Interfacial Dynamics in Dual Channels: Inspired by Cuttlebone
Abstract
:1. Introduction
2. Materials and Methods
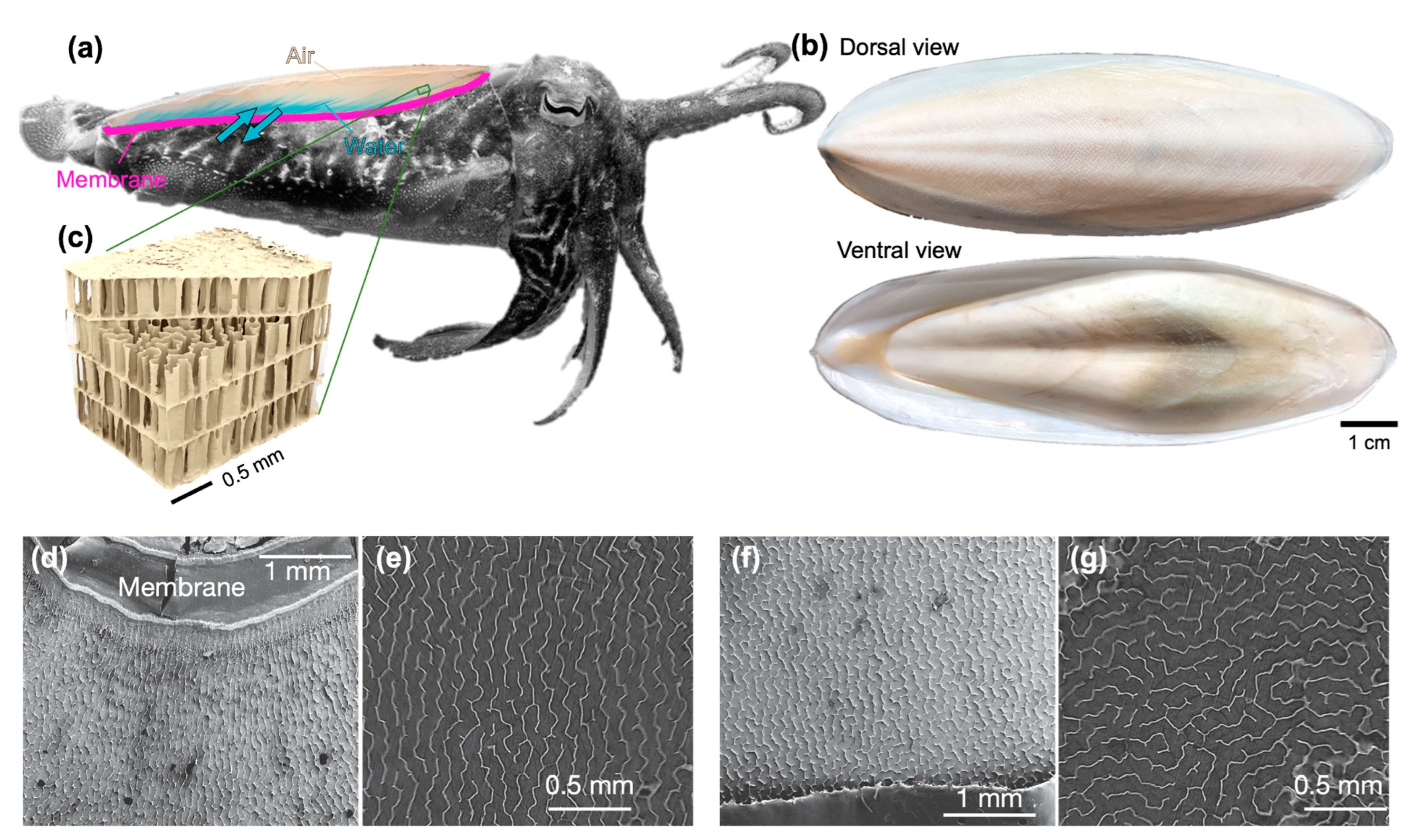
2.1. Cuttlebone Samples
2.2. Tests with Cuttlebone
2.3. Channel Fabrication Using a 3D Printer
2.4. Pushing the Air-Water Interface
3. Results
3.1. Water Penetration in Cuttlebone
3.2. Motion of the Air-Water Interface in Artificial Channels
4. Conclusions and Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, P.; Martin, A.W. On the buoyancy of the pearly Nautilus. J. Exp. Zool. 1978, 205, 5–12. [Google Scholar] [CrossRef]
- Denton, E.J.; Gilpin-Brown, J.B.; Howarth, J.V. The Osmotic Mechanism of the Cuttlebone. J. Mar. Biol. Assoc. UK 1961, 41, 351–363. [Google Scholar] [CrossRef]
- Denton, E.J.; Gilpin-Brown, J.B. The buoyancy of the cuttlefish, sepia officinalis. J. Mar. Biol. Ass. 1961, 41, 319–342. [Google Scholar] [CrossRef]
- Otjacques, E.; Repolho, T.; Paula, J.R.; Simão, S.; Baptista, M.; Rosa, R. Cuttlefish buoyancy in response to food availability and ocean acidification. Biology 2020, 9, 147. [Google Scholar] [CrossRef]
- Birchall, J.D.; Thomas, N.L. On the architecture and function of cuttlefish bone. J. Mater. Sci. 1983, 18, 2081–2086. [Google Scholar] [CrossRef]
- Yang, T.; Jia, Z.; Chen, H.; Deng, Z.; Liu, W.; Chen, L.; Li, L. Mechanical design of the highly porous cuttlebone: A bioceramic hard buoyancy tank for cuttlefish. Proc. Natl. Acad. Sci. USA 2020, 117, 23450–23459. [Google Scholar] [CrossRef]
- Macha, I.J.; Ben-Nissan, B. Marine skeletons: Towards hard tissue repair and regeneration. Mar. Drugs 2018, 16, 225. [Google Scholar] [CrossRef]
- Benito, M.I.; Reolid, M. Comparison of the calcareous shells of belemnitida and sepiida: Is the cuttlebone prong an analogue of the belemnite rostrum solidum? Minerals 2020, 10, 713. [Google Scholar] [CrossRef]
- Ward, P.D.; Von Boletzky, S. Shell implosion depth and implosion morphologies in three species of Sepia (Cephalopoda) from the Mediterranean Sea. J. Mar. Biol. Assoc. U. K. 1984, 64, 955–966. [Google Scholar] [CrossRef]
- Sherrard, K. Cuttlebone morphology limits habitat depth in eleven species of Sepia (Cephalopoda: Sepiidae). Biol. Bull. 2000, 198, 404–414. [Google Scholar] [CrossRef]
- Saffman, P.G.; Taylor, G.I. The penetration of a fluid into a porous medium or Hele-Shaw cell containing a more viscous liquid. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1958, 245, 312–329. [Google Scholar] [CrossRef]
- Al-Housseiny, T.T.; Tsai, P.A.; Stone, H.A. Control of interfacial instabilities using flow geometry. Nat. Phys. 2012, 8, 747–750. [Google Scholar] [CrossRef]
- Jackson, S.; Power, H.; Giddings, D.; Stevens, D. The stability of immiscible viscous fingering in Hele-Shaw cells with spatially varying permeability. Comput. Methods Appl. Mech. Eng. 2017, 320, 606–632. [Google Scholar] [CrossRef]
- Dias, E.O.; Miranda, J.A. Taper-induced control of viscous fingering in variable-gap Hele-Shaw flows. Phys. Rev. E 2013, 87, 053015. [Google Scholar] [CrossRef]
- Pihler-Puzović, D.; Illien, P.; Heil, M.; Juel, A. Suppression of Complex Fingerlike Patterns at the Interface between Air and a Viscous Fluid by Elastic Membranes. Phys. Rev. Lett. 2012, 108, 074502. [Google Scholar] [CrossRef]
- Pihler-Puzović, D.; Périllat, R.; Russell, M.; Juel, A.; Heil, M. Modelling the suppression of viscous fingering in elastic-walled Hele-Shaw cells. J. Fluid Mech. 2013, 731, 162–183. [Google Scholar] [CrossRef]
- Pihler-Puzović, D.; Juel, A.; Heil, M. The interaction between viscous fingering and wrinkling in elastic-walled Hele-Shaw cells. Phys. Fluids 2014, 26, 022102. [Google Scholar] [CrossRef]
- Shokri, H.; Kayhani, M.; Norouzi, M. Saffman–Taylor instability of viscoelastic fluids in anisotropic porous media. Int. J. Mech. Sci. 2018, 135, 1–13. [Google Scholar] [CrossRef]
- Eck, W.; Siekmann, J. On bubble motion in a Hele-Shaw cell, a possibility to study two-phase flows under reduced gravity. Arch. Appl. Mech. 1978, 47, 153–168. [Google Scholar] [CrossRef]
- Kopf-Sill, A.R.; Homsy, G.M. Bubble motion in a Hele–Shaw cell. Phys. Fluids 1988, 31, 18–26. [Google Scholar] [CrossRef]
- Maruvada, S.R.K.; Park, C.-W. Retarded motion of bubbles in Hele–Shaw cells. Phys. Fluids 1996, 8, 3229–3233. [Google Scholar] [CrossRef]
- Zhang, P.; Mines, J.M.; Lee, S.; Jung, S. Particle-bubble interaction inside a Hele-Shaw cell. Phys. Rev. E 2016, 94, 023112. [Google Scholar] [CrossRef]
- Link, D.R.; Anna, S.L.; Weitz, D.A.; Stone, H.A. Geometrically Mediated Breakup of Drops in Microfluidic Devices. Phys. Rev. Lett. 2004, 92, 054503. [Google Scholar] [CrossRef]
- Protière, S.; Bazant, M.Z.; Weitz, D.A.; Stone, H.A. Droplet breakup in flow past an obstacle: A capillary instability due to permeability variations. EPL Europhys. Lett. 2010, 92, 54002. [Google Scholar] [CrossRef]
- Vanapalli, S.A.; Duits, M.H.G.; Mugele, F. Microfluidics as a functional tool for cell mechanics. Biomicrofluidics 2009, 3, 012006. [Google Scholar] [CrossRef]
- Salkin, L.; Courbin, L.; Panizza, P. Microfluidic breakups of confined droplets against a linear obstacle: The importance of the viscosity contrast. Phys. Rev. E 2012, 86, 036317. [Google Scholar] [CrossRef]
- Salkin, L.; Schmit, A.; Courbin, L.; Panizza, P. Passive breakups of isolated drops and one-dimensional assemblies of drops in microfluidic geometries: Experiments and models. Lab a Chip 2013, 13, 3022–3032. [Google Scholar] [CrossRef]
- Xu, J.; Che, P.; Zhang, H.; Zhang, Y.; Wu, J.; Li, W.; He, J.; Ma, Z.; Li, T.; Dong, Y.; et al. Superhydrophobic Modification of Biomass Cuttlebone Applied to Oil Spill Remediation. Materials 2022, 15, 4401. [Google Scholar] [CrossRef]
- Chang, B.; Nave, G.; Jung, S. Drop formation from a wettable nozzle. Commun. Nonlinear Sci. Numer. Simul. 2012, 17, 2045–2051. [Google Scholar] [CrossRef]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. B 1921, 17, 273–283. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Frohlich, K.; Esmaili, E.; Yang, T.; Li, L.; Jung, S. Interfacial Dynamics in Dual Channels: Inspired by Cuttlebone. Biomimetics 2023, 8, 466. https://doi.org/10.3390/biomimetics8060466
Huang M, Frohlich K, Esmaili E, Yang T, Li L, Jung S. Interfacial Dynamics in Dual Channels: Inspired by Cuttlebone. Biomimetics. 2023; 8(6):466. https://doi.org/10.3390/biomimetics8060466
Chicago/Turabian StyleHuang, Matthew, Karl Frohlich, Ehsan Esmaili, Ting Yang, Ling Li, and Sunghwan Jung. 2023. "Interfacial Dynamics in Dual Channels: Inspired by Cuttlebone" Biomimetics 8, no. 6: 466. https://doi.org/10.3390/biomimetics8060466
APA StyleHuang, M., Frohlich, K., Esmaili, E., Yang, T., Li, L., & Jung, S. (2023). Interfacial Dynamics in Dual Channels: Inspired by Cuttlebone. Biomimetics, 8(6), 466. https://doi.org/10.3390/biomimetics8060466




