Repair and Tissue Engineering. Crit. Rev. Oral Biol. Med. 2004, 15, 13–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, M.; Sato, D.; Hino, J.; Akazawa, T.; Tazaki, J.; Ito, K.; Arisue, M. Acid-insoluble human dentin as carrier material for recombinant human BMP-2. J. Biomed. Mater. Res. Part A 2012, 100, 571–577. [Google Scholar] [CrossRef]
- Guo, W.; He, Y.; Zhang, X.; Lu, W.; Wang, C.; Yu, H.; Liu, Y.; Li, Y.; Zhou, Y.; Zhou, J.; et al. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials 2009, 30, 6708–6723. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R.; Silverman, B.F.; Büring, K.; Dubuc, F.L.; Rosenberg, J.M. The bone induction principle. Clin. Orthop. Relat. Res. 1967, 53, 243–284. [Google Scholar] [CrossRef]
- Gomes, M.F.; Destro, M.F.D.S.S.; Banzi, É.C.D.F.; Vieira, E.M.M.; Morosolli, A.R.C.; Goulart, M.D.G.V. Optical density of bone repair after implantation of homogenous demineralized dentin matrix in diabetic rabbits. Braz. Oral Res. 2008, 22, 2275–2280. [Google Scholar] [CrossRef] [Green Version]
- Bakhshalian, N.; Hooshmand, S.; Campbell, S.C.; Kim, J.-S.; Brummel-Smith, K.; Arjmandi, B.H. Biocompatibility and Microstructural Analysis of Osteopromotive Property of Allogenic Demineralized Dentin Matrix. Int. J. Oral Maxillofac. Implant. 2013, 28, 1655–1662. [Google Scholar] [CrossRef]
- De Oliveira, G.; Miziara, M.; da Silva, E.; Ferreira, E.; Biulchi, A.; Alves, J. Enhanced bone formation during healing process of tooth sockets filled with demineralized human dentine matrix. Aust. Dent. J. 2013, 58, 326–332. [Google Scholar]
- Li, R.; Guo, W.; Yang, B.; Guo, L.; Sheng, L.; Chen, G.; Li, Y.; Zou, Q.; Xie, D.; An, X.; et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 2011, 32, 4525–4538. [Google Scholar] [CrossRef]
- Chen, J.; Cui, C.; Qiao, X.; Yang, B.; Yu, M.; Guo, W.; Tian, W. Treated dentin matrix paste as a novel pulp capping agent for dentin regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 3428–3436. [Google Scholar] [CrossRef] [PubMed]
- Epple, M.; Ganesan, K.; Heumann, R.; Klesing, J.; Kovtun, A.; Neumann, S.; Sokolova, V.J.J.C. Application of calcium phosphate nanoparticles in biomedicine. J. Mater. Chem. 2010, 20, 18–23. [Google Scholar] [CrossRef]
- Hench, L.L. An Introduction to Bioceramics; World Scientific: Singapore, 1993. [Google Scholar]
- Legeros, R. Calcium Phosphate Materials in Restorative Dentistry: A Review. Adv. Dent. Res. 1988, 2, 164–180. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef] [Green Version]
- Thamaraiselvi, T.; Rajeswari, S. Biological evaluation of bioceramic materials-a review. Carbon 2004, 24, 172. [Google Scholar]
- Osborn, J.; Newesely, H. The material science of calcium phosphate ceramics. Biomaterials 1980, 1, 108–111. [Google Scholar] [CrossRef]
- Brown, W. A new calcium phosphate, Water-setting cement. Cem. Concr. Res. 1986, 352–379. [Google Scholar]
- Costantino, P.D.; Friedman, C.D. Synthetic bone graft substitutes. Otolaryngol. Clin. N. Am. 1994, 27, 354–361. [Google Scholar] [CrossRef]
- Sugawara, A.; Chow, L.C.; Takagi, S.; Chohayeb, H. In vitro evaluation of the sealing ability of a calcium phosphate cement when used as a root canal sealer-filler. J. Endod. 1990, 16, 162–165. [Google Scholar] [CrossRef]
- Bilginer, S.; Esener, I.T.; Söylemezoğlu, F.; Tiftik, A.M. The investigation of biocompatibility and apical microleakage of tricalcium phosphate based root canal sealers. J. Endod. 1997, 23, 105–109. [Google Scholar] [CrossRef]
- Yadav, A.; Chak, R.K.; Khanna, R. Comparative Evaluation of Mineral Trioxide Aggregate, Biodentine, and Calcium Phosphate Cement in Single Visit Apexification Procedure for Nonvital Immature Permanent Teeth: A Randomized Controlled Trial. Int. J. Clin. Pediatr. Dent. 2020, 13, S1–S13. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Gbureck, U.; Barralet, J. Technological issues for the development of more efficient calcium phosphate bone cements: A critical assessment. Biomaterials 2005, 26, 6423–6429. [Google Scholar] [CrossRef] [PubMed]
- Dickens-Venz, S.H.; Takagi, S.; Chow, L.C.; Bowen, R.L.; Johnston, A.D.; Dickens, B. Physical and chemical properties of resin-reinforced calcium phosphate cements. Dent. Mater. 1994, 10, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, Y.; Ishikawa, K.; Mano, T.; Koyama, T.; Nagatsuka, H.; Matsumura, T.; Suzuki, K. Initial tissue response to anti-washout apatite cement in the rat palatal region: Comparison with conventional apatite cement. J. Biomed. Mater. Res. 2001, 55, 652–660. [Google Scholar] [CrossRef]
- Chang, K.-C.; Chang, C.-C.; Chen, W.-T.; Hsu, C.-K.; Lin, F.-H.; Lin, C.-P. Development of calcium phosphate/sulfate biphasic cement for vital pulp therapy. Dent. Mater. 2014, 30, e362–e370. [Google Scholar] [CrossRef]
- Shieh, T.-M.; Hsu, S.-M.; Chang, K.-C.; Chen, W.-C.; Lin, D.-J. Calcium Phosphate Cement with Antimicrobial Properties and Radiopacity as an Endodontic Material. Materials 2017, 10, 1256. [Google Scholar] [CrossRef] [Green Version]
- Germack, M.; Sedgley, C.M.; Sabbah, W.; Whitten, B. Antibiotic Use in 2016 by Members of the American Association of Endodontists: Report of a National Survey. J. Endod. 2017, 43, 1615–1622. [Google Scholar] [CrossRef]
- Tong, H.J.; Rajan, S.; Bhujel, N.; Kang, J.; Duggal, M.; Nazzal, H. Regenerative Endodontic Therapy in the Management of Nonvital Immature Permanent Teeth: A Systematic Review—Outcome Evaluation and Meta-analysis. J. Endod. 2017, 43, 1453–1464. [Google Scholar] [CrossRef]
- Torabinejad, M.; Nosrat, A.; Verma, P.; Udochukwu, O. Regenerative Endodontic Treatment or Mineral Trioxide Aggregate Apical Plug in Teeth with Necrotic Pulps and Open Apices: A Systematic Review and Meta-analysis. J. Endod. 2017, 43, 1806–1820. [Google Scholar] [CrossRef]
- Chen, Y.P.; Jovani-Sancho, M.d.M.; Sheth, C.C. Is revascularization of immature permanent teeth an effective and reproducible technique? Dent. Traumatol. 2015, 31, 429–436. [Google Scholar]
- Zhang, X.; Li, H.; Sun, J.; Luo, X.; Yang, H.; Xie, L.; Yang, B.; Guo, W.; Tian, W. Cell-derived micro-environment helps dental pulp stem cells promote dental pulp regeneration. Cell Prolif. 2017, 50, e12361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.-J. Characterization of the Apical Papilla and Its Residing Stem Cells from Human Immature Permanent Teeth: A Pilot Study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratwohl, A.; Pasquini, M.C.; Aljurf, M.; Atsuta, Y.; Baldomero, H.; Foeken, L.; Gratwohl, M.; Bouzas, L.F.; Confer, D.; Frauendorfer, K.; et al. One million haemopoietic stem-cell transplants: A retrospective observational study. Lancet Haematol. 2015, 2, e91–e100. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Rama, P.; Rocco, A.; Panaras, A.; Luca, M. Concise Review: Hurdles in a Successful Example of Limbal Stem Cell-based Regenerative Medicine. Stem Cells 2014, 32, 26–34. [Google Scholar] [CrossRef]
- Stefano, F.; Graziella, P.; Tatsuya, M.; Fulvio, M.; Michele, D. Towards a Gene Therapy Clinical Trial for Epidermolysis Bullosa. Rev. Recent Clin. Trials 2006, 1, 155–162. [Google Scholar] [CrossRef]
- Kitaori, T.; Ito, H.; Schwarz, E.M.; Tsutsumi, R.; Yoshitomi, H.; Oishi, S.; Nakano, M.; Fujii, N.; Nagasawa, T.; Nakamura, T. Stromal cell–derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheumatol. 2009, 60, 813–823. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Mantesso, A.; De Bari, C.; Nishiyama, A.; Sharpe, P.T. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc. Natl. Acad. Sci. USA 2011, 108, 6503–6508. [Google Scholar] [CrossRef]
| Advantages [64,65,66,67] | Disadvantages [64,65,66,67] | Prerequisites [30,68,69,70] |
|---|---|---|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, N.; Maher, N.; Amin, F.; Ghabbani, H.; Zafar, M.S.; Rodríguez-Lozano, F.J.; Oñate-Sánchez, R.E. Biomimetic Approaches in Clinical Endodontics. Biomimetics 2022, 7, 229. https://doi.org/10.3390/biomimetics7040229
Kumar N, Maher N, Amin F, Ghabbani H, Zafar MS, Rodríguez-Lozano FJ, Oñate-Sánchez RE. Biomimetic Approaches in Clinical Endodontics. Biomimetics. 2022; 7(4):229. https://doi.org/10.3390/biomimetics7040229
Chicago/Turabian StyleKumar, Naresh, Nazrah Maher, Faiza Amin, Hani Ghabbani, Muhammad Sohail Zafar, Francisco Javier Rodríguez-Lozano, and Ricardo E. Oñate-Sánchez. 2022. "Biomimetic Approaches in Clinical Endodontics" Biomimetics 7, no. 4: 229. https://doi.org/10.3390/biomimetics7040229
APA StyleKumar, N., Maher, N., Amin, F., Ghabbani, H., Zafar, M. S., Rodríguez-Lozano, F. J., & Oñate-Sánchez, R. E. (2022). Biomimetic Approaches in Clinical Endodontics. Biomimetics, 7(4), 229. https://doi.org/10.3390/biomimetics7040229








