Abstract
As the demand for clinically effective fluoride-free oral care products for consumers increases, it is important to document which types of toothpastes have been shown in clinical studies to be effective in improving oral health. In this review, we included different indications, i.e., caries prevention, improving periodontal health, reducing dentin hypersensitivity, protecting against dental erosion, and safely improving tooth whitening in defining what constitutes improvement in oral health. While there are several professional and consumer fluoride-containing formulations fortified with calcium-phosphate-based ingredients, this review focuses on fluoride-free toothpastes containing biomimetic calcium-phosphate-based molecules as the primary active ingredients. Several databases were searched, and only clinical trials in human subjects were included; in vitro and animal studies were excluded. There were 62 oral health clinical trials on biomimetic hydroxyapatite (HAP), 57 on casein phosphopeptide-amorphous calcium phosphate (CPP-ACP), 26 on calcium sodium phosphosilicate (CSPS, or so called Bioglass), and 2 on β-tricalcium phosphate (β-TCP). HAP formulations were tested the most in clinical trials for benefits in preventing caries, dentin hypersensitivity, improving periodontal health, and tooth whitening. Based on the current clinical evidence to date, fluoride-free HAP toothpaste formulations are the most versatile of the calcium phosphate active ingredients in toothpastes for improving oral health.
1. Introduction
Even in the 21st century, poor oral health remains a major human affliction burdening health care systems in countries all over the world. Dental decay (caries) is still the most common affliction of children and very common in adults [1]. Periodontal disease today is the main reason for tooth loss throughout industrialized countries [2]. However, these human afflictions are preventable with improved diets, healthy nutrition, and especially with improved oral hygiene using toothpastes with active ingredients designed to prevent these common health issues [3]. Furthermore, as teeth are expected to last for a lifetime in ageing populations, dental tissues need to be protected from dental erosion. Some oral care products help protect teeth from mineral loss improving the longevity of the dentition [4]. In addition, people today want whiter and healthier looking teeth. Adults value the cosmetic appearance of their teeth; a whiter dentition improves confidence, improves social acceptance and even employment prospects [5]. Therefore, there is a need to develop active ingredients for toothpastes designed to help with one or more of the preventive roles in home oral care.
Fluoride has been the active ingredient most used in toothpastes throughout the world for the prevention of dental caries for a long time. That fluoride toothpaste reduces dental decay has been documented with many placebo-controlled clinical trials [6]. In order to improve fluoride toothpaste formulations to also help prevent gingivitis and lower the risk of periodontal disease, additional ingredients are added. These include pyrophosphates to help reduce calculus formation [7], bicarbonate for dental plaque removal [8], as well as antibacterial agents such as stannous salts [9], zinc salts [10], and chlorhexidine at low concentrations [11]. Natural ingredients such as herbs and plant-based antimicrobials have also been tested mostly in non-fluoride toothpastes [12].
Fluoridated toothpastes pose safety issues for children under age 6 since there is risk of dental fluorosis from fluoride ingestion [13]. Children under age 3 swallow a significant amount of toothpaste even if they are able to rinse and spit [14]. Because of the risk of fluoride ingestion, dentists in the US and Canada are advised to recommend families with children under the age of 3 year to use a pea-sized amount of fluoridated toothpaste [15,16]. In Europe, children up to age 2 should use a rice-size smear, and those aged 2 to 6 years, a pea size amount [17]. However, children, but also their parents when applying toothpaste for their children, still tend to use more toothpaste, and the majority of those ages ≤ 3 years use it 2 times a day or more often [18]. There is no direct evidence that these smaller amounts of toothpaste can prevent cavities [19]. One study showed that the pea-size amount is less effective in cleaning teeth compared to larger toothpaste amounts [20]. Recent concerns about fluoride’s potential neurotoxicity on developing brains [21,22] have also spurred on research to find alternatives to fluoride as an active ingredient in toothpastes. There is now a concerted effort to find effective non-fluoride anti-caries agents. However, because there is also the need to improve general oral health by also reducing the risk of gingivitis, reducing dentin sensitivity, preventing dental mineral loss, and improving on the appearance of teeth, the active ingredient needs to be very versatile and provide more than one benefit. One ingredient, hydroxyapatite (HAP), has been tested clinically as a general multifunctional useful active ingredient [23].
The most promising candidate active ingredients in toothpastes for achieving all these goals in the future are the calcium-phosphate-based molecules [24]. There is a wide range of these inorganic molecules and the most researched ingredients in this class that have already been tested in toothpastes are amorphous calcium derivatives (casein phosphoprotein-amorphous calcium phosphate, or CPP-ACP), hydroxyapatite, calcium sodium phosphosilicate (CSPS, Novamin, Bioglass), and beta-tricalcium phosphate (β-TCP). A recent review on randomized clinical trials comparing calcium-phosphate-based ingredients was published [25], but the authors omitted clinical evidence from in situ trials, where active ingredients are applied to human enamel slabs imbedded in appliances worn by volunteer subjects. Additionally, the authors did not examine the clinical evidence for hydroxyapatite’s usefulness in controlling caries, even though it has been shown to clinically produce calcium phosphate ions required for remineralization and there have been clinical trials published to show reversal of carious lesions [26].
This review was conducted to examine the clinical evidence published for fluoride-free calcium-phosphate-based toothpastes in order to compare them for determining which one might be a versatile, overall effective toothpaste formulation in promoting good overall oral health.
2. Materials and Methods
A PICO framework was used to guide the search. The following question was posed: “Do fluoride-free toothpastes containing calcium-phosphate-based active ingredients help to improve oral health”? The target populations (P) were humans of all ages. The intervention (I) was using one of the following calcium-phosphate-based active ingredients in a human subject clinical trial, including in situ trials using human enamel imbedded in intra-oral appliances worn by human subjects: amorphous calcium derivatives (casein phosphoprotein-amorphous calcium phosphate, or CPP-ACP), hydroxyapatite (HAP), calcium sodium phosphosilicate, (CSPS, Bioglass) and beta-tricalcium phosphate (β-TCP). The controls (C) were untreated teeth or placebo toothpastes, or positive control toothpastes, and the outcome (O) was one of the following: lowered caries or reduction in white spot lesions, reduced dentin hypersensitivity, protection against dental erosion, improvement of gingival or periodontal health, and/or improved appearance of teeth. The literature was searched using the University of Toronto databases PubMed (Medline), Scopus, and Web of Science, as well as Google Scholar, from inception to 1 June 2023. For the active ingredients, the search terms were “hydroxyapatite”, or “nano-hydroxyapatite”; “casein phosphopeptide-amorphous calcium phosphate” or “CPP-ACP”, or “amorphous calcium phosphate” or “ACP”; “calcium sodium phosphosilicate” or “CSPS” or “bioglass” or “novamin”; “beta-tricalcium phosphate” or “β-TCP” or “tricalcium phosphate” or “TCP”. For the vehicle, the search terms were “toothpaste” or “dentifrice”. For the experimental conditions, the search terms were “in vivo”; ”in situ”; “clinical trial”. For the remineralization outcomes, the search terms were “caries” or “white spot lesion” or “WSL”; “remineralization”; “erosion”. For the dentin hypersensitivity outcomes, they were “sensitivity” or “hypersensitivity”. For the gingival health outcomes, the search terms were “gingivitis” or “gingival” or “periodontal” or “periodontitis”. For the tooth whitening outcomes, the search words were “whiten(s)” or “whitening”.
Inclusion and exclusion criteria: The selection of studies was based on the need to focus on only clinical trials that produced direct clinical evidence for the outcomes directly related to oral health improvement. Animal and in vitro studies were excluded, even those that provide support for the mechanisms of how the active ingredients provide benefits since the evidence needs to be gathered from clinical trials in human subjects. In situ studies were included if the enamel slabs imbedded in appliances worn by volunteer subjects were derived from human (not bovine) enamel. In vivo effects on Streptococcus mutans and intra-oral mineral release studies were excluded. All reviews, abstracts, and book chapters were excluded. There were no language restrictions.
Microsoft Excel spreadsheets of the publications were produced by manually downloading the particulars of each publication of interest (authors, title, journal, abstract, key words) or converting “cvs” files generated by the databases, such as Scopus. The studies were ordered alphabetically, and duplicates were manually removed. Even though the collection of papers was obtained systematically, qualitative syntheses (risk of bias) and quantitative syntheses (meta-analysis) were not carried out. Qualitative (risk of bias) and quantitative (meta-analyses) have been conducted elsewhere on hydroxyapatite-containing oral care products [5,26,27], so the aim of this review was to systematically document the studies published for other fluoride-free calcium phosphate toothpastes, in comparison to the current literature on hydroxyapatite toothpastes, in order to determine the volume and extent of this evidence. Qualitative and quantitative meta-analysis of that literature was not the focus of this review.
3. Results
The results of the search are shown in Figure 1.
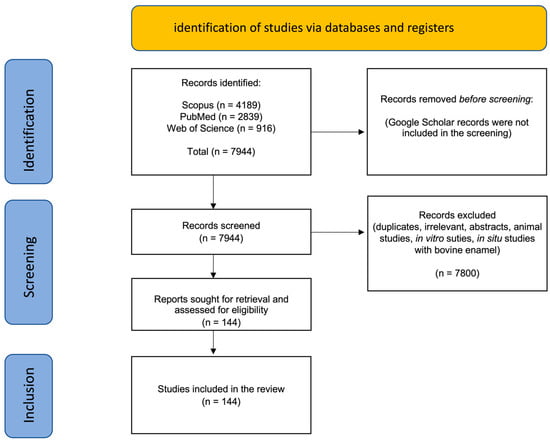
Figure 1.
Summary of the search results showing numbers of publications from each database identified and the total records included after screening and exclusion of records.
A total of 144 clinical trials and in situ clinical studies resulted after applying the exclusion and inclusion criteria. The majority (>80%) of the clinical studies were conducted on HAP- and CPP-ACP-containing toothpastes. Clinical studies on CSPS were mostly on dentin hypersensitivity (DH), and there were only two clinical trials found testing fluoride-free TCP toothpaste. With so many search term combinations, the Google Scholar search yielded an imprecise and excessively large number of titles which, after rapid screening, contained many citations, duplicates, and irrelevant publications. The focus was, therefore, on the titles retrieved in the PubMed, Scopus, and Web of Science databases. Both Scopus and Web of Science permitted “search within results” where subsets of publications were obtained from the large list of publications found using the starting primary search word (e.g., “hydroxyapatite”).
Table S1 shows the distribution of the clinical studies found using the main databases as a result of the various combinations of search terms. The publications that were retrieved in full and carefully read for each of the calcium-phosphate-based toothpaste active ingredients are summarized in Table 1, Table 2, Table 3 and Table 4. Some studies were cited more than once in the tables because they examined more than one aspect of improving oral health in the same study.

Table 1.
(a) Hydroxyapatite (HAP) clinical trials on reducing caries or white spot lesions (WSL) or preventing erosion, listed chronologically. (b) HAP studies in situ using human enamel to measure remineralization or erosion resistance. (c) HAP clinical trials on reducing dentin hypersensitivity listed chronologically. (d) Hydroxyapatite (HAP) clinical trials on improvement of gingival health listed chronologically. (e) Hydroxyapatite (HAP) clinical trials on improving tooth appearance listed chronologically.

Table 2.
(a) Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) clinical trials on reducing caries or white spot lesions (WSL) or preventing erosion, listed chronologically. (b) Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) studies in situ using human enamel to measure remineralization or erosion resistance, listed chronologically. (c) Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) clinical trials on reducing dentin hypersensitivity listed chronologically. (d) Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) clinical trials on improvement of gingival health. (e) Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) clinical trials on improving tooth appearance.

Table 3.
(a) Calcium sodium phosphosilicate (CSPS) clinical trials on reducing caries or white spot lesions (WSL) or preventing erosion, listed chronologically. (b) Calcium sodium phosphosilicate (CSPS) in situ clinical trials on reducing caries on preventing erosion. (c) Calcium sodium phosphosilicate (CSPS) clinical trials on reducing dentin hypersensitivity listed chronologically. (d) Calcium sodium phosphosilicate (CSPS) clinical trials on improvement of gingival health.

Table 4.
(a) Tricalcium phosphate (TCP) clinical trials on reducing caries or white spot lesions (WSL) or preventing erosion. (b) Tricalcium phosphate (TCP) in situ clinical trials on reducing caries on preventing erosion. (c) Tricalcium phosphate (TCP) clinical trials on reducing dentin hypersensitivity. (d) Tricalcium phosphate (TCP) clinical trials on improvement of gingival health. (e) TCP clinical trials on improving tooth appearance.
3.1. Hydroxyapatite (HAP)
The authors of this review have previously published systematic reviews of the clinical evidence that HAP reduces dental caries [26], reduces dentin hypsersenstivity [27], and improves tooth color [5]. That literature has been updated in this review to include the most recent publications. A total of 62 clinical trials were found where HAP toothpaste was shown to reduce caries, remineralize enamel and protect against erosion, reduce dentin hypesensitivity, improve tooth color, and support gingival health (Table 1).
3.2. Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP)
A total of 57 clinical trials were found on CPP-ACP toothpaste showing that this form of calcium-phosphate-based toothpaste reverses white spot lesions, protects against dental erosion and reduces dentin hypersensitivity (Table 2). Only one study was found where CPP-ACP toothpaste was tested to improve gingival health. Several studies were found to show that CPP-ACP reduced dentin hypersensitivity in studies measuring the effectiveness of professional peroxide bleaching products and that the CPP-ACP did not interfere with the whitening process, but none were found where the active ingredient CPP-ACP was tested on its own in a toothpaste for whitening teeth.
3.3. Calcium Sodium Phosphosilicate (CSPS, Novamin, Biomin, Bioglass)
There have been several studies on fluoride toothpastes fortified with Novamin (CSPS), but those were not summarized in this review since the focus was on fluoride-free toothpastes. Recently, two studies examined CSPS as an active ingredient in fluoride-free toothpastes for controlling caries or white spot lesions [122,145]. There were 23 clinical studies found showing that CSPS was also capable of reducing dentin hypersensitivity. One study was found where CSPS as an isolated active ingredient was able to control gingival health. No studies were found where CSPS toothpastes were tested to improve the color of teeth. These studies are summarized in Table 3.
3.4. Beta-Tricalcium Phosphate (β-TCP)
The clinical literature on tricalcium phosphate toothpaste in improving oral health was very limited. While there were a number of in vitro studies and studies conducted on fluoride toothpaste with added TCP (called ‘functionalized’ TCP), only one clinical trial was found where a fluoride-free TCP toothpaste was tested in a clinical trial for reducing caries, and one clinical trial examined how fluorid-free TCP in toothpaste affected dentin hypersensitivity (Table 4).
4. Discussion
This systematic review was conducted to compare the clinical evidence that has been published on the calcium-phosphate-containing toothpastes designed to improve oral health. We were interested in comparing the calcium-phosphate-based active ingredients without fluoride. Many fluoride toothpaste formulations contain calcium phosphate additives in an attempt to improve the remineralization and protection of tooth enamel, but recent studies have shown that some ingredients, such as hydroxyapatite, perform as well if not better than fluoridated toothpaste [24,26,27]. Dental fluorosis has been an increasing concern, particularly in those countries that continue to fluoridate their drinking water supplies [169]. In addition, there are concerns that prenatal and even postnatal exposure to fluoride is linked to interference with brain function during early development and growth [170]. For these reasons, it is worthwhile to seek alternatives to fluoridated toothpaste.
The fluoride-free, calcium-phosphate-containing toothpaste formulations tested in the studies summarized in this review show great promise in that they have been shown in clinical trials to prevent dental decay, reverse white spot lesions, remineralize tooth enamel, protecting it from erosion, desensitize hypersensitive root surfaces and even improve gingival health, all while whitening and brightening the dentition.
There were 62 clinical studies found where HAP was the active ingredient and almost an equal number of clinical studies conducted on CPP-ACP. The vast majority of them used fluoride-toothpaste as positive controls. No study was conducted to compare HAP vs. CPP-ACP in a head-to-head clinical trial. Toothpastes containing CPP-ACP, which contains casein peptides, cannot be used in patients who are allergic to milk proteins. Neither can that toothpaste be given a ‘vegan’ designation. Calcium phosphate ingredients, if accidentally swallowed, are considered safe since they dissociate in the stomach into their constituent inorganic components (calcium and phosphate ions), which are not only harmless but actually contribute to needed dietary sources [171].
One other fluoride-free calcium-phosphate active ingredient that should have been considered but not included in the search was calcium glycerophosphate (CaGP), an active ingredient mentioned in the review by Enax et al. [172] on the remineralization strategies of molar incisor hypocalcification. While this ingredient is used mainly to fortify fluoride toothpaste, it has only been tested in three clinical trials as an active ingredient without fluoride [173,174,175]. In those recent trials, it has been shown to be effective on its own and should really be counted as the fifth active ingredient for fluoride-free calcium-phosphate-containing toothpaste with the potential to reverse white spot lesions.
5. Future Directions
While the clinical evidence to date on the effectiveness of biomimetic fluoride-free calcium-phosphate ingredients in oral care products is already quite extensive and based on dozens of clinical trials, the development of new strategies and products for the prevention and control of oral diseases and maintaining good oral health should continue. Randomized clinical trials (RCTs) where calcium-phosphate-based toothpaste formulations are tested in head-to-head experiments have not been conducted. These would be useful in order to determine which active ingredients most meet the needs of the average consumer in improving overall oral health. Additional clinical trials are required using subjects in susceptible populations and in all age groups.
6. Conclusions
Because of the concern by families of the lasting negative effects of fluoride ingestion with the use of fluoridated toothpaste, there is increased interest by researchers in preventive dentistry to clinically test fluoride-free toothpastes for the potential to be effective in improving oral health. While there is extensive clinical evidence that the biomimetic approach of using hydroxyapatite, casein phopshopeptide-amorphous calcium phosphate, or calcium sodium phosphosilicate has proven successful, additional clinical studies would help identify the most effective active ingredients so that dentists can tailor targeted preventive regimens best suited for patients’ needs. Based on the current clinical evidence to date, fluoride-free hydroxyapatite seems to be an all-round, versatile, and effective agent for improving oral health, in comparison to the other calcium phosphate active ingredients in toothpastes tested clinically.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomimetics8040331/s1, Table S1: Search results using designated search terms.
Author Contributions
Conceptualization, H.L., F.M. and J.E.; literature search, H.L., F.M. and J.E.; qualitative synthesis and writing, H.L.; review and editing, F.M. and J.E.; supervision, final editing, corresponding author, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this review was published data in the studies referenced. Online information was referenced and accessed as shown in the reference list. No new data were created.
Conflicts of Interest
J.E. and F.M. are senior scientists and employees of Dr. Kurt Wolff GmbH & Co. KG in Germany.
References
- Meyer, F.; Enax, J. Early childhood caries: Epidemiology, aetiology, and prevention. Int. J. Dent. 2018, 2018, 1415873. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Fraihat, N.; Madae’en, S.; Bencze, Z.; Herczeg, A.; Varga, O. Clinical Effectiveness and Cost-Effectiveness of Oral-Health Promotion in Dental Caries Prevention among Children: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 2668. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, R.F.; Caneppele, T.M.F.; Scaramucci, T.; El Dib, R.; Maia, L.C.; Ferreira, D.M.T.P.; Borges, A.B. Protective effect of fluorides on erosion and erosion/abrasion in enamel: A systematic review and meta-analysis of randomized in situ trials. Arch. Oral Biol. 2020, 120, 104945. [Google Scholar] [CrossRef]
- Limeback, H.; Meyer, F.; Enax, J. Tooth Whitening with Hydroxyapatite: A Systematic Review. Dent. J. 2023, 11, 50. [Google Scholar] [CrossRef]
- Walsh, T.; Worthington, H.V.; Glenny, A.M.; Marinho, V.C.; Jeroncic, A. Fluoride toothpastes of different concentrations for preventing dental caries. Cochrane Database Syst. Rev. 2019, 3, CD007868. [Google Scholar] [CrossRef]
- Hong, I.; Lee, H.G.; Keum, H.L.; Kim, M.J.; Jung, U.W.; Kim, K.; Kim, S.Y.; Park, T.; Kim, H.J.; Kim, J.J.; et al. Clinical and Microbiological Efficacy of Pyrophosphate Containing Toothpaste: A Double-Blinded Placebo-Controlled Randomized Clinical Trial. Microorganisms 2020, 8, 1806. [Google Scholar] [CrossRef]
- Taschieri, S.; Tumedei, M.; Francetti, L.; Corbella, S.; Del Fabbro, M. Efficacy of 67% sodium bicarbonate toothpaste for plaque control and gingivitis control. A systematic review and meta-analysis. J. Evid. Based Dent. Pract. 2022, 22, 101709. [Google Scholar] [CrossRef]
- Lorenz, K.; Hoffmann, T.; Heumann, C.; Noack, B. Effect of toothpaste containing amine fluoride and stannous chloride on the reduction of dental plaque and gingival inflammation. A randomized controlled 12-week home-use study. Int. J. Dent. Hyg. 2019, 17, 237–243. [Google Scholar] [CrossRef]
- Prasad, K.V.; Therathil, S.G.; Agnihotri, A.; Sreenivasan, P.K.; Mateo, L.R.; Cummins, D. The Effects of Two New Dual Zinc plus Arginine Dentifrices in Reducing Oral Bacteria in Multiple Locations in the Mouth: 12-Hour Whole Mouth Antibacterial Protection for Whole Mouth Health. J. Clin. Dent. 2018, 29, A25–A32. [Google Scholar]
- Yates, R.; Jenkins, S.; Newcombe, R.; Wade, W.; Moran, J.; Addy, M. A 6-month home usage trial of a 1% chlorhexidine toothpaste (1). Effects on plaque, gingivitis, calculus and toothstaining. J. Clin. Periodontol. 1993, 20, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, M.; Trivedi, H.M.; Chen, D.; Gajendrareddy, P.; Chen, L. Recent Development of Active Ingredients in Mouthwashes and Toothpastes for Periodontal Diseases. Molecules 2021, 26, 2001. [Google Scholar] [CrossRef]
- Santos, A.P.; Oliveira, B.H.; Nadanovsky, P. Effects of low and standard fluoride toothpastes on caries and fluorosis: Systematic review and meta-analysis. Caries Res. 2013, 47, 382–390. [Google Scholar] [CrossRef] [PubMed]
- van Loveren, C.; Ketley, C.E.; Cochran, J.; Duckworth, R.M.; O’Mullane, D.M. Fluoride ingestion from toothpaste: Fluoride recovered from the toothbrush, the expectorate and the after-brush rinses. Commun. Dent. Oral. Epid. 2004, 32 (Suppl. S1), 54–61. [Google Scholar] [CrossRef] [PubMed]
- American Dental Association Council on Scientific Affairs. Fluoride Toothpaste Use for Young Children. J. Am. Dent. Assoc. 2014, 145, 190–191. Available online: https://jada.ada.org/article/S0002-8177(14)60226-9/pdf (accessed on 4 May 2023).
- Canadian Dental Association. CDA Position on Fluoride. February 2021. Available online: https://www.cda-adc.ca/_files/position_statements/fluoride.pdf (accessed on 4 May 2023).
- Toumba, K.J.; Twetman, S.; Splieth, C.; van Loveren, C.; Lygidakis, N.A. Guidelines on the use of fluoride for caries prevention in children: And updated EAPD policy document. Eur. Arch. Paed. Dent. 2019, 20, 507–516. [Google Scholar] [CrossRef]
- Thornton-Evans, G.; Junger, M.L.; Lin, M.; Wei, L.; Espinosa, L.; Beltran-Aguilar, E. Use of toothpaste and toothbrushing among children and adolescents—United States, 2013–2016. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 87–90. [Google Scholar] [CrossRef]
- Creeth, J.; Bosma, M.L.; Govier, K. How much is a ‘pea-sized amount’? A study of dentifrice dosing by parents in three countries. Int. Dent. J. 2013, 63 (Suppl. S2), 25–30. [Google Scholar] [CrossRef]
- Sarembe, S.; Ufer, C.; Kiesow, A.; Limeback, H.; Meyer, F.; Fuhrmann, I.; Enax, J. Influence of the Amount of Toothpaste on Cleaning Efficacy: An In Vitro Study. Eur. J. Dent. 2022, 17, 497–503. [Google Scholar] [CrossRef]
- Till, C.; Green, R. Controversy: The evolving science of fluoride: When new evidence doesn’t conform with existing beliefs. Pediatr. Res. 2020, 90, 1093–1095. [Google Scholar]
- National Toxicology Program. NTP Board of Scientific Counselors Working Group Report on the Draft State of the Science Monograph and the Draft Meta-Analysis Manuscript on Fluoride. 2023. Available online: https://ntp.niehs.nih.gov/sites/default/files/2023-04/wgrptBSC20230400.pdf (accessed on 4 May 2023).
- Steinert, S.; Zwanzig, K.; Doenges, H.; Kuchenbecker, J.; Meyer, F.; Enax, J. Daily application of a toothpaste with biomimetic hydroxyapatite and its subjective impact on dentin hypersensitivity, tooth smoothness, tooth whitening, gum bleeding, and feeling of freshness. Biomimetics 2020, 5, 17. [Google Scholar] [CrossRef]
- Meyer, F.; Amaechi, B.T.; Fabritius, H.-O.; Enax, J. Overview of Calcium Phosphates in Biomimetic Oral Care. Open Dent. J. 2018, 12, 406–423. [Google Scholar] [CrossRef] [PubMed]
- Singal, K.; Sharda, S.; Gupta, A.; Malik, V.S.; Singh, M.; Chauhan, A.; Agarwal, A.; Pradhan, P.; Singh, M. Effectiveness-of Calcium Phosphate derivative agents on the prevention and remineralization of caries among children- A systematic review & meta-analysis of randomized controlled trials. J. Evid. Based Dent. Pract. 2022, 22, 101746. [Google Scholar] [PubMed]
- Limeback, H.; Enax, J.; Meyer, F. Biomimetic hydroxyapatite and caries prevention: A systematic review and meta-analysis. Can. J. Dent. Hyg. 2021, 55, 148–159. [Google Scholar] [PubMed]
- Limeback, H.; Enax, J.; Meyer, F. Clinical Evidence of Biomimetic Hydroxyapatite in Oral Care Products for Reducing Dentin Hypersensitivity: An Updated Systematic Review and Meta-Analysis. Biomimetics 2023, 8, 23. [Google Scholar] [CrossRef]
- Kani, K.; Kani, M.; Isozaki, A.; Shintani, H.; Ohashi, T.; Tokumoto, T. Effect of apatite-containing dentifrices on dental caries in school children. J. Dent. Health 1989, 19, 104–109. [Google Scholar] [CrossRef]
- Lelli, M.; Putignano, A.; Marchetti, M.; Foltran, I.; Mangani, F.; Procaccini, M.; Roveri, N.; Orsini, G. Remineralization and repair of enamel surface by biomimetic Zn-carbonate hydroxyapatite containing toothpaste: A comparative in vivo study. Front. Physiol. 2014, 5, 333. [Google Scholar] [CrossRef]
- Makeeva, I.M.; Polyakova, M.A.; Avdeenko, O.E.; Paramonov, Y.O.; Kondrati’ev, S.A.; Pilyagina, A.A. Evaluation of the effectiveness of long-term use of Apadent Total Care toothpaste containing medical nano-hydroxyapatite. Stomatologiia 2016, 95, 34–36. [Google Scholar] [CrossRef]
- Schlagenhauf, U.; Kunzelmann, K.H.; Hannig, C.; May, T.W.; Hösl, H.; Gratza, M.; Viergutz, G.; Nazet, M.; Schamberger, S.; Proff, P. Impact of a non-fluoridated microcrystalline hydroxyapatite dentifrice on enamel caries progression in highly caries-susceptible orthodontic patients: A randomized, controlled 6-month trial. J. Investig. Clin. Dent. 2019, 10, e12399. [Google Scholar] [CrossRef]
- Bossù, M.; Saccucci, M.; Salucci, A.; Giorgio, G.D.; Bruni, E.; Uccelletti, D.; Sarto, M.S.; Familiari, G.; Relucenti, M.; Polimeni, A. Enamel remineralization and repair results of biomimetic hydroxyapatite toothpaste on deciduous teeth: An effective option to fluoride toothpaste. J. Nanobiotech 2019, 17, 17. [Google Scholar] [CrossRef]
- Badiee, M.; Jafari, N.; Fatemi, S.; Ameli, N.; Kasraei, S.; Ebadifar, A. Comparison of the effects of toothpastes containing nanohydroxyapatite and fluoride on white spot lesions in orthodontic patients: A randomized clinical trial. Dent. Res. J. 2020, 17, 354–359. [Google Scholar]
- Grocholewicz, K.; Matkowska-Cichocka, G.; Makowiecki, P.; Droździk, A.; Ey-Chmielewska, H.; Dziewulska, A.; Tomaski, M.; Trybek, G.; Janiszewska-Olszowska, J. Effect of nano-hydroxyapatite and ozone on approximal initial caries: A randomized clinical trial. Sci. Rep. 2020, 10, 11192. [Google Scholar] [CrossRef]
- Paszynska, E.; Pawinska, M.; Gawriolek, M.; Kaminska, I.; Otulakowska-Skrzynska, J.; Marczuk-Kolada, G.; Rzatowski, S.; Sokolowskaq, K.; Olszewska, A.; Schlagenhauf, U.; et al. Impact of a toothpaste with microcrystalline hydroxyapatite on the occurrence of early childhood caries: A 1-year randomized clinical trial. Sci. Rep. 2021, 11, 2650. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Pandian, S.M. Bionic effects of nano hydroxyapatite dentifrice on demineralised surface of enamel post orthodontic debonding: In-vivo split mouth study. Prog. Orthod. 2021, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Pascadopoli, M.; Gallo, S.; Lelli, M.; Tarterini, F.; Giglia, F.; Scribante, A. SEM/EDS Evaluation of the Mineral Deposition on a Polymeric Composite Resin of a Toothpaste Containing Biomimetic Zn-Carbonate Hydroxyapatite (microRepair®) in Oral Environment: A Randomized Clinical Trial. Polymers 2021, 13, 2740. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Montasser, M.A.; Abd El Latief, M.H.; Modica, G.G.; Scribante, A. Home Oral Care with Biomimetic Hydroxyapatite vs. Conventional Fluoridated Toothpaste for the Remineralization and Desensitizing of White Spot Lesions: Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 8676. [Google Scholar] [CrossRef]
- Paszynska, E.; Pawinska, M.; Enax, J.; Meyer, F.; Schulze zur Wiesche, E.; May, T.W.; Amaechi, B.T.; Limeback, H.; Hernik, A.; Otulakowska-Skrzynska, J.; et al. Caries-preventing effect of a hydroxyapatite-toothpaste in adults: A 18 months double-blinded randomized clinical trial. Front. Public Health 2023, 11, 1199728. [Google Scholar] [CrossRef]
- Najibfard, K.; Ramalingam, K.; Chedjieu, I.; Amaechi, B.T. Remineralization of early caries by a nano-hydroxyapatite dentifrice. J. Clin. Dent. 2011, 22, 139–143. [Google Scholar]
- Amaechi, B.T.; AbdulAzees, P.A.; Alshareif, D.O.; Shehata, M.A.; Lima, P.P.d.C.S.; Abdollahi, A.; Kalkhorani, P.S.; Evans, V. Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children. BDJ Open 2019, 5, 18. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Alshareif, D.O.; AbdulAzees, P.A.; Shehata, M.A.; Lima, P.P.; Abdollahi, A.; Kalkhorani, P.S.; Evans, V.; Bagheri, A.; Okoye, L.O. Anti-caries evaluation of a nano-hydroxyapatite dental lotion for use after toothbrushing: An in situ study. J. Dent. 2021, 115, 103863. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Farah, R.; Liu, J.A.; Phillips, T.S.; Perozo, B.I.; Kataoka, Y.; Meyer, F.; Enax, J. Remineralization of molar incisor hypomineralization (MIH) with a hydroxyapatite toothpaste: An in-situ study. BDJ Open 2022, 8, 33. [Google Scholar] [CrossRef]
- Hüttemann, R.W.; Dönges, H. Investigations for treating hypersensitive necks of teeth with hydroxyapatite. Dtsch. Zahnärztl Z. 1987, 42, 486–488. [Google Scholar]
- Barone, M.; Malpassi, M. Clinical trial of a 15% supermicronized hydroxyapatite gel for dentin hypersensitivity. G. Ital. Endod. 1991, 5, 43–47. [Google Scholar] [PubMed]
- Park, J.J.; Park, J.B.; Kwon, Y.H.; Herr, Y.; Chung, J.H. The effects of microcrystalline hydroxyapatite containing toothpaste in the control of tooth hypersensitivity. J. Korean Acad. Periodontol. 2005, 35, 577–590. [Google Scholar] [CrossRef][Green Version]
- Kim, M.S.; Chae, G.J.; Choi, S.H.; Chai, J.K.; Kim, C.K.; Cho, K.S. Effect of hydroxyapatite containing dentifrice on teeth hypersensitivity after periodontal therapy. J. Korean Acad. Periodontol. 2008, 38, 1–6. [Google Scholar] [CrossRef][Green Version]
- Kang, S.J.; Kwon, Y.H.; Park, J.B.; Herr, Y.; Chung, J.H. The effects of hydroxyapatite toothpaste on tooth hypersensitivity. J. Korean Acad. Periodontol. 2009, 39, 9–16. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, J.B.; Lee, C.W.; Koo, K.T.; Kim, T.I.; Seol, Y.J.; Lee, Y.M.; Ku, Y.; Chung, C.P.; Rhyu, I.C. The clinical effects of a hydroxyapatite containing toothpaste for dentine hypersensitivity. J. Korean Acad. Periodontol. 2009, 39, 87–94. [Google Scholar] [CrossRef]
- Orsini, G.; Procaccini, M.; Manzoli, L.; Giuliodori, F.; Lorenzini, A.; Putignano, A. A double-blind randomized-controlled trial comparing the desensitizing efficacy of a new dentifrice containing carbonate/hydroxyapatite nanocrystals and a sodium fluoride/potassium nitrate dentifrice. J. Clin. Periodontol. 2010, 37, 510–517. [Google Scholar] [CrossRef]
- Shetty, S.; Kohad, R.; Yeltiwar, R. Hydroxyapatite as an in-office agent for tooth hypersensitivity: A clinical and scanning electron microscopic study. J. Periodontol. 2010, 81, 1781–1789. [Google Scholar] [CrossRef]
- Browning, W.D.; Cho, S.D.; Deschepper, E.J. Effect of a nano-hydroxyapatite paste on bleaching-related tooth sensitivity. J. Esthet. Restor. Dent. 2012, 24, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Procaccini, M.; Manzoli, L.; Sparabombe, S.; Tiriduzzi, P.; Bambini, F.; Putignano, A. A 3-day randomized clinical trial to investigate the desensitizing properties of three dentifrices. J. Periodontol. 2013, 84, e65–e73. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.; Shashirekha, G. Comparison of efficacy of three different desensitizing agents for in-office relief of dentin hypersensitivity: A 4 weeks clinical study. J. Conserv. Dent. 2015, 18, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Pinojj, A.; Shetty, A.; Shetty, D.; Shetty, S. A comparison of clinical efficacy of dentifrices containing calcium sodium phosphosilicate, nanoparticle hydroxyapatite and a dentifrice containing casein phosphopeptide amorphous calcium phosphate on dentinal hypersensitivity: A comparative triple blind randomized study. Adv. Hum. Biol. 2014, 4, 57–64. [Google Scholar]
- Reddy, S.; Prasad, M.G.S.; Prasad, S.; Bhowmik, N.; Ashwini, N.; Sravya, L.; Singh, S. The effect of pro-argin technology vs nano technology using commercially available dentifrice: A comparative study. Int. J. Appl. Dent. Sci. 2014, 1, 26–30. [Google Scholar]
- Vano, M.; Derchi, G.; Barone, A.; Covani, U. Effectiveness of nano-hydroxyapatite toothpaste in reducing dentin hypersensitivity: A double-blind randomized controlled trial. Quintessence Int. 2014, 45, 703–710. [Google Scholar]
- Narmatha, V.J.; Thakur, S. An in-vivo comparative study of the efficacy of Propolis, nano-hydroxyapatite and potassium nitrate-containing desensitizing agents. RRJDS 2014, 2, 113–118. [Google Scholar]
- Amin, M.; Mehta, R.; Duseja, S.; Desai, K. Evaluation of the efficacy of commercially available nano hydroxypatite paste (Aclaim) as a desensitizing agent. Adv. Human Biol. 2015, 5, 34–38. [Google Scholar]
- Gopinath, N.M.; John, J.; Nagappan, N.; Prabhu, S.; Kumar, E.S. Evaluation of dentifrice containing nano-hydroxyapatite for dentinal hypersensitivity: A randomized controlled trial. J. Int. Oral Health 2015, 7, 118–122. [Google Scholar]
- Lee, S.-Y.; Jung, H.-I.; Jung, B.-Y.; Cho, Y.-S.; Kwon, H.-K.; Kim, B.-I. Desensitizing efficacy of nano-carbonate apatite dentifrice and Er,Cr:YSGG laser: A randomized clinical trial. Photomed. Laser Surg. 2015, 33, 9–14. [Google Scholar] [CrossRef]
- Vano, M.; Derchi, G.; Barone, A.; Genovesi, A.; Covani, U. Tooth bleaching with hydrogen peroxide and nano-hydroxyapatite: A 9-month follow-up randomized clinical trial. Int. J. Dent. Hyg. 2015, 13, 301–307. [Google Scholar] [CrossRef]
- Anand, S.; Rejula, F.; Sam, J.V.G.; Christaline, R.; Nair, M.G.; Dinakaran, S. Comparative evaluation of effect of nano-hydroxyapatite and 8% arginine containing toothpastes in managing dentin hypersensitivity: Double blind randomized clinical trial. Acta Medica 2017, 60, 114–119. [Google Scholar] [CrossRef]
- Makeeva, I.M.; Polyakova, M.A.; Doroshina, V.Y.; Sokhova, I.A.; Arakelyan, M.G.; Makeeva, M.K. Efficiency of paste and suspension with nano-hydroxyapatite on the sensitivity of teeth with gingival recession. Stomatologiia 2018, 97, 23–27. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Lemke, K.C.; Saha, S.; Gelfond, J. Clinical efficacy in relieving dentin hypersensitivity of nanohydroxyapatite-containing cream: A randomized controlled trial. Open Dent. J. 2018, 12, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Vano, M.; Derchi, G.; Barone, A.; Pinna, R.; Usai, P.; Covani, U. Reducing dentine hypersensitivity with nano-hydroxyapatite toothpaste: A double-blind randomized controlled trial. Clin. Oral Investig. 2018, 22, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Al Asmari, D.; Khan, M.K. Evaluate efficacy of desensitizing toothpaste containing zinc-carbonate hydroxyapatite nanocrystals: Non-comparative eight-week clinical study. J. Int. Soc. Prev. Community Dent. 2019, 9, 566–570. [Google Scholar] [PubMed]
- Kondyurova, E.V.; Lisevtsova, J.V.; Eliseykina, E.V.; Vilikotskiy, A.E.; Zakirova, S.A. Clinical evaluation of a dentifrice containing Nhap for the reduction of dentin hypersensitivity. Int. J. Oral Dent. Health 2019, 5, 104. [Google Scholar] [CrossRef]
- Alencar, C.D.; Ortiz, M.I.; Silva, F.A.; Alves, E.B.; Araújo, J.L.; Silva, C.M. Effect of nanohydroxyapatite associated with photobiomodulation in the control of dentin hypersensitivity: A randomized, double-blind, placebo-controlled clinical trial. Am. J. Dent. 2020, 33, 138–144. [Google Scholar]
- Ding, P.H.; Dai, A.; Hu, H.J.; Huang, J.P.; Liu, J.M.; Chen, L.L. Efficacy of nano-carbonate apatite dentifrice in relief from dentine hypersensitivity following non-surgical periodontal therapy: A randomized controlled trial. BMC Oral Health 2020, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- Alharith, D.N.; Al-Omari, M.; Almnea, R.; Basri, R.; Alshehri, A.H.; Al-Nufiee, A.A. Clinical efficacy of single application of plain nano-hydroxyapatite paste in reducing dentine hypersensitivity—A randomized clinical trial. Saudi Endod. J. 2021, 11, 24–30. [Google Scholar]
- Amaechi, B.T.; Lemke, K.C.; Saha, S.; Luong, M.N.; Gelfond, J. Clinical efficacy of nanohydroxyapatite-containing toothpaste at relieving dentin hypersensitivity: An 8 weeks randomized control trial. BDJ Open 2021, 7, 23. [Google Scholar] [CrossRef]
- Ehlers, V.; Reuter, A.K.; Kehl, E.B.; Enax, J.; Meyer, F.; Schlecht, J.; Schmidtmann, I.; Deschner, J. Efficacy of a toothpaste based on microcrystalline hydroxyapatite on children with hypersensitivity caused by MIH: A randomised controlled trial. Oral Health Prev. Dent. 2021, 19, 647–658. [Google Scholar]
- Polyakova, M.; Sokhova, I.; Doroshina, V.; Arakelyan, M.; Novozhilova, N.; Babina, K. The effect of toothpastes containing hydroxyapatite, fluoroapatite, and Zn-Mg-hydroxyapatite nanocrystals on dentin hypersensitivity: A randomized clinical trial. J. Int. Soc. Prev. Commun. Dent. 2022, 12, 252. [Google Scholar]
- Vlasova, N.; Samusenkov, V.; Novikova, I.; Nikolenko, D.; Nikolashvili, N.; Gor, I.; Danilina, A. Clinical efficacy of hydroxyapatite toothpaste containing Polyol Germanium Complex (PGC) with threonine in the treatment of dentine hypersensitivity. Saudi Dent. J. 2022, 34, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Trapani, B.; Gallo, S.; Radu, M.; Scribante, A. Biomimetic hydroxyapatite paste for molar-incisor hypomineralization: A randomized clinical trial. Oral Dis. 2022, preprint. [Google Scholar] [CrossRef] [PubMed]
- Harks, I.; Jockel-Schneider, Y.; Schlagenhauf, U.; May, T.W.; Gravemeier, M.; Prior, K.; Petersilka, G.; Ehmke, B. Impact of the Daily Use of a Microcrystal Hydroxyapatite Dentifrice on De Novo Plaque Formation and Clinical/Microbiological Parameters of Periodontal Health. A Randomized Trial. PLoS ONE 2016, 11, e0160142. [Google Scholar] [CrossRef]
- Doroshina, V.Y.; Sokhova, I.A.; Polyakova, M.A.; Margaryan, E.G. Comparative evaluation of the effectiveness of oral care products in inflammatory disease of the oral cavity, accompanied by teeth hyperesthesia. New Am. Med. J. 2019, 13, 34–40. [Google Scholar]
- Monterubbianesi, R.; Sparabombe, S.; Tosco, V.; Profili, F.; Mascitti, M.; Hosein, A.; Putignano, A.; Orsini, G. Can Desensitizing Toothpastes Also Have an Effect on Gingival Inflammation? A Double-Blind, Three-Treatment Crossover Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 8927. [Google Scholar] [CrossRef]
- Brauner, E.; Di Cosola, M.; Ambrosino, M.; Cazzolla, A.P.; Dioguardi, M.; Nocini, R.; Topi, S.; Mancini, A.; Maggiore, M.E.; Scacco, S.; et al. Efficacy of bioactivated anticalculus toothpaste on oral health: A single-blind, parallel-group clinical study. Minerva Dent. Oral Sci. 2022, 71, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Andrea, B.; Lanteri, V.; Martina, B.; Fabio, D.F.; Eleonora, F.; Annamaria, G. Proactive therapy in maintenance of periodontal orthodontic patient. Int. J. Clin. Dent. 2022, 15, 39–47. [Google Scholar]
- Niwa, M.; Sato, T.; Li, W.; Aoki, H.; Aoki, H.; Daisaku, T. Polishing and whitening properties of toothpaste containing hydroxyapatite. J. Mater. Sci. Mater. Med. 2001, 12, 277–281. [Google Scholar] [CrossRef]
- Raoufi, S.; Birkhed, D. Effect of whitening toothpastes on tooth staining using two different colour-measuring devices-a 12-week clinical trial. Int. Dent. J. 2010, 60, 419–423. [Google Scholar] [PubMed]
- Woo, G.-J.; Kim, E.-K.; Jeong, S.-H.; Song, K.-B.; Goo, H.-J.; Jeon, E.-S.; Choi, Y.-H. Comparison of the whitening effect of toothpastes containing 0.25% hydroxyapatite and 0.75% hydrogen peroxide. J. Korean Acad. Oral Health 2014, 38, 3–9. [Google Scholar] [CrossRef]
- Bommer, C.; Flessa, H.P.; Xu, X.; Kunzelmann, K.H. Hydroxyapatite and self-assembling peptide matrix for non-oxidizing tooth whitening. J. Clin. Dent. 2018, 29, 57–63. [Google Scholar]
- Steinert, S.; Kuchenbecker, J.; Meyer, F.; Simader, B.; Zwanzig, K.; Enax, J. Whitening effects of a novel oral care gel with biomimetic hydroxyapatite: A 4-week observational pilot study. Biomimetics 2020, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Sköld-Larsson, K.; Hallgren, A.; Petersson, L.G.; Twetman, S. Effect of a dental cream containing amorphous cream phosphate complexes on white spot lesion regression assessed by laser fluorescence. Oral Health Prev. Dent. 2007, 5, 229–233. [Google Scholar] [PubMed]
- Bailey, D.L.; Adams, G.G.; Tsao, C.E.; Hyslop, A.; Escobar, K.; Manton, D.J.; Reynolds, E.C.; Morgan, M.V. Regression of post-orthodontic lesions by a remineralizing cream. J. Dent. Res. 2009, 88, 1148–1153. [Google Scholar] [CrossRef]
- Rao, S.K.; Bhat, G.S.; Aradhya, S.; Devi, A.; Bhat, M. Study of the efficacy of toothpaste containing casein phosphopeptide in the prevention of dental caries: A randomized controlled trial in 12- to 15-year-old high caries risk children in Bangalore, India. Caries Res. 2009, 43, 430–435. [Google Scholar] [CrossRef]
- Uysal, T.; Amasyali, M.; Koyuturk, A.E.; Ozcan, S. Effects of different topical agents on enamel demineralization around orthodontic brackets: An in vivo and in vitro study. Aust. Dent. J. 2010, 55, 268–274. [Google Scholar] [CrossRef]
- Bröchner, A.; Christensen, C.; Kristensen, B.; Tranæus, S.; Karlsson, L.; Sonnesen, L.; Twetman, S. Treatment of post-orthodontic white spot lesions with casein phosphopeptide-stabilised amorphous calcium phosphate. Clin. Oral Investig. 2011, 15, 369–373. [Google Scholar] [CrossRef]
- Akin, M.; Basciftci, F.A. Can white spot lesions be treated effectively? Angle Orthod. 2012, 82, 770–775. [Google Scholar] [CrossRef]
- Sitthisettapong, T.; Phantumvanit, P.; Huebner, C.; Derouen, T. Effect of CPP-ACP paste on dental caries in primary teeth: A randomized trial. J. Dent. Res. 2012, 91, 847–852. [Google Scholar] [CrossRef]
- Wang, J.X.; Yan, Y.; Wang, X.J. Clinical evaluation of remineralization potential of casein phosphopeptide amorphous calcium phosphate nanocomplexes for enamel decalcification in orthodontics. Chin. Med. J. 2012, 125, 4018–4021. [Google Scholar] [PubMed]
- Krithikadatta, J.; Fredrick, C.; Abarajithan, M.; Kandaswamy, D. Remineralisation of occlusal white spot lesion with a combination of 10% CPP-ACP and 0.2% sodium fluoride evaluated using Diagnodent: A pilot study. Oral Health Prev. Dent. 2013, 11, 191–196. [Google Scholar] [PubMed]
- Plonka, K.A.; Pukallus, M.L.; Holcombe, T.F.; Barnett, A.G.; Walsh, L.J.; Seow, W.K. Randomized controlled trial: A randomized controlled clinical trial comparing a remineralizing paste with an antibacterial gel to prevent early childhood caries. Pediatr. Dent. 2013, 35, 8–12. [Google Scholar] [PubMed]
- Aykut-Yetkiner, A.; Kara, N.; Ateş, M.; Ersin, N.; Ertuğrul, F. Does casein phosphopeptide amorphous calcium phosphate provide remineralization on white spot lesions and inhibition of Streptococcus mutans? J. Clin. Pediatr. Dent. 2014, 38, 302–306. [Google Scholar] [CrossRef]
- Yazıcıoğlu, O.; Ulukapi, H. The investigation of non-invasive techniques for treating early approximal carious lesions: An in vivo study. Int. Dent. J. 2014, 64, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zou, J.; Yang, R.; Zhou, X. Remineralization effects of casein phosphopeptide-amorphous calcium phosphate crème on artificial early enamel lesions of primary teeth. Int. J. Paediatr. Dent. 2011, 21, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Llena, C.; Leyda, A.M.; Forner, L. CPP-ACP and CPP-ACFP versus fluoride varnish in remineralisation of early caries lesions. A prospective study. Eur. J. Paediatr. Dent. 2015, 16, 181–186. [Google Scholar]
- Memarpour, M.; Fakhraei, E.; Dadaein, S.; Vossoughi, M. Efficacy of fluoride varnish and casein phosphopeptide-amorphous calcium phosphate for remineralization of primary teeth: A randomized clinical trial. Med. Princ. Pract. 2015, 24, 231–237. [Google Scholar] [CrossRef]
- Sitthisettapong, T.; Doi, T.; Nishida, Y.; Kambara, M.; Phantumvanit, P. Effect of CPP-ACP Paste on Enamel Carious Lesion of Primary Upper Anterior Teeth Assessed by Quantitative Light-Induced Fluorescence: A One-Year Clinical Trial. Caries Res. 2015, 49, 434–441. [Google Scholar] [CrossRef]
- Sim, C.P.C.; Wee, J.; Xu, Y.; Cheung, Y.-B.; Soong, Y.-L.; Manton, D.J. Anti-caries effect of CPP-ACP in irradiated nasopharyngeal carcinoma patients. Clin. Oral Investig. 2015, 19, 1005–1011. [Google Scholar] [CrossRef]
- Esenlik, E.; Uzer Çelik, E.; Bolat, E. Efficacy of a casein phosphopeptide amorphous calcium phosphate (CPP-ACP) paste in preventing white spot lesions in patients with fixed orthodontic appliances: A prospective clinical trial. Eur. J. Paediatr. Dent. 2016, 17, 274–280. [Google Scholar]
- Güçlü, Z.A.; Alaçam, A.; Coleman, N.J. A 12-Week Assessment of the Treatment of White Spot Lesions with CPP-ACP Paste and/or Fluoride Varnish. Biomed. Res. Int. 2016, 2016, 8357621. [Google Scholar] [CrossRef]
- Munjal, D.; Garg, S.; Dhindsa, A.; Sidhu, G.K.; Sethi, H.S. Assessment of White Spot Lesions and In-Vivo Evaluation of the Effect of CPP-ACP on White Spot Lesions in Permanent Molars of Children. J. Clin. Diagn. Res. 2016, 10, ZC149-54. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.P.; Goyal, A.; Utreja, A.K.; Jena, A.K. Effects of various remineralizing agents on the outcome of post-orthodontic white spot lesions (WSLs): A clinical trial. Prog. Orthod. 2016, 17, 25. [Google Scholar] [CrossRef]
- Karabekiroğlu, S.; Ünlü, N.; Küçükyilmaz, E.; Şener, S.; Botsali, M.S.; Malkoç, S. Treatment of post-orthodontic white spot lesions with CPP-ACP paste: A three year follow up study. Dent. Mater. J. 2017, 36, 791–797. [Google Scholar] [CrossRef]
- Mendes, A.C.; Restrepo, M.; Bussaneli, D.; Zuanon, A.C. Use of Casein Amorphous Calcium Phosphate (CPP-ACP) on White-spot Lesions: Randomised Clinical Trial. Oral Health Prev. Dent. 2018, 16, 27–31. [Google Scholar] [PubMed]
- Wang, Y.H.; Liu, F.; Liu, H.N.; Wang, Q.X.; Xing, W.Z.; Li, Z.C. Impact assessment on enamel remineralization after orthodontic treatment with casein phosphopeptide calcium phosphate complex. Shanghai Kou Qiang Yi Xue 2018, 27, 382–385. [Google Scholar] [PubMed]
- Bobu, L.; Murariu, A.; Topor, G.; Beznea, A.; Vasluianu, R. Comparative evaluation of casein phosphopeptide—Amorphous calcium phosphate and fluoride in managing early caries lesions. Rev. Chim. 2019, 70, 3746–3749. [Google Scholar] [CrossRef]
- Tingyun, W.; Detang, W.; Youjia, Z.; Qiong, R.; Aimin, W.; Shangqun, H.; Xiaofang, Z. Effects of different types of fluoride-free toothpaste on the remineralization of enamel after acid erosion: An in vivo study. Chin. J. Tiss. Eng. Res. 2019, 23, 2842–2846. [Google Scholar]
- Al-Batayneh, O.B.; Bani Hmood, E.I.; Al-Khateeb, S.N. Assessment of the effects of a fluoride dentifrice and GC Tooth Mousse on early caries lesions in primary anterior teeth using quantitative light-induced fluorescence: A randomised clinical trial. Eur. Arch. Paediatr. Dent. 2020, 21, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Bangi, S.L.; Konda, P.; Talapaneni, A.K.; Fatima, A.; Hussain, A. Evaluation of three commercially available materials in reducing the white spot lesions during fixed orthodontic treatment: A prospective randomized controlled trial. J. Ind. Orthod. Soc. 2020, 54, 100–105. [Google Scholar]
- Perić, T.; Marković, D.; Tomić-Spirić, V.; Petrović, B.; Perić-Popadić, A.; Marković, E. Clinical efficacy of casein phosphopeptide-amorphous calcium phosphate and casein phosphopeptide-amorphous calcium fluoride phosphate and their influence on the quality of life in patients with Sjögren’s syndrome. Srp. Arh. Celok. Lek. 2020, 148, 528–534. [Google Scholar] [CrossRef]
- Ashour, D.G.; Farid, M.R.; Mosallam, R.S.; Abouauf, E.A. Effect of CPP-ACP pastes with/without fluoride on white spot lesion progression, salivary pH, and fluoride release in high caries risk patients: A randomized clinical trial. J. Int. Oral Health 2021, 13, 336–343. [Google Scholar]
- Juárez-López, M.L.A.; Gómez-Rivas, Y.C.; Murrieta-Pruneda, F. Casein-phosphopeptide-amorphous-calcium-phosphate plus brushing with fluoride toothpaste for remineralization of early caries. Acta Pediátrica México 2021, 42, 272–279. [Google Scholar]
- El-Sherif, S.A.; Shaalan, O.O.; Hamza, N.K.; El Baz, M.A. Remineralization Potential of Pearl Powder Compared to Casein Phosphopeptide Amorphous Calcium Phosphate on Enamel White Spot Lesions (Randomized Clinical Trial). J. Pharm. Negat. Results 2022, 13, 1662–1671. [Google Scholar]
- Hamdi, K.; Hamama, H.H.; Motawea, A.; Fawzy, A.; Mahmoud, S.H. Long-term evaluation of early-enamel lesions treated with novel experimental tricalcium silicate paste: A 2-year randomized clinical trial. J. Esthet. Restor. Dent. 2022, 34, 1113–1121. [Google Scholar] [CrossRef]
- Olgen, I.C.; Sonmez, H.; Bezgin, T. Effects of different remineralization agents on MIH defects: A randomized clinical study. Clin. Oral. Investig. 2022, 26, 3227–3238. [Google Scholar] [CrossRef]
- Salah, R.; Afifi, R.R.; Kehela, H.A.; Aly, N.M.; Rashwan, M. Efficacy of a novel bioactive glass in the treatment of enamel white spot lesions: A randomized controlled trial. J. Evid. Based Dent. Pract. 2022, 22, 101725. [Google Scholar] [CrossRef]
- Simon, L.S.; Dash, J.K.; U, D.; Philip, S.; Sarangi, S. Management of Post Orthodontic White Spot Lesions Using Resin Infiltration and CPP-ACP Materials- A Clinical Study. J. Clin. Paeditr. Dent. 2022, 46, 70–74. [Google Scholar]
- Srinivasan, N.; Kavitha, M.; Loganathan, S.C. Comparison of the remineralization potential of CPP-ACP and CPP-ACP with 900 ppm fluoride on eroded human enamel: An in situ study. Arch. Oral. Biol. 2010, 55, 541–544. [Google Scholar] [CrossRef]
- Shen, P.; Walker, G.D.; Yuan, Y.; Reynolds, C.; Stanton, D.P.; Fernando, J.R.; Reynolds, E.C. Importance of bioavailable calcium in fluoride dentifrices for enamel remineralization. J. Dent. 2018, 78, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Perić, T.; Markovic, D.; Petrovic, B.; Radojevic, V.; Todorovic, T.; Radicevic, B.A.; Heinemann, R.J.; Susic, G.; Popadic, A.P.; Spiric, V.T. Efficacy of pastes containing CPP-ACP and CPP-ACFP in patients with Sjögren’s syndrome. Clin. Oral Investig. 2015, 19, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Garry, A.P.; Flannigan, N.L.; Cooper, L.; Komarov, G.; Burnside, G.; Higham, S.M. A randomised controlled trial to investigate the remineralising potential of Tooth Mousse™ in orthodontic patients. J. Orthod. 2017, 44, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zawaideh, F.I.; Owais, A.I.; Mushtaha, S. Effect of CPP-ACP or a potassium nitrate sodium fluoride dentifrice on enamel erosion prevention. J. Clin. Paediatr. Dent. 2017, 41, 135–140. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, N.-W.; Ye, X.-Y.; Zheng, H.-Y.; Attin, T.; Cheng, H. In situ effect of Tooth Mousse containing CPP-ACP on human enamel subjected to in vivo acid attacks. J. Dent. 2018, 76, 40–45. [Google Scholar] [CrossRef]
- de Oliveira, P.R.A.; Barboza, C.M.; Barreto, L.S.C.; Tostes, M.A. Effect of CPP-ACP on remineralization of artificial caries-like lesion: An in situ study. Braz. Oral Res. 2020, 34, e061. [Google Scholar] [CrossRef]
- de Oliveira, P.R.A.; Barreto, L.S.D.C.; Tostes, M.A. Effectiveness of CPP-ACP and fluoride products in tooth remineralization. Int. J. Dent. Hyg. 2022, 20, 635–642. [Google Scholar] [CrossRef]
- Kumar, A.; Goyal, A.; Gauba, K.; Kapur, A.; Singh, S.K.; Mehta, S.K. An evaluation of remineralised MIH using CPP-ACP and fluoride varnish: An in-situ and in-vitro study. Eur. Arch. Paediatr. Dent. 2022, 23, 79–97. [Google Scholar] [CrossRef]
- Borges, B.C.; Borges, J.S.; de Melo, C.D.; Pinheiro, I.V.; Santos, A.J.; Braz, R.; Montes, M.A. Efficacy of a novel at-home bleaching technique with carbamide peroxides modified by CPP-ACP and its effect on the microhardness of bleached enamel. Oper. Dent. 2011, 36, 521–528. [Google Scholar] [CrossRef]
- Özgül, B.M.; Saat, S.; Sönmez, H.; Oz, F.T. Clinical evaluation of desensitizing treatment for incisor teeth affected by molar-incisor hypomineralization. J. Clin. Pediatr. Dent. 2013, 38, 101–105. [Google Scholar] [CrossRef]
- Maghaireh, G.A.; Alzraikat, H.; Guidoum, A. Assessment of the effect of casein phosphopeptide-amorphous calcium phosphate on postoperative sensitivity associated with in-office vital tooth whitening. Oper. Dent. 2014, 39, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Mahesuti, A.; Duan, Y.L.; Wang, G.; Cheng, X.R.; Matis, B.A. Short-term Efficacy of Agents Containing KNO3 or CPP-ACP in Treatment of Dentin Hypersensitivity. Chin. J. Dent. Res. 2014, 17, 43–47. [Google Scholar] [PubMed]
- Zhang, D.; Zhang, X.; Peng, H.; Sun, H. Casein phosphopeptide-amorphous calcium phosphate nanocomplexes as a preventive agent for radiation caries and dental sensitivity in irradiated head and neck cancer patients. Chin. J. Clin. Oncol. 2014, 41, 1293–1296. [Google Scholar]
- Konekeri, V.; Bennadi, D.; Manjunath, M.; Kshetrimayum, N.; Siluvai, S.; Reddy, C.V.K. A clinical study to assess the effectiveness of CPP-ACP (casein phosphopeptide-amorphous calcium phosphate) versus potassium-nitrate (KNO3) on cervical dentine hypersensitivity. J. Young Pharm. 2015, 7, 217–224. [Google Scholar] [CrossRef]
- Nanjundasetty, J.K.; Ashrafulla, M. Efficacy of desensitizing agents on postoperative sensitivity following an in-office vital tooth bleaching: A randomized controlled clinical trial. J. Conserv. Dent. 2016, 19, 207–211. [Google Scholar] [CrossRef]
- Tarique, N.; Awan, R.; Saleh, M.I. Vital tooth bleaching and management of post operative sensitivity: A clinical trial evaluating the efficacy of different desensitizing materials. Pak. J. Med. Health Sci. 2017, 11, 1564–1567. [Google Scholar]
- Pasini, M.; Giuca, M.R.; Scatena, M.; Gatto, R.; Caruso, S. Molar incisor hypomineralization treatment with casein phosphopeptide and amorphous calcium phosphate in children. Minerva Stomatol. 2018, 67, 20–25. [Google Scholar] [CrossRef]
- Yassin, O.; Milly, H. Effect of CPP-ACP on efficacy and postoperative sensitivity associated with at-home vital tooth bleaching using 20% carbamide peroxide. Clin. Oral Investig. 2019, 23, 1555–1559. [Google Scholar] [CrossRef]
- Adil, H.M.; Jouhar, R.; Ahmed, M.A.; Basha, S.; Ahmed, N.; Abbasi, M.S.; Maqsood, A.; Nagarajappa, A.K.; Alam, M.K. Comparison of casein phosphopeptide with potassium nitrate and sodium monofluorophosphate desensitizing efficacy after in-office vital bleaching—A randomized trial. Appl. Sci. 2021, 11, 9291. [Google Scholar] [CrossRef]
- Gümüştaş, B.; Dikmen, B. Effectiveness of remineralization agents on the prevention of dental bleaching induced sensitivity: A randomized clinical trial. Int. J. Dent. Hyg. 2021, 20, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Jain, R.K. Comparative evaluation of white spot lesions incidence between NovaMin, Probiotic and fluoride containing dentifrices during orthodontic treatment using laser fluorescence- a prospective randomized controlled trial. Clin. Investig. Orthog. 2023, 82, 75–82. [Google Scholar] [CrossRef]
- Du, M.Q.; Bian, Z.; Jiang, H.; Greenspan, D.C.; Burwell, A.K.; Zhong, J.; Tai, B.J. Clinical evaluation of a dentifrice containing calcium sodium phosphosilicate (NovaMin) for the treatment of dentin hypersensitivity. Am. J. Dent. 2008, 21, 210–214. [Google Scholar]
- Litkowski, L.; Greenspan, D.C. A clinical study of the effect of calcium sodium phosphosilicate on dentin hypersensitivity-Proof of principle. J. Clin. Dent. 2010, 21, 77–81. [Google Scholar] [PubMed]
- Narongdej, T.; Sakoolnamarka, R.; Boonroung, T. The effectiveness of a calcium sodium phosphosilicate desensitizer in reducing cervical dentin hypersensitivity: A pilot study. J. Am. Dent. Assoc. 2010, 141, 995–999. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Sharma, A. Comparison of clinical efficacy of a dentifrice containing calcium sodium phosphosilicate to a dentifrice containing potassium nitrate and to a placebo on dentinal hypersensitivity: A randomized clinical trial. J. Periodontol. 2010, 81, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Salian, S.; Thakur, S.; Kulkarni, S.; Latorre, G. A randomized controlled clinical study evaluating the efficacy of two desensitizing dentifrices. J. Clin. Dent. 2010, 21, 82–87. [Google Scholar] [PubMed]
- Sharma, N.; Roy, S.; Kakar, A.; Greenspan, D.C.; Scott, R. A clinical study comparing oral formulations containing 7.5% calcium sodium phosphosilicate (Novamin®), 5% potassium nitrate, and 0.4% stannous fluoride for the management of dentin hypersensitivity. J. Clin. Dent. 2010, 21, 88–92. [Google Scholar]
- West, N.X.; Macdonald, E.L.; Jones, S.B.; Claydon, N.C.A.; Hughes, N.; Jeffery, P. Randomized in situ clinical study comparing the ability of two new desensitizing toothpaste technologies to occlude patent dentin tubules. J. Clin. Dent. 2011, 22, 82–89. [Google Scholar] [PubMed]
- Pradeep, A.R.; Agarwal, E.; Naik, S.B.; Bajaj, P.; Kalra, N. Comparison of efficacy of three commercially available dentrifices on dentinal hypersensitivity: A randomized clinical trial. Aust. Dent. J. 2012, 57, 429–434. [Google Scholar] [CrossRef]
- Rajesh, K.S.; Hedge, S.; Kumar, A.; Shetty, D.G. Evaluation of the efficacy of a 5% calcium sodium phosphosilicate (Novamin®) containing dentifrice for the relief of dentinal hypersensitivity: A clinical study. Ind. J. Dent. Res. 2012, 23, 363–367. [Google Scholar]
- Surve, S.M.; Acharya, A.B.; Shetty, A.; Thakur, S.L. Efficacy of calcium sodium phosphosilicate in managing dentinal hypersensitivity. Gen. Dent. 2012, 60, e308–e311. [Google Scholar] [PubMed]
- Acharya, A.B.; Surve, S.M.; Thakur, S.L. A clinical study of the effect of calcium sodium phosphosilicate on dentin hypersensitivity. J. Clin. Exp. Dent. 2013, 5, e18–e22. [Google Scholar] [CrossRef]
- Pintado-Palomino, K.; Peitl Filho, O.; Zanotto, E.D.; Tirapelli, C. A clinical, randomized, controlled study on the use of desensitizing agents during tooth bleaching. J. Dent. 2015, 43, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.R.; Khatri, S.G.; Acharya, S.; Patil, S.T. Evaluation of instant desensitization after a single topical application over 30 days: A randomized trial. Aust. Dent. J. 2015, 60, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Majji, P.; Murthy, K.R. Clinical efficacy of four interventions in the reduction of dentinal hypersensitivity: A 2-month study. Ind. J. Dent. Res. 2016, 27, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Sufi, F.; Hall, C.; Mason, S.; Shaw, D.; Kennedy, L.; Gallob, J.T. Efficacy of an experimental toothpaste containing 5% calcium sodium phosphosilicate in the relief of dentin hypersensitivity: An 8-week randomized study (Study 1). Am. J. Dent. 2016, 29, 93–100. [Google Scholar]
- Sufi, F.; Hall, C.; Mason, S.; Shaw, D.; Milleman, J.; Milleman, K. Efficacy of an experimental toothpaste containing 5% calcium sodium phosphosilicate in the relief of dentin hypersensitivity: An 8-week randomized study (Study 2). Am. J. Dent. 2016, 29, 101–109. [Google Scholar]
- Athuluru, D.; Reddy, C.; Sudhir, K.; Kumar, K.; Gomasani, S.; Nagarakanti, S. Evaluation and comparison of efficacy of three desensitizing dentifrices on dentinal hypersensitivity and salivary biochemical characteristics: A randomized controlled trial. Dent. Res. J. 2017, 14, 150–157. [Google Scholar]
- Hall, C.; Mason, S.; Cooke, J. Exploratory randomised controlled clinical study to evaluate the comparative efficacy of two occluding toothpastes—A 5% calcium sodium phosphosilicate toothpaste and an 8% arginine/calcium carbonate toothpaste—for the longer-term relief of dentine hypersensitivity. J. Dent. 2017, 60, 36–43. [Google Scholar]
- Fu, Y.; Sufi, F.; Wang, N.; Young, S.; Feng, X. An exploratory randomised study to evaluate the efficacy of an experimental occlusion-based dentifrice in the relief of dentin hypersensitivity. Oral Health Prev. Dent. 2019, 17, 107–115. [Google Scholar]
- Alsherbiney, H.; El-Deeb, H.; Alsherbiney, A.; Abdou, A.; Mobarak, E.; Hamza, O. Effect of calcium sodium phosphosilicate-containing compared to nanohydroxyapatite-containing toothpastes on dentinal tubule occlusion: A randomized clinical in situ study. J. Int. Oral Health 2020, 12, 305–312. [Google Scholar]
- Bhowmik, E.; Pawar Chandrashekhar, D.; Sharma Hareesha, M. Comparative evaluation of fluorinol and calcium sodium phosphosilicate-containing toothpastes in the treatment of dentin hypersensitivity. Int. J. Dent. Hyg. 2021, 19, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ongphichetmetha, N.; Lertpimonchai, A.; Champaiboon, C. Bioactive glass and arginine dentifrices immediately relieved dentine hypersensitivity following non-surgical periodontal therapy: A randomized controlled trial. J. Periodontol. 2022, 93, 246–255. [Google Scholar] [CrossRef]
- Detsomboonrat, P.; Trairatvorakul, C.; Pisarnturakit, P.P. Similar 1-year caries increment after use of fluoride or non-fluoride toothpaste in infants and toddlers. Fluoride 2016, 49, 313–326. [Google Scholar]
- Jang, J.-H.; Oh, S.; Kim, H.-J.; Kim, D.-S. A randomized clinical trial for comparing the efficacy of desensitizing toothpastes on the relief of dentin hypersensitivity. Nat. Sci. Rep. 2023, 13, 5171. [Google Scholar] [CrossRef] [PubMed]
- Neurath, C.; Limeback, H.; Osmunson, B.; Connett, M.; Kanter, V.; Wells, C.R. Dental Fluorosis Trends in US Oral Health Surveys: 1986 to 2012. JDR Clin. Trans. Res. 2019, 4, 298–308. [Google Scholar] [CrossRef]
- Farmus, L.; Till, C.; Green, R.; Hornung, R.; Martinez Mier, E.A.; Ayotte, P.; Muckle, G.; Lanphear, B.P.; Flora, D.B. Critical windows of fluoride neurotoxicity in Canadian children. Environ. Res. 2021, 200, 111315. [Google Scholar] [CrossRef]
- Enax, J.; Meyer, F.; Schulze Zur Wiesche, E.; Epple, M. On the Application of Calcium Phosphate Micro- and Nanoparticles as Food Additive. Nanomaterials 2022, 12, 4075. [Google Scholar] [CrossRef]
- Enax, J.; Amaechi, B.T.; Farah, R.; Liu, J.A.; Schulze zur Wiesche, E.; Meyer, F. Remineralization Strategies for Teeth with Molar Incisor Hypomineralization (MIH): A Literature Review. Dent. J. 2023, 11, 80. [Google Scholar] [CrossRef]
- Sezer, B.; Kargul, B. Effect of Remineralization Agents on Molar-Incisor Hypomineralization-Affected Incisors: A Randomized Controlled Clinical Trial. J. Clin. Pediatr. Dent. 2022, 46, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Sezer, B.; Tuğcu, N.; Calışkan, C.; Baş Durmus, B.; Kupets, T.; Bekiroğlu, N.; Kargül, B.; Bourgeois, D. Effect of casein phosphopeptide amorphous calcium fluoride phosphate and calcium glycerophosphate on incisors with molar-incisor hypomineralization: A cross-over, randomized clinical trial. Bio-Med. Mater. Eng. 2022, 33, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.A.; Yildiz, P.K.; Gokkaya, B.; Bilsel, S.O.; Kargul, B. The effect of a novel toothpaste in children with white spot lesions. J. Pak. Med. Assoc. 2022, 72, 2170–2174. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).