Biomimetic Enamel Regeneration Using Self-Assembling Peptide P11-4
Abstract
:1. Introduction
2. Regeneration of Dental Tissues
3. Self-Assembling Peptide P11-4 for Enamel Regeneration
4. Safety, Biocompatibility, and Clinical Feasibility of P11-4
5. In Vitro Studies on Remineralization Effect of P11-4
6. In Vivo and Clinical Studies on Therapeutic Effect of P11-4
7. Perspectives for Treatments with P11-4
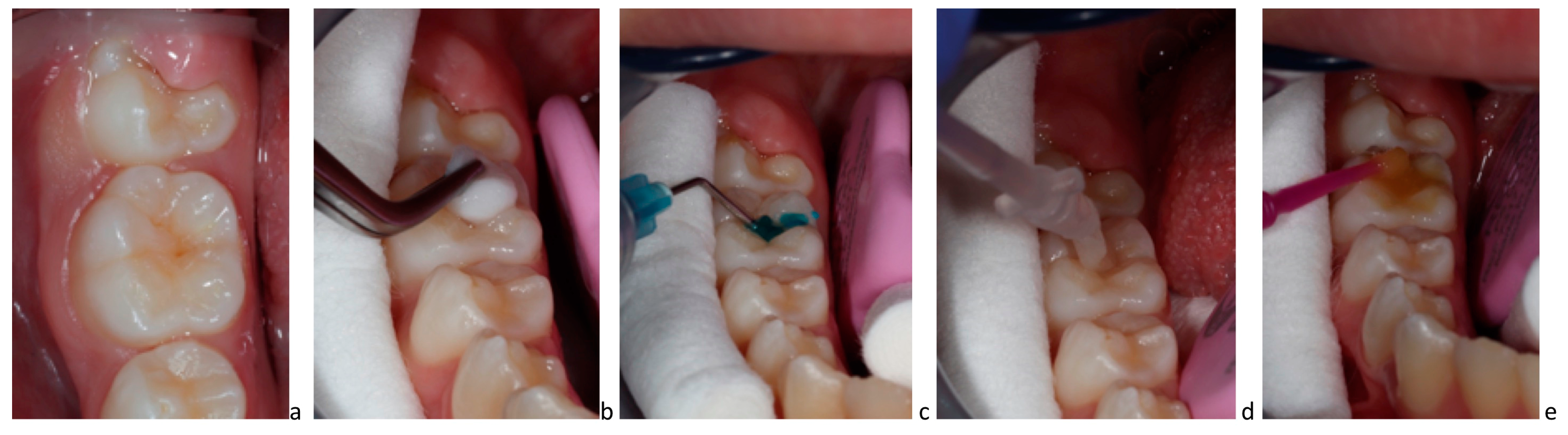
8. Clinical Application of P11-4 on Carious Lesions
9. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Marthaler, T.M. Changes in dental caries 1953–2003. Caries Res. 2004, 38, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcenes, W.; Kassebaum, N.; Bernabé, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C. Global burden of oral conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, S. Understanding and managing dental caries: A medical approach. Alpha Omegan 2007, 100, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Mejàre, I.; Stenlund, H.; Zelezny-Holmlund, C. Caries incidence and lesion progression from adolescence to young adulthood: A prospective 15-year cohort study in Sweden. Caries Res. 2004, 38, 130–141. [Google Scholar] [CrossRef]
- Splieth, C.; Ekstrand, K.; Alkilzy, M.; Clarkson, J.; Meyer-Lueckel, H.; Martignon, S.; Paris, S.; Pitts, N.; Ricketts, D.; van Loveren, C. Sealants in dentistry: Outcomes of the ORCA Saturday Afternoon Symposium 2007. Caries Res. 2010, 44, 3–13. [Google Scholar] [CrossRef]
- Marinho, V.C.; Higgins, J.P.; Logan, S.; Sheiham, A. Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2003, 2003, CD002782. [Google Scholar] [CrossRef]
- Marinho, V.C.C.; Worthington, H.V.; Walsh, T.; Clarkson, J.E. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2013, 7, CD002279. [Google Scholar] [CrossRef]
- Gupta, N.; Marya, C.M.; Nagpal, R.; Oberoi, S.S.; Dhingra, C. A Review of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) and Enamel Remineralization. Compend. Contin. Educ. Dent. 2016, 37, 36–39. [Google Scholar]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef]
- Cate Ten, J.M. Recent Advances in the Study of Dental Calculus; Irl Press: Oxford, UK, 1989; Volume 290. [Google Scholar]
- Editorial, Advancing regenerative medicine. Nat. Med. 2014, 20, 795. [CrossRef] [Green Version]
- Kirkham, J.; Firth, A.; Vernals, D.; Boden, N.; Robinson, C.; Shore, R.; Brookes, S.; Aggeli, A. Self-assembling peptide scaffolds promote enamel remineralization. J. Dent. Res. 2007, 86, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Brunton, P.A.; Davies, R.P.W.; Burke, J.L.; Smith, A.; Aggeli, A.; Brookes, S.J.; Kirkham, J. Treatment of early caries lesions using biomimetic self-assembling peptides--a clinical safety trial. Br. Dent. J. 2013, 215, E6. [Google Scholar] [CrossRef] [Green Version]
- Kirkham, J.; Brookes, S.J.; Shore, R.C.; Wood, S.R.; Smith, D.; Zhang, J.; Chen, H.; Robinson, C. Physico-chemical properties of crystal surfaces in matrix–mineral interactions during mammalian biomineralisation. Curr. Opin. Colloid Interface Sci. 2002, 7, 124–132. [Google Scholar] [CrossRef]
- Sindhura, V.; Uloopi, K.; Vinay, C.; Chandrasekhar, R. Evaluation of enamel remineralizing potential of self-assembling peptide P11-4 on artificially induced enamel lesions in vitro. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 352–356. [Google Scholar] [CrossRef]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Lutz, R.; Neukam, F.W.; Simion, M.; Schmitt, C.M. Long-term outcomes of bone augmentation on soft and hard-tissue stability: A systematic review. Clin. Oral Implant. Res. 2015, 26 (Suppl. 11), 103–122. [Google Scholar] [CrossRef]
- Felton, S.H. Self-Assembling B-Sheet Peptide Networks as Smart Scaffolds for Tissue Engineering, in Chemistry; University of Leeds: Leeds, UK, 2005; Volume 526, p. 184. [Google Scholar]
- Kyle, S.; Aggeli, A.; Ingham, E.; McPherson, M.J. Recombinant self-assembling peptides as biomaterials for tissue engineering. Biomaterials 2010, 31, 9395–9405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.-Q.; Wang, S.; Zhao, T.; Li, Y.; Yang, J.; Liu, Y.; Zhang, H.; Miao, L.; Sun, W. Biomimetic oligopeptide formed enamel-like tissue and dentin tubule occlusion via mineralization for dentin hypersensitivity treatment. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211005384. [Google Scholar] [CrossRef]
- Kind, L.; Stevanovic, S.; Wuttig, S.; Wimberger, S.; Hofer, J.; Müller, B.; Pieles, U. Biomimetic Remineralization of Carious Lesions by Self-Assembling Peptide. J. Dent. Res. 2017, 96, 790–797. [Google Scholar] [CrossRef]
- Alkilzy, M.; Tarabaih, A.; Santamaria, R.; Splieth, C. Self-assembling Peptide P11-4 and Fluoride for Regenerating Enamel. J. Dent. Res. 2018, 97, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.; Özcan, M.; Maleki Dizaj, S.; Sharifi, S.; Al-Haj Husain, N.; Eftekhari, A.; Ahmadian, E. A review on potential toxicity of dental material and screening their biocompatibility. Toxicol. Mech. Methods 2019, 29, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.L. In Situ Engineering of Skeletal Tissues Using Self-Assembled Biomimetic Scaffolds. Ph.D. Thesis, University of Leeds, Leeds, UK, 2011. [Google Scholar]
- Bröseler, F.; Tietmann, C.; Bommer, C.; Drechsel, T.; Heinzel-Gutenbrunner, M.; Jepsen, S. Randomised clinical trial investigating self-assembling peptide P11-4 in the treatment of early caries. Clin. Oral Investig. 2020, 24, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Martins, L.F.; de Sousa, J.P.; de Castilho, A.R.F.; Puppin-Rontani, J.; Davies, R.P.; Puppin-Rontani, R.M. Enhancing bond strength on demineralized dentin by pre-treatment with selective remineralising agents. J. Mech. Behav. Biomed. Mater. 2018, 81, 214–221. [Google Scholar] [CrossRef]
- Deyhle, H.; Dziadowiec, I.; Kind, L.; Thalmann, P.; Schulz, G.; Müller, B. Mineralization of Early Stage Carious Lesions In Vitro-A Quantitative Approach. Dent. J. 2015, 3, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, P.; Kochhar, A.S.; Mengi, R.; Gajare, S.M.; Nanda, S.S.; Wani, S.A. Evaluation of Remineralizing Capacity of P11-4, CPP-ACP, Silver Diamine Fluoride, and NovaMin: An In Vitro Study. J. Contemp. Dent. Pract. 2021, 22, 357–360. [Google Scholar] [CrossRef]
- Schlee, M.; Schad, T.; Koch, J.H.; Cattin, P.C.; Rathe, F. Clinical performance of self-assembling peptide P11-4 in the treatment of initial proximal carious lesions: A practice-based case series. J. Investig. Clin. Dent. 2018, 9, e12286. [Google Scholar] [CrossRef]
- Doberdoli, D.; Bommer, C.; Begzati, A.; Haliti, F.; Heinzel-Gutenbrunner, M.; Juric, H. Randomized Clinical Trial investigating Self-Assembling Peptide P11-4 for Treatment of Early Occlusal Caries. Sci. Rep. 2020, 10, 4195. [Google Scholar] [CrossRef] [Green Version]
- Alkilzy, M.; Santamaria, R.; Schmoeckel, J.; Splieth, C. Treatment of Carious Lesions Using Self-Assembling Peptides. Adv. Dent. Res. 2018, 29, 42–47. [Google Scholar] [CrossRef]
- Kondelova, P.S.; Mannaa, A.; Bommer, C.; Abdelaziz, M.; Daeniker, L.; di Bella, E.; Krejci, I. Efficacy of P11-4 for the treatment of initial buccal caries: A randomized clinical trial. Sci. Rep. 2020, 10, 20211. [Google Scholar] [CrossRef]
- Welk, A.; Ratzmann, A.; Reich, M.; Krey, K.F.; Schwahn, C. Effect of self-assembling peptide P11-4 on orthodontic treatment-induced carious lesions. Sci. Rep. 2020, 10, 6819. [Google Scholar] [CrossRef] [Green Version]
- Gohar, R.A.A.E.G.; Ibrahim, S.H.; Safwat, O.M. Evaluation of the remineralizing effect of biomimetic self-assembling peptides in post-orthodontic white spot lesions compared to fluoride-based delivery systems: Randomized controlled trial. Clin. Oral Investig. 2023, 27, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Kobeissi, R.; Badr, S.B.; Osman, E. Effectiveness of Self-assembling Peptide P11-4 Compared to Tricalcium Phosphate Fluoride Varnish in Remineralization of White Spot Lesions: A Clinical Randomized Trial. Int. J. Clin. Pediatr. Dent. 2020, 13, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Gözetici, B.; Öztürk-Bozkurt, F.; Toz-Akalın, T. Comparative Evaluation of Resin Infiltration and Remineralisation of Noncavitated Smooth Surface Caries Lesions: 6-month Results. Oral Health Prev. Dent. 2019, 17, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Rathore, M.; Goyal, A. Assesment of Remineralization of Hypomineralized Enamel Lesions Using Self- Assembling Peptide Using Laser Fluorescence—A Pilot Study. Saudi J. Oral Dent. Res. 2021, 6, 498–501. [Google Scholar]
- Kamh, R.A.; Niazy, M.A.; El-Yasaky, M.A. Clinical Performance and Remineralization Potential of Different Biomimitic Materials on White Spot Lesions. Al-Azhar Dent. J. Girls 2018, 5, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Abdel Aziz, F.; Marei, T.E.; Elmalt, M.A. Assessment of self-assembling peptide P 11-4 in the treatment of white spot lesions after orthodontic treatment. Egypt. Orthod. J. 2016, 50, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Riad, M.F.; Raafat, R.; Nabil Amin, A.M. Comparative Study Using Biomimetic Remineralization Versus Fluoride Varnish in Management of White Spot Lesion in Post Orthodontic Treated Patient: Split Mouth Randomized Clinical Trial. Indian J. Public Health Res. Dev. 2020, 11, 646–652. [Google Scholar]
- Metwally, N.; Niazy, M.; El-Malt, M. Remineralization of Early Carious Lesions using Biomimetic Selfassembling Peptides Versus Fluoride agent. (In vitro and In vivo study). Al-Azhar Dent. J. Girls 2017, 4, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Jablonski-Momeni, A.; Korbmacher-Steiner, H.; Heinzel-Gutenbrunner, M.; Jablonski, B.; Jaquet, W.; Bottenberg, P. Randomised in situ clinical trial investigating self-assembling peptide matrix P11-4 in the prevention of artificial caries lesions. Sci. Rep. 2019, 9, 269. [Google Scholar] [CrossRef] [Green Version]
- Aparna, B.K.; Yashoda, R.; Puranik, M.P. Remineralization of early enamel caries lesions using self-assembling peptides P11-4: Systematic review and meta-analysis. J. Oral Biol. Craniofacial Res. 2022, 12, 324–331. [Google Scholar] [CrossRef]
- Schlee, M.; Rathe, F.; Bommer, C.; Broseler, F.; Kind, L. Self-assembling peptide matrix for treatment of dentin hypersensitivity: A randomized controlled clinical trial. J. Periodontol. 2018, 89, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Bommer, C.; Flessa, H.-P.; Xu, X.; Kunzelmann, K.-H. Hydroxyapatite and Self-Assembling Peptide Matrix for Non-Oxidizing Tooth Whitening. J. Clin. Dent. 2018, 29, 57–63. [Google Scholar] [PubMed]
- Jablonski-Momeni, A.; Nothelfer, R.; Morawietz, M.; Kiesow, A.; Korbmacher-Steiner, H. Impact of self-assembling peptides in remineralisation of artificial early enamel lesions adjacent to orthodontic brackets. Sci. Rep. 2020, 10, 15132. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, J.; Carvalho, R.; Barbosa-Martins, L.; Torquato, R.; Mugnol, K.; Nascimento, F.; Tersariol, I.; Puppin-Rontani, R. The Self-Assembling Peptide P11-4 Prevents Collagen Proteolysis in Dentin. J. Dent. Res. 2019, 98, 347–354. [Google Scholar] [CrossRef]
- Splieth, C. Self-assembling peptides: A perfectly engineered preventive and therapeutic tool. In Innovations in Preventive Dentistry, 1st ed.; Alkilzy, M., Splieth, C.H., Eds.; Quintessenz Verlag: Berlin, Germany, 2021. [Google Scholar]


| Author, Year | Type of the Study | Main Conclusions |
|---|---|---|
| Kirkham et al., 2007 [13] | In vitro | P11-4 enhances remineralization and induces de novo hydroxyapatite nucleation |
| Brunton et al., 2013 [14] | In vivo | P11-4 is safe and associated with significant enamel regeneration by promoting mineral deposition within the subsurface tissue |
| Kind et al., 2017 [22] | In vitro | P11-4 diffuses into the depth of carious lesion |
| Metwally et al., 2017 [42] | In vitro/in vivo | Self-assembling peptides (Curodont Repair) were successful as a remineralizing agent in young permanent teeth with white spot lesions |
| Schlee et al., 2018 [30] | Practice-based case series | Radiographic and digital subtraction analyses suggested that initial proximal carious lesions can regress after treatment with P11-4 |
| Alkilzy et al., 2018 [23] | Randomized clinical trial | P11-4 in combination with fluoride application is a simple, safe, and effective noninvasive treatment for early carious lesions, which is superior to the fluoride alone |
| Sindhura et al., 2018 [16] | In vitro | SAP P11-4 exhibited superior remineralization with uniform mineral deposition compared to CPP/ACP at the 3 month interval |
| Jablonski-Momeni et al., 2019 [43] | In situ | The self-assembling peptide P11-4 may represent a preventive or therapeutic measure in accompanying orthodontic treatments to prevent the formation of white spots around orthodontic brackets or to treat and mask them |
| Doberdoli et al., 2020 [31] | Randomized clinical trial | SAP P11-4, applied in combination with fluoride varnish or twice-weekly SAPM, was a superior treatment for early caries compared to fluoride varnish alone |
| Bröseler et al., 2020 [26] | Randomized clinical trial | The size of early carious lesions treated with P11-4 was significantly reduced; this result was superior to that of fluoride varnish |
| Sedlakova et al., 2020 [33] | Controlled, blinded split-mouth clinical trial | P11-4 supports the formation of de novo hydroxyapatite crystals deep within and throughout the carious lesion body; it offers the clinician a new, effective, non-aerosol-generating, and noninvasive treatment option |
| Welk et al., 2020 [34] | Controlled clinical trial | Treatment of white spot lesions with self-assembling peptide P11-4 led to superior remineralization of the subsurface lesions compared with the control teeth |
| Kobeissi et al., 2020 [36] | Split-mouth controlled trial | P11-4 showed superiority in treatment of post-orthodontic lesions due to its guided enamel regeneration potential |
| Aparna et al., 2022 [44] | Systematic review and meta-analysis | In vitro and in vivo studies showed evidence of superior biomimetic remineralization in the P11-4 group compared to other remineralizing agents |
| Gohar et al., 2023 [35] | Randomized clinical trial | Biomimetic remineralization promoted by self-assembling peptide P11-4 achieved successful subsurface remineralization, making the material a promising guide to lesion regression in post-orthodontic therapy |
| Indications and Conditions for Treatment with P11-4 | Contraindications and Limitations for Treatment with P11-4 |
|---|---|
| Early carious lesion without cavitation | Carious lesion with cavitation |
| Patients with no/low compliance with tooth brushing and dental hygiene | Good patient compliance with dental hygiene |
| Patients with moderate caries risk and activity | Patients with low caries risk |
| Age groups accompanied with caries activity (adolescents and young adults) | Elderly patients with slow caries progression or already arrested lesions |
| Lesion progression in spite of preventive measures | Allergy to the product |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkilzy, M.; Qadri, G.; Splieth, C.H.; Santamaría, R.M. Biomimetic Enamel Regeneration Using Self-Assembling Peptide P11-4. Biomimetics 2023, 8, 290. https://doi.org/10.3390/biomimetics8030290
Alkilzy M, Qadri G, Splieth CH, Santamaría RM. Biomimetic Enamel Regeneration Using Self-Assembling Peptide P11-4. Biomimetics. 2023; 8(3):290. https://doi.org/10.3390/biomimetics8030290
Chicago/Turabian StyleAlkilzy, Mohammad, Ghalib Qadri, Christian H. Splieth, and Ruth M. Santamaría. 2023. "Biomimetic Enamel Regeneration Using Self-Assembling Peptide P11-4" Biomimetics 8, no. 3: 290. https://doi.org/10.3390/biomimetics8030290







