The 3D-McMap Guidelines: Three-Dimensional Multicomposite Microsphere Adaptive Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. PLA Microsphere Production (200 µm)
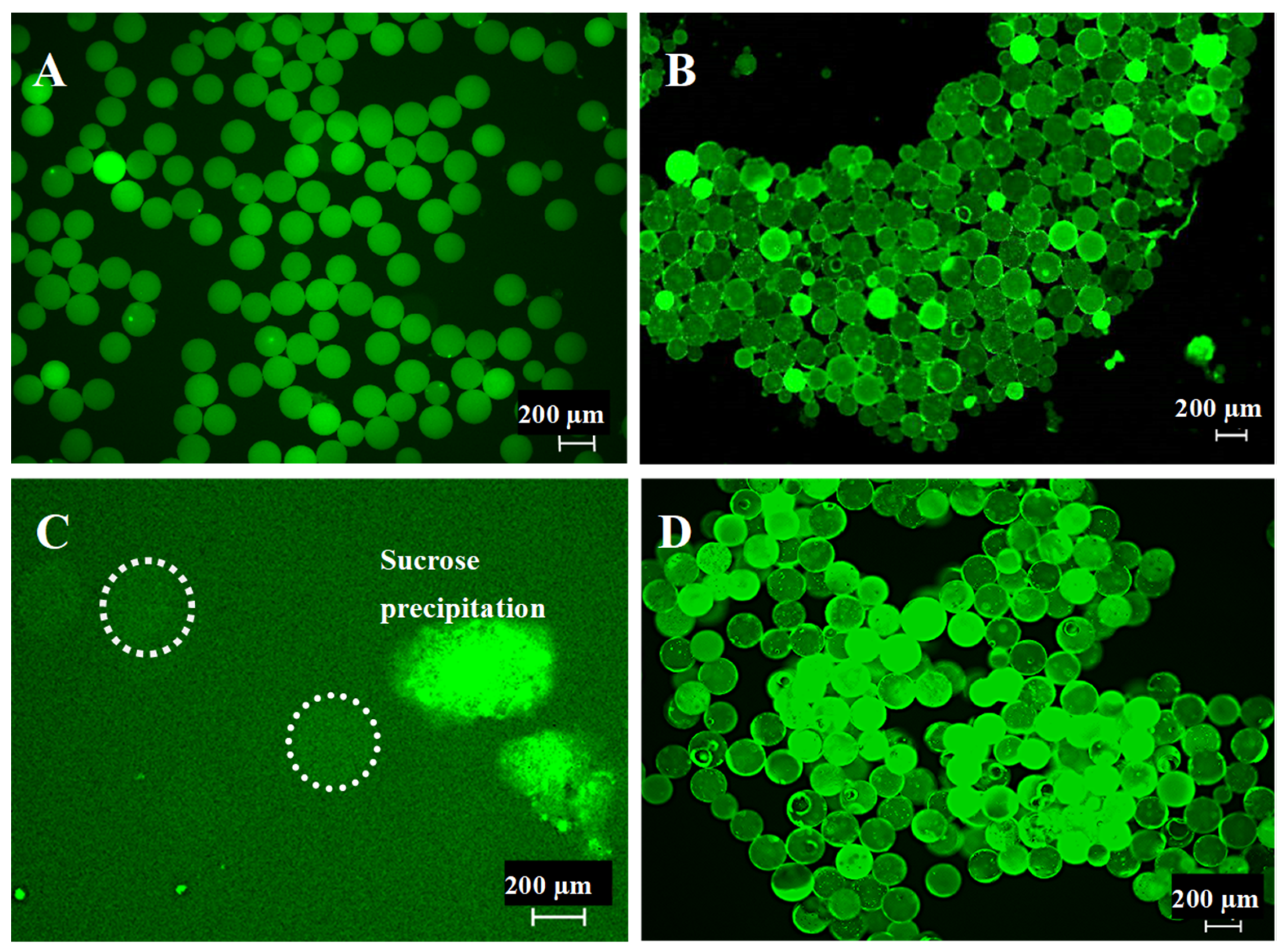
2.2. Microsphere Quality Control
2.3. Bioink Preparation
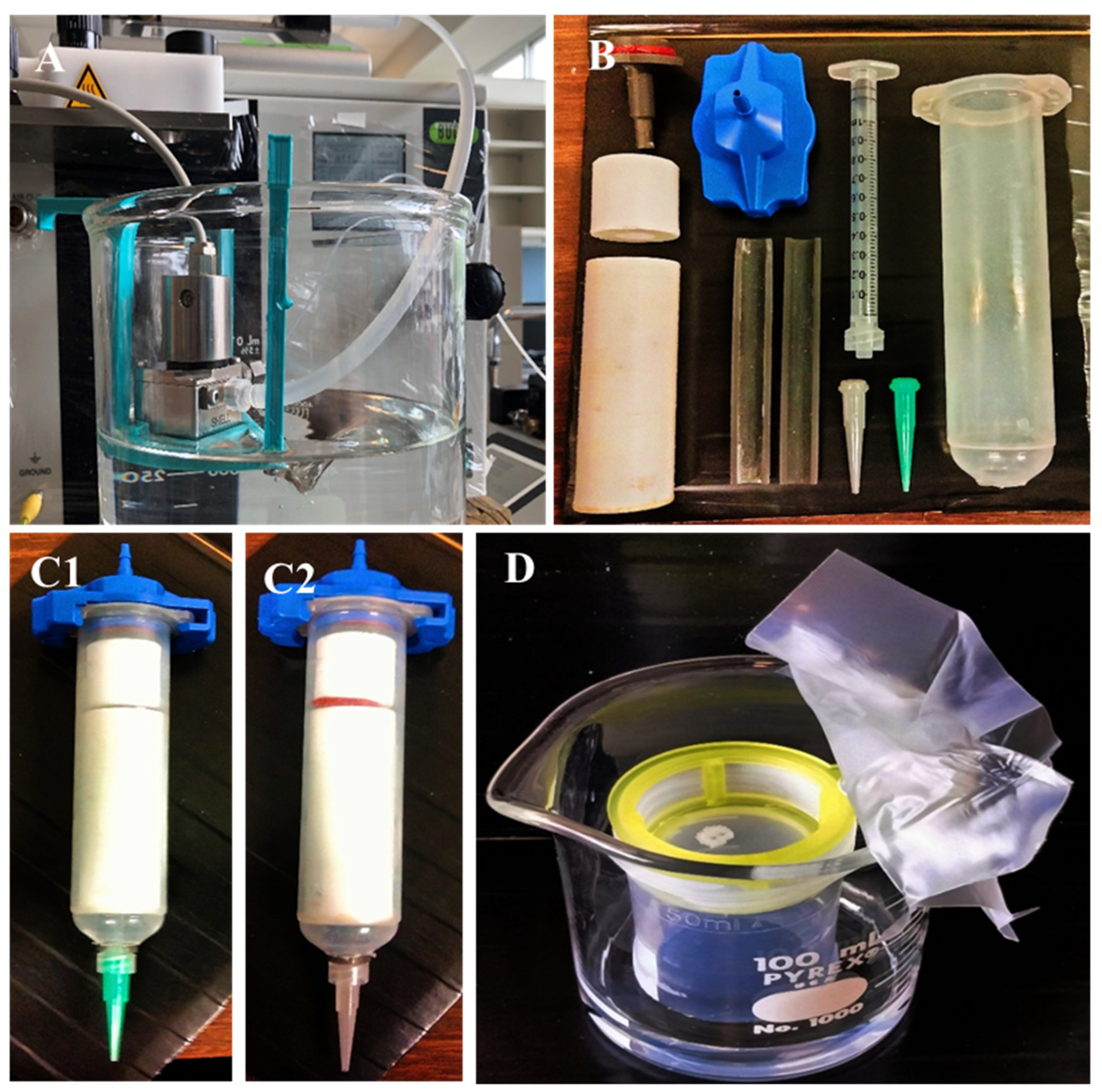
2.4. Three-Dimensional (3D) Printing
2.5. Vapor Sintering (Dichloromethane)
2.6. Scanning Electron Microscopy
2.7. Micro-Computed Tomography
2.8. Mechanical Stability Testing
3. Results
3.1. Generation of 200 µm Diameter Microspheres
3.2. 3D-McMap Method/Guide
- A continuous uninterrupted printing cycle;
- Printing stage temperature remained at 21 °C;
- Extrusion pressure was unaltered;
- Needle offsets were not varied.
- The pressure required to extrude the ink changed during the printing process, often increasing by 0.5–1 bar per extruded 0.25 mL of bioink. An analysis of the material showed that the microsphere ink was drying out in the syringe, losing its flow characteristics. Altering the specified ratio of microspheres to CMC and/or altering the concentration of the CMC prevented the proper flow of the ink.
- After two layers, the added weight of subsequent layers onto previous ones caused the first two layers slowly to collapse, as the relative wettish nature of the bioink could not withstand the pressure. During this process, any pores created by design and printed into the scaffolds were filled (Figure 3(A2,A3)).
- On the non-heated print bed, the printed layers did not dry quickly enough to stabilize their shapes to prevent the issues raised in point 2.
- Scaffolds printed on polyimide tape for better adhesion during printing stuck strongly to the tape after 30 min drying time already and could not be removed from the platform without breakage.
- Cooling of the printhead with the microsphere/CMC bioink to 4 °C ensured that the bioink continued to properly lubricate the microspheres, which greatly reduced the need for extrusion pressures adjustments during printing. A small pressure change was required only after two layers had been printed. Thereafter, the extrusion pressure needed no further changes. It was observed that only the bioink at the tip of the extrusion syringe, which was outside the cooled area, dried out over time. This issue was counteracted by pausing the printing process as necessary and briefly dabbing it with a sterile water-wetted tissue paper for 5 s.
- The print stage was kept between 50 °C and 60 °C, and the printing process was paused every two layers of each shape for up to 10 s. This action resulted in a distinct improvement in the stability of both external and internal geometric structures (Figure 3(B,C1–C3) and Figure 4) despite the added weight of additional layers.
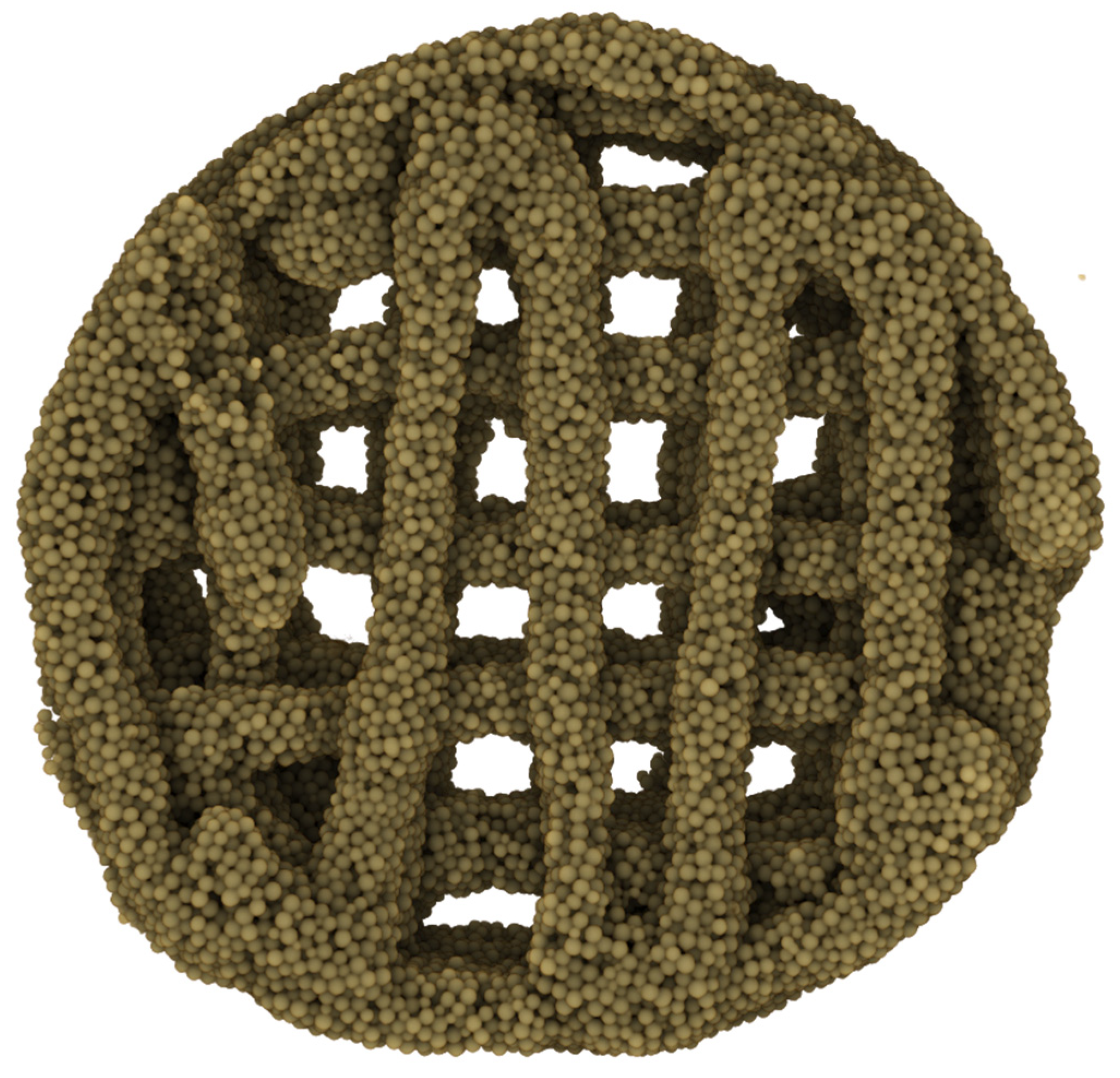
- The implementation of this drying step, however, also resulted in shrinkage of 0.1–0.15 mm in the printed scaffold every two layers, causing the follow-on layers to be out of alignment. To compensate for this, a cylinder was designed that comprised multiple-cylinder sections, with each section being two layers thick and taking into consideration the 0.1–0.15 mm shrinkage of the drying step. This ensured that all layers connected up properly, and the final 3D structure maintained its overall shape-integrity, producing a symmetrical 3D-printed shape each time (Figure 3(B,C1)).
- The polyimide tape was replaced with aluminum foil. The printed structure could easily be removed from the foil, even if only partially dried after 30 min. This made the collection of scaffolds very simple.
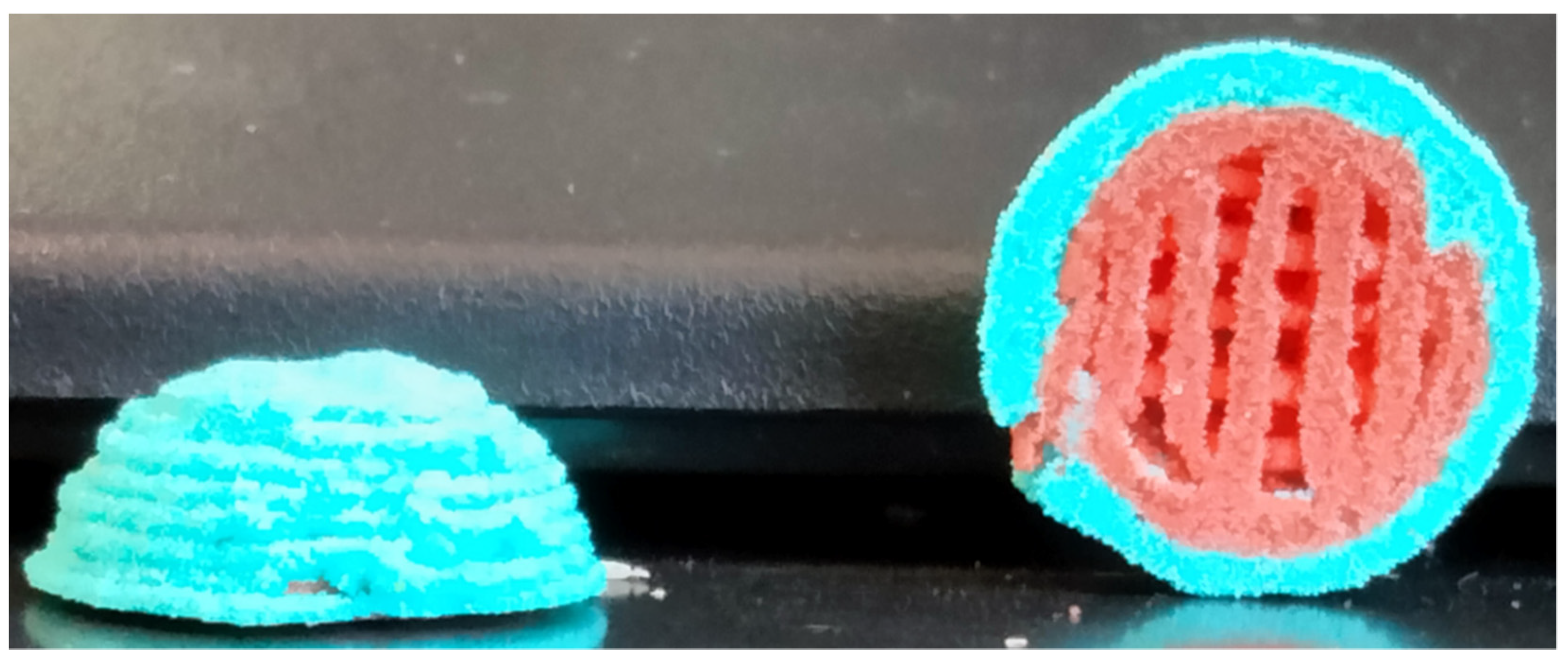
3.3. Multicomposite 3D-Printed Hemisphere
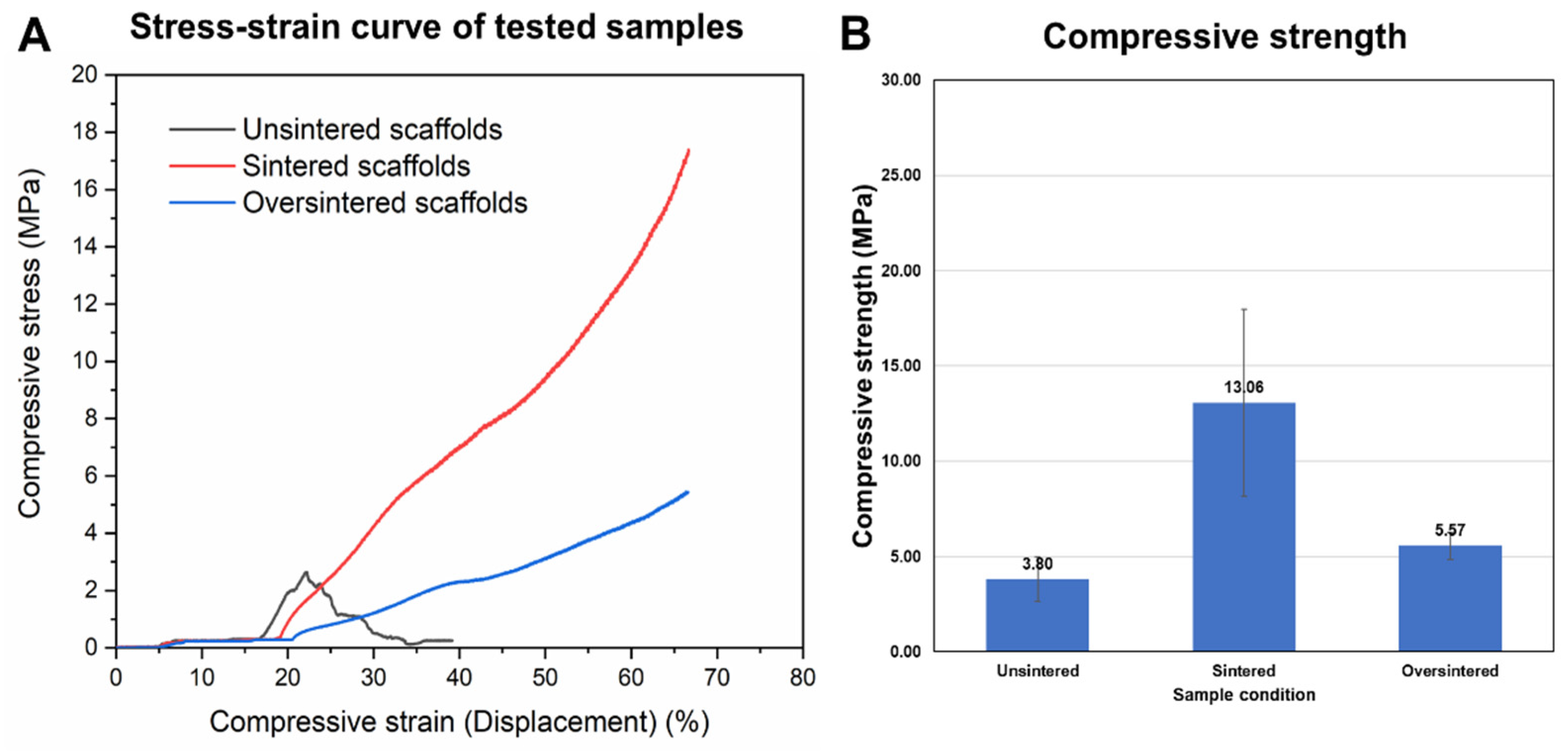
3.4. Sintering Periods and Mechanical Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mm | Millimeters |
| µm | Micrometers |
| PLGA | Poly (lactic-co-glycolic) acid |
| PLA | Poly-(lactic) acid |
| SEM | Scanning Electron Microscope |
| MicroCT/µCT | Micro Computed Tomography |
| 3D | Three Dimensional |
| w/v | Weight/Volume (%) |
| v/v | Volume/Volume (%) |
| RPM | Revolutions Per Minute |
| PVA | Polyvinyl Alcohol |
| Hz | Hertz |
| s | Seconds |
| Min | Minutes |
| Amp | Amplitude |
| g | Grams |
| °C | Degrees Celsius |
| CMC | Carboxymethyl cellulose |
| 16G-18G | 16 Gauge/18 Gauge |
| mm/s | Millimeter/second |
| SE | Secondary electron |
| XL30 ESEM-FEG | Environmental Scanning Electron Microscope |
| Au-Pd | Gold-palladium |
| kV | kilovolts |
| μA | Micro-ampere |
| mL | Milliliters |
| DCM | Dichloromethane |
| TGF | Transforming Growth Factor |
| AI | Artificial intelligence |
| DNA | Deoxyribose nucleic acid |
References
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef]
- Wang, X.; Wenk, E.; Zhang, X.; Meinel, L.; Vunjak-Novakovic, G.; Kaplan, D.L. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J. Control. Release 2009, 134, 81–90. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Rodrigues, M.T.; Silva, S.S.; Malafaya, P.B.; Gomes, M.E.; Viegas, C.A.; Dias, I.R.; Azevedo, J.T.; Mano, J.F.; Reis, R.L. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials 2006, 27, 6123–6137. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Sheu, C.; Fong, Y.T.; Liao, H.T.; Chen, J.P. Microsphere-Based Hierarchically Juxtapositioned Biphasic Scaffolds Prepared from Poly(Lactic-co-Glycolic Acid) and Nanohydroxyapatite for Osteochondral Tissue Engineering. Polymers 2016, 8, 429. [Google Scholar] [CrossRef]
- Chi, J.; Wang, M.; Chen, J.; Hu, L.; Chen, Z.; Backman, L.J.; Zhang, W. Topographic Orientation of Scaffolds for Tissue Regeneration: Recent Advances in Biomaterial Design and Applications. Biomimetics 2022, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Khan, Y.; Berkland, C.J.; Laurencin, C.T.; Detamore, M.S. Microsphere-Based Scaffolds in Regenerative Engineering. Annu. Rev. Biomed. Eng. 2017, 19, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Li, B.; Chen, L. Controlled dual delivery of low doses of BMP-2 and VEGF in a silk fibroin-nanohydroxyapatite scaffold for vascularized bone regeneration. J. Mater. Chem. B 2017, 5, 6963–6972. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, S.; Koch, A.; Jun, Y.; Chou, C.; Awadallah, M.R.; Lee, C.H. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication 2016, 8, 025003. [Google Scholar] [CrossRef] [PubMed]
- Legemate, K.; Tarafder, S.; Jun, Y.; Lee, C.H. Engineering Human TMJ Discs with Protein-Releasing 3D-Printed Scaffolds. J. Dent. Res. 2016, 95, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Esposito Corcione, C.; Gervaso, F.; Scalera, F.; Padmanabhan, S.K.; Madaghiele, M.; Montagna, F.; Sannino, A.; Licciulli, A.; Maffezzoli, A. Highly loaded hydroxyapatite microsphere/PLA porous scaffolds obtained by fused deposition modelling. Ceram. Int. 2019, 45, 2803–2810. [Google Scholar] [CrossRef]
- Visscher, L.E.; Dang, H.P.; Knackstedt, M.A.; Hutmacher, D.W.; Tran, P.A. 3D printed Polycaprolactone scaffolds with dual macro-microporosity for applications in local delivery of antibiotics. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 87, 78–89. [Google Scholar] [CrossRef]
- Dang, H.P.; Shabab, T.; Shafiee, A.; Peiffer, Q.C.; Fox, K.; Tran, N.; Dargaville, T.R.; Hutmacher, D.W.; Tran, P.A. 3D printed dual macro-, microscale porous network as a tissue engineering scaffold with drug delivering function. Biofabrication 2019, 11, 035014. [Google Scholar] [CrossRef]
- Hing, K.A.; Annaz, B.; Saeed, S.; Revell, P.A.; Buckland, T. Microporosity enhances bioactivity of synthetic bone graft substitutes. J. Mater. Sci. Mater. Med. 2005, 16, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.; Schirmer, L.; Segura, T. Granular hydrogels: Emergent properties of jammed hydrogel microparticles and their applications in tissue repair and regeneration. Curr. Opin. Biotechnol. 2019, 60, 1–8. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Kalaji, N.; Sheibat-Othman, N.; Saadaoui, H.; Elaissari, A.; Fessi, H. Colloidal and physicochemical characterization of proteincontaining poly(lactide-co-glycolide) (PLGA) microspheres before and after drying. e-Polymers 2009, 9, 1–12. [Google Scholar] [CrossRef]
- Jiang, Z.; Diggle, B.; Tan, M.L.; Viktorova, J.; Bennett, C.W.; Connal, L.A. Extrusion 3D Printing of Polymeric Materials with Advanced Properties. Adv. Sci. 2020, 7, 2001379. [Google Scholar] [CrossRef]
- Nava-Arzaluz, M.G.; Piñón-Segundo, E.; Ganem-Rondero, A. Sucrose Esters as Transdermal Permeation Enhancers. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2015; pp. 273–290. [Google Scholar]
- Klar, R.M. The Induction of Bone Formation: The Translation Enigma. Front. Bioeng. Biotechnol. 2018, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- BaoLin, G.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Seitz, D.; Konig, F.; Muller, P.E.; Jansson, V.; Klar, R.M. Induction of Articular Chondrogenesis by Chitosan/Hyaluronic-Acid-Based Biomimetic Matrices Using Human Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2019, 20, 4487. [Google Scholar] [CrossRef]
- Xiong, F.; Hausdorf, J.; Niethammer, T.R.; Jansson, V.A.; Klar, R.M. Temporal TGF-beta Supergene Family Signalling Cues Modulating Tissue Morphogenesis: Chondrogenesis within a Muscle Tissue Model? Int. J. Mol. Sci. 2020, 21, 4863. [Google Scholar] [CrossRef]
- Liu, H.; Muller, P.E.; Aszodi, A.; Klar, R.M. Osteochondrogenesis by TGF-beta3, BMP-2 and noggin growth factor combinations in an ex vivo muscle tissue model: Temporal function changes affecting tissue morphogenesis. Front. Bioeng. Biotechnol. 2023, 11, 1140118. [Google Scholar] [CrossRef]
- Chen, J.; Pan, P.; Zhang, Y.; Zhong, S.; Zhang, Q. Preparation of chitosan/nano hydroxyapatite organic-inorganic hybrid microspheres for bone repair. Colloids Surf. B Biointerfaces 2015, 134, 401–407. [Google Scholar] [CrossRef]
- Li, Q.; Chang, B.; Dong, H.; Liu, X. Functional microspheres for tissue regeneration. Bioact. Mater. 2023, 25, 485–499. [Google Scholar] [CrossRef]
- Becerra, J.; Rodriguez, M.; Leal, D.; Noris-Suarez, K.; Gonzalez, G. Chitosan-collagen-hydroxyapatite membranes for tissue engineering. J. Mater. Sci. Mater. Med. 2022, 33, 18. [Google Scholar] [CrossRef] [PubMed]
- Faruq, O.; Kim, B.; Padalhin, A.R.; Lee, G.H.; Lee, B.T. A hybrid composite system of biphasic calcium phosphate granules loaded with hyaluronic acid-gelatin hydrogel for bone regeneration. J. Biomater. Appl. 2017, 32, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Lee, B.T. A combination of biphasic calcium phosphate scaffold with hyaluronic acid-gelatin hydrogel as a new tool for bone regeneration. Tissue Eng. Part A 2014, 20, 1993–2004. [Google Scholar] [CrossRef]
- Zhang, F.; King, M.W. Biodegradable Polymers as the Pivotal Player in the Design of Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2020, 9, e1901358. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Zhang, R.; Huang, Y.; Li, Y.; Shan, M.; Zhong, X.; Xing, Y.; Wang, M.; Zhang, Y.; et al. Cartilage Extracellular Matrix Scaffold with Kartogenin-Encapsulated PLGA Microspheres for Cartilage Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 600103. [Google Scholar] [CrossRef]
- Sims-Lucas, S.; Good, M.; Vainio, S.J. Organogenesis: From Development to Disease. Front. Cell Dev. Biol. 2017, 5, 85. [Google Scholar] [CrossRef]
- Safavi, H.R.; Amiri, A.; Baniassadi, M.; Zolfagharian, A.; Baghani, M. An anisotropic constitutive model for fiber reinforced salt-sensitive hydrogels. Mech. Adv. Mater. Struct. 2023, 30, 4814–4827. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kouzani, A.Z.; Bodaghi, M.; Xiang, Y.; Zolfagharian, A. 3D-Printed Phase-Change Artificial Muscles with Autonomous Vibration Control. Adv. Mater. Technol. 2023, 8, 2300199. [Google Scholar] [CrossRef]
- Singh, M.; Morris, C.P.; Ellis, R.J.; Detamore, M.S.; Berkland, C. Microsphere-Based Seamless Scaffolds Containing Macroscopic Gradients of Encapsulated Factors for Tissue Engineering. Tissue Eng. Part C Methods 2008, 14, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Kacarevic, Z.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanisevic, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mandal, S.S.; Bauri, S.; Maiti, P. 3D bioprinting and its innovative approach for biomedical applications. MedComm 2023, 4, e194. [Google Scholar] [CrossRef] [PubMed]
- Giroux, V.; Rustgi, A.K. Metaplasia: Tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat. Rev. Cancer 2017, 17, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Torgovnick, A.; Schumacher, B. DNA repair mechanisms in cancer development and therapy. Front. Genet. 2015, 6, 157. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Gillispie, G.; Prim, P.; Lee, S.J. Physical and Chemical Factors Influencing the Printability of Hydrogel-based Extrusion Bioinks. Chem. Rev. 2020, 120, 10834–10886. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.S.; Datta, P.; Nesaei, S.; Ozbolat, V.; Ozbolat, I.T. 3D Coaxial Bioprinting: Process Mechanisms, Bioinks and Applications. Prog. Biomed. Eng. 2022, 4, 022003. [Google Scholar] [CrossRef]
- Wahlberg, B.; Ghuman, H.; Liu, J.R.; Modo, M. Ex vivo biomechanical characterization of syringe-needle ejections for intracerebral cell delivery. Sci. Rep. 2018, 8, 9194. [Google Scholar] [CrossRef] [PubMed]
- Blau, P.J. The significance and use of the friction coefficient. Tribol. Int. 2001, 34, 585–591. [Google Scholar] [CrossRef]
- Wartlick, O.; Kicheva, A.; Gonzalez-Gaitan, M. Morphogen gradient formation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001255. [Google Scholar] [CrossRef]
- Shifrin, Y.; Pinto, V.I.; Hassanali, A.; Arora, P.D.; McCulloch, C.A. Force-induced apoptosis mediated by the Rac/Pak/p38 signalling pathway is regulated by filamin A. Biochem. J. 2012, 445, 57–67. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klar, R.M.; Cox, J.; Raja, N.; Lohfeld, S. The 3D-McMap Guidelines: Three-Dimensional Multicomposite Microsphere Adaptive Printing. Biomimetics 2024, 9, 94. https://doi.org/10.3390/biomimetics9020094
Klar RM, Cox J, Raja N, Lohfeld S. The 3D-McMap Guidelines: Three-Dimensional Multicomposite Microsphere Adaptive Printing. Biomimetics. 2024; 9(2):94. https://doi.org/10.3390/biomimetics9020094
Chicago/Turabian StyleKlar, Roland M., James Cox, Naren Raja, and Stefan Lohfeld. 2024. "The 3D-McMap Guidelines: Three-Dimensional Multicomposite Microsphere Adaptive Printing" Biomimetics 9, no. 2: 94. https://doi.org/10.3390/biomimetics9020094
APA StyleKlar, R. M., Cox, J., Raja, N., & Lohfeld, S. (2024). The 3D-McMap Guidelines: Three-Dimensional Multicomposite Microsphere Adaptive Printing. Biomimetics, 9(2), 94. https://doi.org/10.3390/biomimetics9020094




