A Contemporary Review of Trachea, Nose, and Ear Cartilage Bioengineering and Additive Manufacturing
Abstract
:1. Introduction
2. Tissue Bioengineering
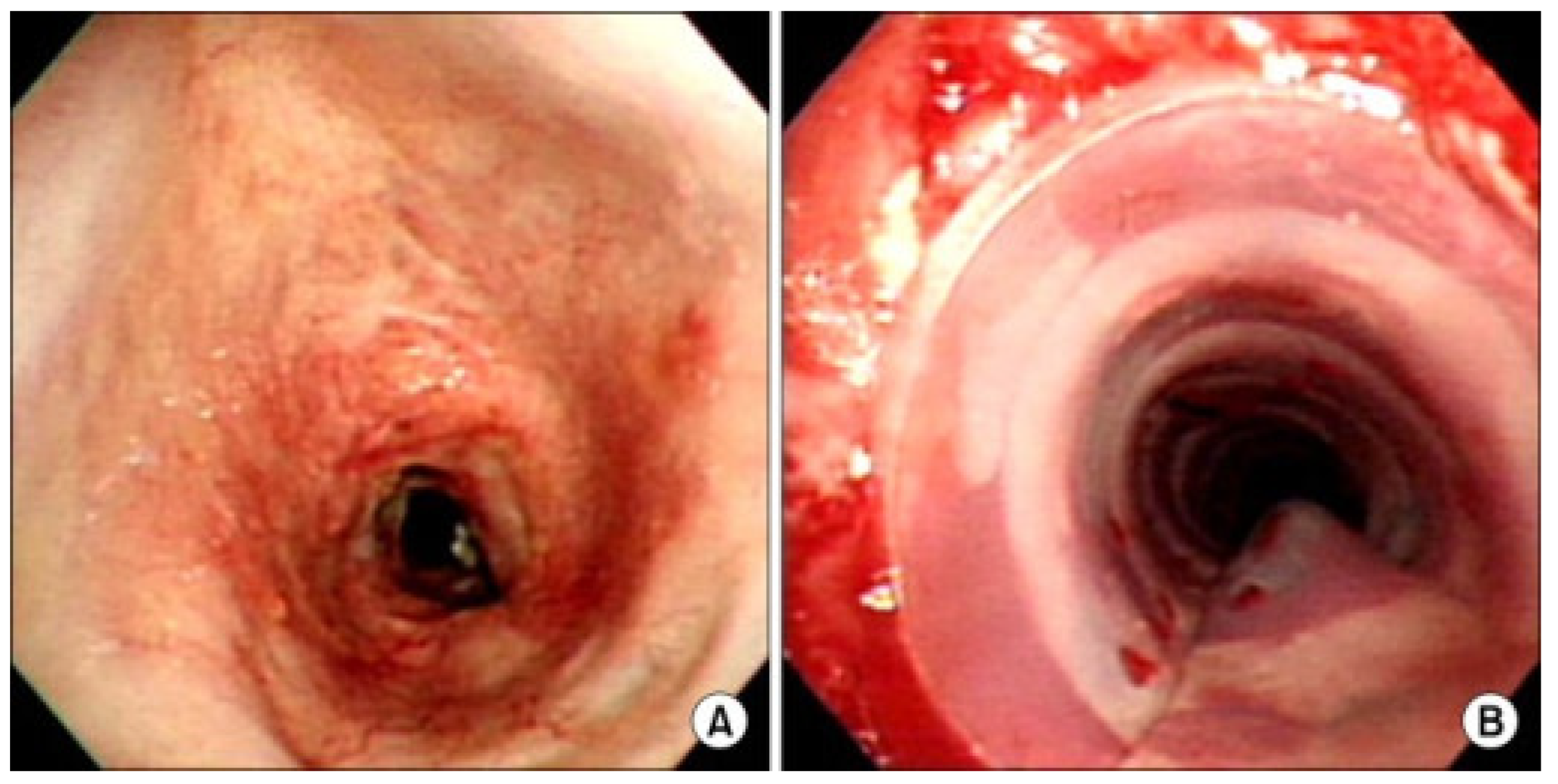
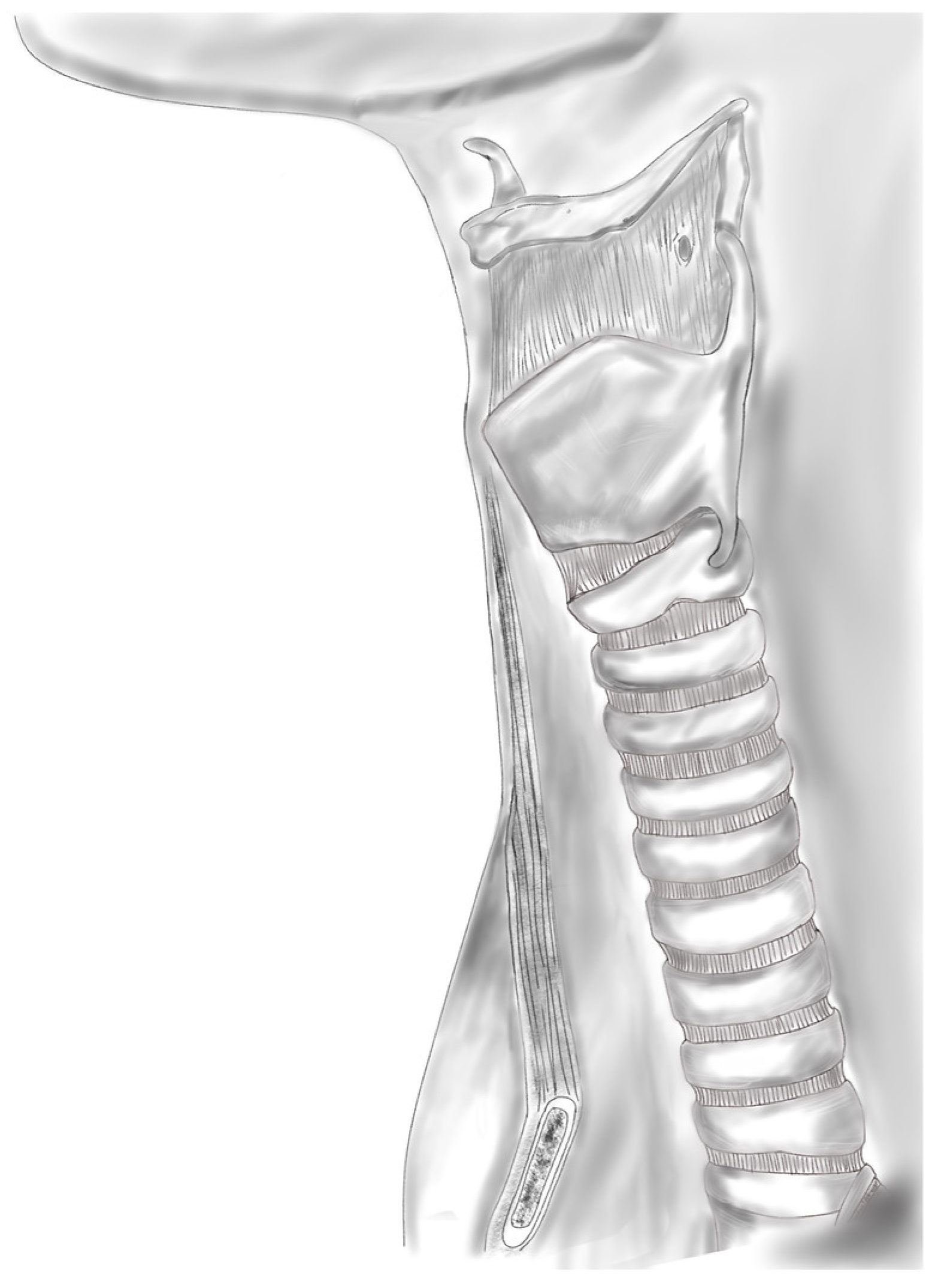
3. Trachea
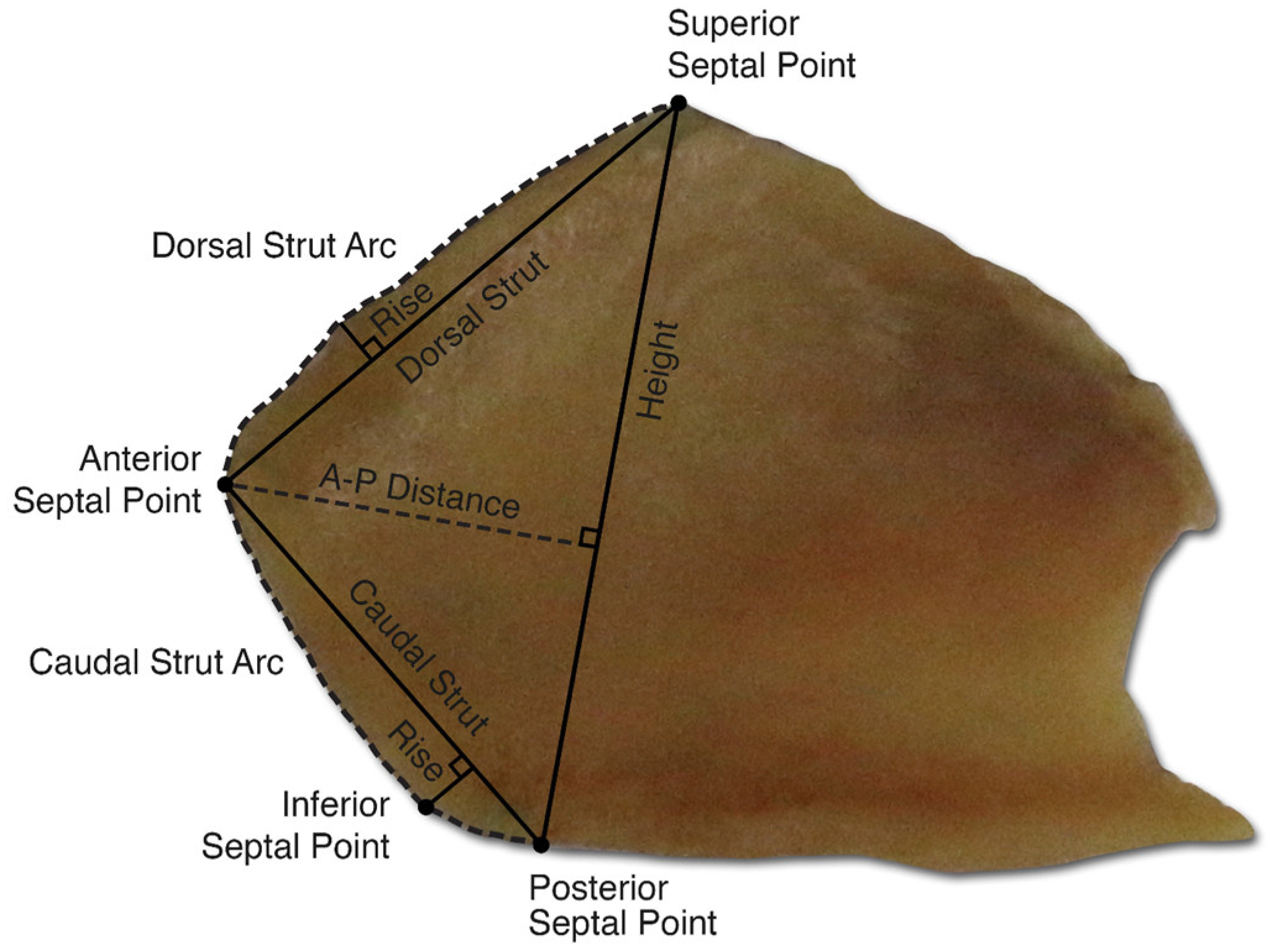
4. Nose
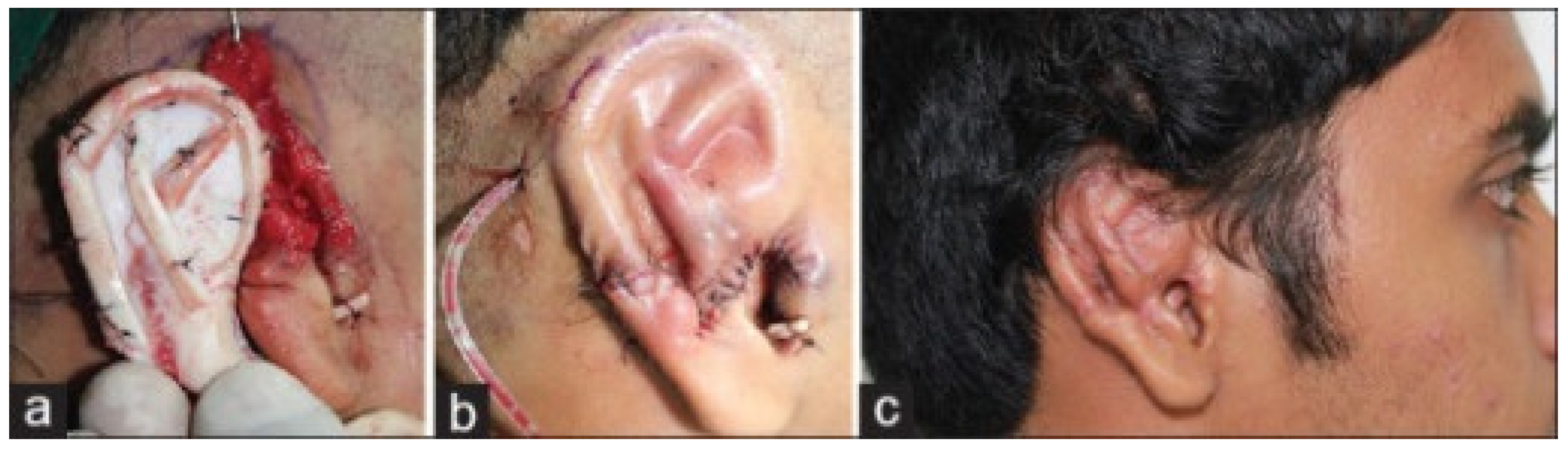
5. Ear
6. Current Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bewley, A.F.; Farwell, D.G. Laryngeal Transplantation. In Advances in Oto-Rhino-Laryngology; S. Karger AG: Basel, Switzerland, 2020; Volume 85, pp. 125–132. ISBN 978-3-318-06627-2. [Google Scholar] [CrossRef]
- Siemionow, M. The past the present and the future of face transplantation. Curr. Opin. Organ Transplant. 2020, 25, 568–575. [Google Scholar] [CrossRef]
- Niermeyer, W.L.; Rodman, C.; Li, M.M.; Chiang, T. Tissue engineering applications in otolaryngology—The state of translation. Laryngoscope Investig. Otolaryngol. 2020, 5, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Vacanti, C.A. Tissue engineering in the trachea. Anat. Rec. 2014, 297, 44–50. [Google Scholar] [CrossRef]
- Fishman, J.M.; Lowdell, M.; Birchall, M.A. Stem cell-based organ replacements-airway and lung tissue engineering. Semin. Pediatr. Surg. 2014, 23, 119–126. [Google Scholar] [CrossRef]
- Fuchs, J.R.; Hannouche, D.; Terada, S.; Vacanti, J.P.; Fauza, D.O. Fetal tracheal augmentation with cartilage engineered from bone marrow-derived mesenchymal progenitor cells. J. Pediatr. Surg. 2003, 38, 984–987. [Google Scholar] [CrossRef]
- Bergman, M.; Harwood, J.; Liu, L.; Shontz, K.M.; Chan, C.; Chiang, T. Long-Term Chondrocyte Retention in Partially Decellularized Tracheal Grafts. Otolaryngol.-Head Neck Surg. 2024, 170, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Canseco, J.A.; Kojima, K.; Penvose, A.R.; Ross, J.D.; Obokata, H.; Gomoll, A.H.; Vacanti, C.A. Effect on ligament marker expression by direct-contact co-culture of mesenchymal stem cells and anterior cruciate ligament cells. Tissue Eng. Part A 2012, 18, 2549–2558. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.; Zhang, Q.; Chang, E.I.; Mathur, A.B.; Yu, P. Decellularized tracheal matrix scaffold for tracheal tissue engineering: In vivo host response. Plast. Reconstr. Surg. 2013, 132, 549e–559e. [Google Scholar] [CrossRef] [PubMed]
- Khalid, T.; Soriano, L.; Lemoine, M.; Cryan, S.-A.; O’Brien, F.J.; O’Leary, C. Development of tissue-engineered tracheal scaffold with refined mechanical properties and vascularisation for tracheal regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1187500. [Google Scholar] [CrossRef]
- Verzeletti, V.; Mammana, M.; Zambello, G.; Dell’Amore, A.; Rea, F. Human tracheal transplantation: A systematic review of case reports. Clin. Transplant. 2024, 38, e15238. [Google Scholar] [CrossRef]
- Haliloĝlu, T.; Onar, V.; Yildirim, G.; Sapçi, T.; Savci, N.; Kahvecioĝlu, O.; Karavus, A. Tracheal Reconstruction with Porous High-Density Polyethylene Tracheal Prosthesis. Ann. Otol. Rhinol. Laryngol. 2000, 109, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Feng, X.; Mao, T.; Feng, X.; Ouyang, H.-W.; Zhao, G.; Chen, F. Engineering of human tracheal tissue with collagen-enforced poly-lactic-glycolic acid non-woven mesh: A preliminary study in nude mice. Br. J. Oral Maxillofac. Surg. 2007, 45, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Best, C.A.; Pepper, V.K.; Ohst, D.; Bodnyk, K.; Heuer, E.; Onwuka, E.A.; King, N.; Strouse, R.; Grischkan, J.; Breuer, C.K.; et al. Designing a tissue-engineered tracheal scaffold for preclinical evaluation. Int. J. Pediatr. Otorhinolaryngol. 2018, 104, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, Á.; Cano-Vicent, A.; Sabater I Serra, R.; El-Tanani, M.; Aljabali, A.; Tambuwala, M.M.; Mishra, Y.K. Scaffolds in the microbial resistant era: Fabrication, materials, properties and tissue engineering applications. Mater. Today Bio 2022, 16, 100412. [Google Scholar] [CrossRef]
- Park, J.-H.; Yoon, J.-K.; Lee, J.B.; Shin, Y.M.; Lee, K.-W.; Bae, S.-W.; Lee, J.; Yu, J.; Jung, C.-R.; Youn, Y.-N.; et al. Experimental Tracheal Replacement Using 3-dimensional Bioprinted Artificial Trachea with Autologous Epithelial Cells and Chondrocytes. Sci. Rep. 2019, 9, 2103. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hong, H.; Ajiteru, O.; Sultan, M.T.; Lee, Y.J.; Lee, J.S.; Lee, O.J.; Lee, H.; Park, H.S.; Choi, K.Y.; et al. 3D bioprinted silk fibroin hydrogels for tissue engineering. Nat. Protoc. 2021, 16, 5484–5532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, G.; Lin, H.; Shang, Y.; Liu, N.; Zhen, Y.; An, Y. Cartilage 3D bioprinting for rhinoplasty using adipose-derived stem cells as seed cells: Review and recent advances. Cell Prolif. 2023, 56, e13417. [Google Scholar] [CrossRef] [PubMed]
- Vunjak-Novakovic, G.; Martin, I.; Obradovic, B.; Treppo, S.; Grodzinsky, A.J.; Langer, R.; Freed, L.E. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J. Orthop. Res. 1999, 17, 130–138. [Google Scholar] [CrossRef]
- Bader, A.; Macchiarini, P. Moving towards in situ tracheal regeneration: The bionic tissue engineered transplantation approach. J. Cell. Mol. Med. 2010, 14, 1877–1889. [Google Scholar] [CrossRef]
- Keller, B.; Yang, T.; Chen, Y.; Munivez, E.; Bertin, T.; Zabel, B.; Lee, B. Interaction of TGFβ and BMP signaling pathways during chondrogenesis. PLoS ONE 2011, 6, e16421. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-G.; Tang, R.-F.; Qi, Y.-Y.; Chen, W.-P.; Xiong, Y.; Wu, L.-D. Restoration of cartilage defects using a superparamagnetic iron oxide-labeled adipose-derived mesenchymal stem cell and TGF-β3-loaded bilayer PLGA construct. Regen. Med. 2020, 15, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.M.; Gonzalez Vazquez, A.G.; Chen, G.; O’Brien, F.J. Activation of the SOX-5, SOX-6, and SOX-9 Trio of Transcription Factors Using a Gene-Activated Scaffold Stimulates Mesenchymal Stromal Cell Chondrogenesis and Inhibits Endochondral Ossification. Adv. Healthc. Mater. 2020, 9, 1901827. [Google Scholar] [CrossRef] [PubMed]
- Gamez, C.; Schneider-Wald, B.; Schuette, A.; Mack, M.; Hauk, L.; Khan, A.U.M.; Gretz, N.; Stoffel, M.; Bieback, K.; Schwarz, M.L. Bioreactor for mobilization of mesenchymal stem/stromal cells into scaffolds under mechanical stimulation: Preliminary results. PLoS ONE 2020, 15, e0227553. [Google Scholar] [CrossRef] [PubMed]
- Merceron, C.; Vinatier, C.; Portron, S.; Masson, M.; Amiaud, J.; Guigand, L.; Chérel, Y.; Weiss, P.; Guicheux, J. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am. J. Physiol.-Cell Physiol. 2010, 298, C355–C364. [Google Scholar] [CrossRef] [PubMed]
- Shearier, E.; Xing, Q.; Qian, Z.; Zhao, F. Physiologically Low Oxygen Enhances Biomolecule Production and Stemness of Mesenchymal Stem Cell Spheroids. Tissue Eng. Part C Methods 2016, 22, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Perryman, M.C.; Kraft, S.M.; Kavookjian, H.L. Laryngotracheal Reconstruction for Subglottic and Tracheal Stenosis. Otolaryngol. Clin. N. Am. 2023, 56, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Brigger, M.T.; Boseley, M.E. Management of tracheal stenosis. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.G. Tracheal reconstruction. Semin. Pediatr. Surg. 1994, 3, 244–252. [Google Scholar]
- Kim, H.J.; Kim, S.W.; Lee, H.Y.; Kang, H.H.; Kang, J.Y.; Kim, J.S.; Kim, M.S.; Kim, S.S.; Kim, J.W.; Yun, H.G.; et al. Clinical Experience of Rigid Bronchoscopy in Single Center. Tuberc. Respir. Dis. 2012, 72, 486–492. [Google Scholar] [CrossRef]
- Furlow, P.W.; Mathisen, D.J. Surgical anatomy of the trachea. Ann. Cardiothorac. Surg. 2018, 7, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Law, J.X.; Liau, L.L.; Aminuddin, B.S.; Ruszymah, B.H.I. Tissue-engineered trachea: A review. Int. J. Pediatr. Otorhinolaryngol. 2016, 91, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Bogan, S.L.; Teoh, G.Z.; Birchall, M.A. Tissue Engineered Airways: A Prospects Article. J. Cell. Biochem. 2016, 117, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, M.M.A.; Willemse, J.; van den Hoek, S.; Kremers, G.-J.; Luider, T.M.; van Huizen, N.A.; Willemssen, F.E.J.A.; Metselaar, H.J.; IJzermans, J.N.M.; van der Laan, L.J.W.; et al. Decellularization of Whole Human Liver Grafts Using Controlled Perfusion for Transplantable Organ Bioscaffolds. Stem Cells Dev. 2017, 26, 1304–1315. [Google Scholar] [CrossRef]
- Abedin, E.; Lari, R.; Mahdavi Shahri, N.; Fereidoni, M. Development of a demineralized and decellularized human epiphyseal bone scaffold for tissue engineering: A histological study. Tissue Cell 2018, 55, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Ding, F.; Gong, L.; Gu, X. Extracellular Matrix Scaffolds for Tissue Engineering and Regenerative Medicine. Curr. Stem Cell Res. Ther. 2017, 12, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Conconi, M.T.; De Coppi, P.; Di Liddo, R.; Vigolo, S.; Zanon, G.F.; Parnigotto, P.P.; Nussdorfer, G.G. Tracheal matrices, obtained by a detergent-enzymatic method, support in vitro the adhesion of chondrocytes and tracheal epithelial cells. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2005, 18, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Liu, Y.; Li, D.; Yin, Z.; Huo, Y.; Jiang, G.; Yang, Y.; Wang, Z.; Li, Y.; et al. Porous decellularized trachea scaffold prepared by a laser micropore technique. J. Mech. Behav. Biomed. Mater. 2019, 90, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, D.; Yin, Z.; He, A.; Lin, M.; Jiang, G.; Song, X.; Hu, X.; Liu, Y.; Wang, J.; et al. Tissue-engineered trachea regeneration using decellularized trachea matrix treated with laser micropore technique. Acta Biomater. 2017, 58, 113–121. [Google Scholar] [CrossRef]
- Frejo, L.; Grande, D.A. 3D-bioprinted tracheal reconstruction: An overview. Bioelectron. Med. 2019, 5, 15. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, H.; Dong, W.; Bai, J.; Gao, B.; Xia, D.; Feng, B.; Chen, M.; He, X.; Yin, M.; et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair. Sci. Rep. 2017, 7, 5246. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Jing, H.; Gao, M.; Wang, S.; Fu, W.; Zhang, X.; He, X.; Zheng, J. Long-segmental tracheal reconstruction in rabbits with pedicled Tissue-engineered trachea based on a 3D-printed scaffold. Acta Biomater. 2019, 97, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Jin, D.; Wang, Q.; Gao, M.; Zhang, J.; Zhang, H.; Bai, J.; Feng, B.; Chen, M.; Huang, Y.; et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair in a goat model. J. Tissue Eng. Regen. Med. 2019, 13, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.-W.; Lee, K.-W.; Park, J.-H.; Lee, J.; Jung, C.-R.; Yu, J.; Kim, H.-Y.; Kim, D.-H. 3D Bioprinted Artificial Trachea with Epithelial Cells and Chondrogenic-Differentiated Bone Marrow-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 1624. [Google Scholar] [CrossRef]
- Weidenbecher, M.; Tucker, H.M.; Gilpin, D.A.; Dennis, J.E. Tissue-engineered trachea for airway reconstruction. Laryngoscope 2009, 119, 2118–2123. [Google Scholar] [CrossRef] [PubMed]
- Delaere, P.; Vranckx, J.; Verleden, G.; De Leyn, P.; Van Raemdonck, D. Leuven Tracheal Transplant Group Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N. Engl. J. Med. 2010, 362, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Klepetko, W.; Marta, G.M.; Wisser, W.; Melis, E.; Kocher, A.; Seebacher, G.; Aigner, C.; Mazhar, S. Heterotopic tracheal transplantation with omentum wrapping in the abdominal position preserves functional and structural integrity of a human tracheal allograft. J. Thorac. Cardiovasc. Surg. 2004, 127, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.J.; De Coppi, P.; Speggiorin, S.; Roebuck, D.; Butler, C.R.; Samuel, E.; Crowley, C.; McLaren, C.; Fierens, A.; Vondrys, D.; et al. Stem-cell-based, tissue engineered tracheal replacement in a child: A 2-year follow-up study. Lancet 2012, 380, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Delaere, P.R.; Van Raemdonck, D. The trachea: The first tissue-engineered organ? J. Thorac. Cardiovasc. Surg. 2014, 147, 1128–1132. [Google Scholar] [CrossRef]
- Macchiarini, P.; Jungebluth, P.; Go, T.; Asnaghi, M.A.; Rees, L.E.; Cogan, T.A.; Dodson, A.; Martorell, J.; Bellini, S.; Parnigotto, P.P.; et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008, 372, 2023–2030. [Google Scholar] [CrossRef]
- Gonfiotti, A.; Jaus, M.O.; Barale, D.; Baiguera, S.; Comin, C.; Lavorini, F.; Fontana, G.; Sibila, O.; Rombolà, G.; Jungebluth, P.; et al. The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet 2014, 383, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Macchiarini: Lancet retracts two papers on first tissue engineered trachea transplant. BMJ 2023, 383, 2529. [Google Scholar] [CrossRef] [PubMed]
- Paterlini, M. Paolo Macchiarini: Disgraced surgeon is sentenced to 30 months in prison. BMJ 2023, 381, 1442. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.J.; Butler, C.R.; Varanou-Jenkins, A.; Partington, L.; Carvalho, C.; Samuel, E.; Crowley, C.; Lange, P.; Hamilton, N.J.; Hynds, R.E.; et al. Tracheal Replacement Therapy with a Stem Cell-Seeded Graft: Lessons from Compassionate Use Application of a GMP-Compliant Tissue-Engineered Medicine. Stem Cells Transl. Med. 2017, 6, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. Nasal Anatomy and Function. Facial Plast. Surg. 2017, 33, 003–008. [Google Scholar] [CrossRef]
- Bloom, J.D.; Antunes, M.B.; Becker, D.G. Anatomy, Physiology, and General Concepts in Nasal Reconstruction. Facial Plast. Surg. Clin. N. Am. 2011, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lavernia, L.; Brown, W.E.; Wong, B.J.F.; Hu, J.C.; Athanasiou, K.A. Toward tissue-engineering of nasal cartilages. Acta Biomater. 2019, 88, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Fulco, I.; Miot, S.; Haug, M.D.; Barbero, A.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Jundt, G.; Marsano, A.; Farhadi, J.; et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: An observational first-in-human trial. Lancet 2014, 384, 337–346. [Google Scholar] [CrossRef]
- Fisher, M.; Alba, B.; Ahmad, J.; Robotti, E.; Cerkes, N.; Gruber, R.P.; Rohrich, R.J.; Bradley, J.P.; Tanna, N. Current Practices in Dorsal Augmentation Rhinoplasty. Plast. Reconstr. Surg. 2022, 149, 1088–1102. [Google Scholar] [CrossRef]
- Na, H.; Jang, Y. Dorsal Augmentation using Alloplastic Implants. Facial Plast. Surg. 2017, 33, 189–194. [Google Scholar] [CrossRef]
- Liang, X.; Wang, K.; Malay, S.; Chung, K.C.; Ma, J. A systematic review and meta-analysis of comparison between autologous costal cartilage and alloplastic materials in rhinoplasty. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yu, W.; Jin, Y.; Chen, H.; Ma, G.; Chang, S.; Lin, X. Safety and Efficacy of Cosmetic Augmentation of the Nasal Tip and Nasal Dorsum With Expanded Polytetrafluoroethylene: A Randomized Clinical Trial. JAMA Facial Plast. Surg. 2018, 20, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.H.; Jang, Y.J. Comparison of the Surgical Outcomes of Dorsal Augmentation Using Expanded Polytetrafluoroethylene or Autologous Costal Cartilage. JAMA Facial Plast. Surg. 2016, 18, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Popko, M.; Bleys, R.L.A.W.; De Groot, J.-W.; Huizing, E.H. Histological structure of the nasal cartilages and their perichondrial envelope. I. The septal and lobular cartilage. Rhinology 2007, 45, 148–152. [Google Scholar] [PubMed]
- Wu, Y.; Ayan, B.; Moncal, K.K.; Kang, Y.; Dhawan, A.; Koduru, S.V.; Ravnic, D.J.; Kamal, F.; Ozbolat, I.T. Hybrid Bioprinting of Zonally Stratified Human Articular Cartilage Using Scaffold-Free Tissue Strands as Building Blocks. Adv. Healthc. Mater. 2020, 9, 2001657. [Google Scholar] [CrossRef] [PubMed]
- Möller, T.; Amoroso, M.; Hägg, D.; Brantsing, C.; Rotter, N.; Apelgren, P.; Lindahl, A.; Kölby, L.; Gatenholm, P. In Vivo Chondrogenesis in 3D Bioprinted Human Cell-laden Hydrogel Constructs. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1227. [Google Scholar] [CrossRef] [PubMed]
- Apelgren, P.; Amoroso, M.; Lindahl, A.; Brantsing, C.; Rotter, N.; Gatenholm, P.; Kölby, L. Chondrocytes and stem cells in 3D-bioprinted structures create human cartilage in vivo. PLoS ONE 2017, 12, e0189428. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Liang, Y.; Vyhlidal, M.; Erkut, E.J.; Kunze, M.; Mulet-Sierra, A.; Osswald, M.; Ansari, K.; Seikaly, H.; Boluk, Y.; et al. In vitro maturation and in vivo stability of bioprinted human nasal cartilage. J. Tissue Eng. 2022, 13, 20417314221086370. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.L.; Sidle, D.M. Cosmetic Otoplasty. Facial Plast. Surg. Clin. N. Am. 2018, 26, 19–29. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Bulstrode, N.; Chang, K.W.; Cho, Y.S.; Frenzel, H.; Jiang, D.; Kesser, B.W.; Siegert, R.; Triglia, J.M. International Consensus Recommendations on Microtia, Aural Atresia and Functional Ear Reconstruction. J. Int. Adv. Otol. 2019, 15, 204–208. [Google Scholar] [CrossRef]
- Wilkes, G.H.; Wong, J.; Guilfoyle, R. Microtia Reconstruction. Plast. Reconstr. Surg. 2014, 134, 464e–479e. [Google Scholar] [CrossRef]
- Im, D.D.; Paskhover, B.; Staffenberg, D.A.; Jarrahy, R. Current Management of Microtia: A National Survey. Aesthetic Plast. Surg. 2013, 37, 402–408. [Google Scholar] [CrossRef]
- Balaji, S.M. Two stage ear/microtia reconstruction using costal cartilage. Ann. Maxillofac. Surg. 2015, 5, 163–167. [Google Scholar] [CrossRef]
- Van Osch, G.J.V.M.; Van Der Veen, S.W.; Burger, E.H.; Verwoerd-Verhoef, H.L. Chondrogenic Potential of in Vitro Multiplied Rabbit Perichondrium Cells Cultured in Alginate Beads in Defined Medium. Tissue Eng. 2000, 6, 321–330. [Google Scholar] [CrossRef]
- Togo, T.; Utani, A.; Naitoh, M.; Ohta, M.; Tsuji, Y.; Morikawa, N.; Nakamura, M.; Suzuki, S. Identification of cartilage progenitor cells in the adult ear perichondrium: Utilization for cartilage reconstruction. Lab. Investig. 2006, 86, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-W.; Johnson, T.S.; Motarjem, P.M.; Peretti, G.M.; Randolph, M.A.; Yaremchuk, M.J. Tissue-engineered flexible ear-shaped cartilage. Plast. Reconstr. Surg. 2005, 115, 1633–1641. [Google Scholar] [CrossRef]
- Cao, Y.; Rodriguez, A.; Vacanti, M.; Ibarra, C.; Arevalo, C.; Vacanti, C.A. Comparative study of the use of poly(glycolic acid), calcium alginate and pluronics in the engineering of autologous porcine cartilage. J. Biomater. Sci. Polym. Ed. 1998, 9, 475–487. [Google Scholar] [CrossRef]
- Chang, J.; Rasamny, J.J.; Park, S.S. Injectable Tissue-Engineered Cartilage Using a Fibrin Sealant. Arch. Facial Plast. Surg. 2007, 9, 161–166. [Google Scholar] [CrossRef]
- Yue, H.; Pathak, J.L.; Zou, R.; Qin, L.; Liao, T.; Hu, Y.; Kuang, W.; Zhou, L. Fabrication of chondrocytes/chondrocyte-microtissues laden fibrin gel auricular scaffold for microtia reconstruction. J. Biomater. Appl. 2021, 35, 838–848. [Google Scholar] [CrossRef]
- Elisseeff, J.; McIntosh, W.; Anseth, K.; Riley, S.; Ragan, P.; Langer, R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J. Biomed. Mater. Res. 2000, 51, 164–171. [Google Scholar] [CrossRef]
- Chetty, A.; Steynberg, T.; Moolman, S.; Nilen, R.; Joubert, A.; Richter, W. Hydroxyapatite-coated polyurethane for auricular cartilage replacement: An in vitro study. J. Biomed. Mater. Res. A 2008, 84, 475–482. [Google Scholar] [CrossRef]
- Jeon, Y.H.; Choi, J.H.; Sung, J.K.; Kim, T.K.; Cho, B.C.; Chung, H.Y. Different effects of PLGA and chitosan scaffolds on human cartilage tissue engineering. J. Craniofac. Surg. 2007, 18, 1249–1258. [Google Scholar] [CrossRef]
- García-López, J.; Garciadiego-Cázares, D.; Melgarejo-Ramírez, Y.; Sánchez-Sánchez, R.; Solís-Arrieta, L.; García-Carvajal, Z.; Sánchez-Betancourt, J.I.; Ibarra, C.; Luna-Bárcenas, G.; Velasquillo, C. Chondrocyte differentiation for auricular cartilage reconstruction using a chitosan based hydrogel. Histol. Histopathol. 2015, 30, 1477–1485. [Google Scholar] [CrossRef]
- Tan, H.; Gong, Y.; Lao, L.; Mao, Z.; Gao, C. Gelatin/chitosan/hyaluronan ternary complex scaffold containing basic fibroblast growth factor for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2007, 18, 1961–1968. [Google Scholar] [CrossRef]
- Wu, W.; Chen, F.; Liu, Y.; Ma, Q.; Mao, T. Autologous injectable tissue-engineered cartilage by using platelet-rich plasma: Experimental study in a rabbit model. J. Oral Maxillofac. Surg. 2007, 65, 1951–1957. [Google Scholar] [CrossRef]
- Ahmed, N.; Gan, L.; Nagy, A.; Zheng, J.; Wang, C.; Kandel, R.A. Cartilage tissue formation using redifferentiated passaged chondrocytes in vitro. Tissue Eng. Part A 2009, 15, 665–673. [Google Scholar] [CrossRef]
- Gan, L.; Kandel, R.A. In vitro cartilage tissue formation by Co-culture of primary and passaged chondrocytes. Tissue Eng. 2007, 13, 831–842. [Google Scholar] [CrossRef]
- Tseng, A.; Pomerantseva, I.; Cronce, M.J.; Kimura, A.M.; Neville, C.M.; Randolph, M.A.; Vacanti, J.P.; Sundback, C.A. Extensively Expanded Auricular Chondrocytes Form Neocartilage In Vivo. Cartilage 2014, 5, 241–251. [Google Scholar] [CrossRef]
- Martin, I.; Vunjak-Novakovic, G.; Yang, J.; Langer, R.; Freed, L.E. Mammalian chondrocytes expanded in the presence of fibroblast growth factor 2 maintain the ability to differentiate and regenerate three-dimensional cartilaginous tissue. Exp. Cell Res. 1999, 253, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Vacanti, J.P.; Paige, K.T.; Upton, J.; Vacanti, C.A. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast. Reconstr. Surg. 1997, 100, 297–302; discussion 303–304. [Google Scholar] [CrossRef] [PubMed]
- Kamil, S.H.; Kojima, K.; Vacanti, M.P.; Bonassar, L.J.; Vacanti, C.A.; Eavey, R.D. In vitro tissue engineering to generate a human-sized auricle and nasal tip. Laryngoscope 2003, 113, 90–94. [Google Scholar] [CrossRef]
- Bhamare, N.; Tardalkar, K.; Parulekar, P.; Khadilkar, A.; Joshi, M. 3D printing of human ear pinna using cartilage specific ink. Biomed. Mater. 2021, 16, 055008. [Google Scholar] [CrossRef]
- Naumann, A.; Aigner, J.; Staudenmaier, R.; Seemann, M.; Bruening, R.; Englmeier, K.H.; Kadegge, G.; Pavesio, A.; Kastenbauer, E.; Berghaus, A. Clinical aspects and strategy for biomaterial engineering of an auricle based on three-dimensional stereolithography. Eur. Arch. Oto-Rhino-Laryngol. 2003, 260, 568–575. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, H.; Yin, Z.; Liu, Y.; Zhang, Q.; Zhang, C.; Pan, B.; Zhou, J.; Zhou, X.; Sun, H.; et al. In Vitro Regeneration of Patient-specific Ear-shaped Cartilage and Its First Clinical Application for Auricular Reconstruction. eBioMedicine 2018, 28, 287–302. [Google Scholar] [CrossRef]
- Vogel, G. Report finds misconduct by surgeon. Science 2015, 348, 954–955. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L.; Hansson, G.K. Tracheobronchial transplantation: The Royal Swedish Academy of Sciences’ concerns. Lancet 2016, 387, 942. [Google Scholar] [CrossRef]
- Delaere, P.R.; Van Raemdonck, D. Commentary: The sobering truth about tracheal regeneration. J. Thorac. Cardiovasc. Surg. 2020, 159, 2537–2539. [Google Scholar] [CrossRef]
- Fux, T.; Österholm, C.; Themudo, R.; Simonson, O.; Grinnemo, K.-H.; Corbascio, M. Synthetic tracheal grafts seeded with bone marrow cells fail to generate functional tracheae: First long-term follow-up study. J. Thorac. Cardiovasc. Surg. 2020, 159, 2525–2537.e23. [Google Scholar] [CrossRef]
- Delaere, P.; Van Raemdonck, D. Tracheal replacement. J. Thorac. Dis. 2016, 8 (Suppl. 2), S186–S196. [Google Scholar] [CrossRef]
- Jodat, Y.A.; Kiaee, K.; Vela Jarquin, D.; De La Garza Hernández, R.L.; Wang, T.; Joshi, S.; Rezaei, Z.; De Melo, B.A.G.; Ge, D.; Mannoor, M.S.; et al. A 3D-Printed Hybrid Nasal Cartilage with Functional Electronic Olfaction. Adv. Sci. 2020, 7, 1901878. [Google Scholar] [CrossRef]
- Lotz, A.S.; Havla, J.B.; Richter, E.; Frölich, K.; Staudenmaier, R.; Hagen, R.; Kleinsasser, N.H. Cytotoxic and genotoxic effects of matrices for cartilage tissue engineering. Toxicol. Lett. 2009, 190, 128–133. [Google Scholar] [CrossRef] [PubMed]

| Tracheal Cartilage | Nasal Cartilage | Auricular Cartilage | |
|---|---|---|---|
| Biomechanical Properties | Flexible yet rigid hyaline cartilage capable of withstanding dynamic collapse | Multiple segments of hyaline cartilage with varying levels of thickness and rigidity | Single piece of elastic cartilage of uniform thickness but highly varied topography |
| Cell Sources | Autologous chondrocytes and MSCs | Autologous chondrocytes and MSCs | Autologous chondrocytes, perichondrocytes, and MSCs |
| Scaffold Materials | Decellularized donor trachea, PGA, PLA, PCL, PCLA, Pluronic F-127, collagen gel, polypropylene, polytetrafluoroethylene | PCL, PLGA, cellulose-based hydrogels, alginate-based hydrogels, type 1 and 2 collagen hydrogel | PLA, PGA, collagen, gelatin, keratin, fibronectin, alginate, cellulose, HA, chitosan, GAG, human fibrin, PU, chitosan, PRP |
| Scaffold Manufacturing | Decellularized donor trachea, injection molding, electrospinning, 3D bioprinting | 3D bioprinting | Injection molding, photopolymerization hydrogel system, external stenting, stereolithography |
| Current Human Applications | Repair of circumferential tracheal defects, repair of bronchial defects | Repair of LLC and ULC defects | Auricular cartilage framework for microtia repair |
| Current Limitations | Poor revascularization after implantation, lack of cellular ingrowth after implantation, inability to fully monitor graft after implantation, exposure to microbial organisms after implantation, recurrent granulation tissue formation and luminal collapse at short-term follow-up, anastomotic breakdown and fistulae formation at long-term follow-up | Lack of studies investigating human use, lack of FDA guidance on bioengineered nasal cartilage, large amount of heterogeneity in manufacturing methods limiting large-scale translation | Low chondrocyte cell numbers from initial donor cell harvest, lack of long-term follow-up studies, potential cytotoxic effects of scaffold breakdown products |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, M.; Ahmed, K.H.; Punjabi, N.; Inman, J.C. A Contemporary Review of Trachea, Nose, and Ear Cartilage Bioengineering and Additive Manufacturing. Biomimetics 2024, 9, 327. https://doi.org/10.3390/biomimetics9060327
Feng M, Ahmed KH, Punjabi N, Inman JC. A Contemporary Review of Trachea, Nose, and Ear Cartilage Bioengineering and Additive Manufacturing. Biomimetics. 2024; 9(6):327. https://doi.org/10.3390/biomimetics9060327
Chicago/Turabian StyleFeng, Max, Khwaja Hamzah Ahmed, Nihal Punjabi, and Jared C. Inman. 2024. "A Contemporary Review of Trachea, Nose, and Ear Cartilage Bioengineering and Additive Manufacturing" Biomimetics 9, no. 6: 327. https://doi.org/10.3390/biomimetics9060327
APA StyleFeng, M., Ahmed, K. H., Punjabi, N., & Inman, J. C. (2024). A Contemporary Review of Trachea, Nose, and Ear Cartilage Bioengineering and Additive Manufacturing. Biomimetics, 9(6), 327. https://doi.org/10.3390/biomimetics9060327







