Calcium Phosphate Biomaterials for 3D Bioprinting in Bone Tissue Engineering
Abstract
:1. Introduction
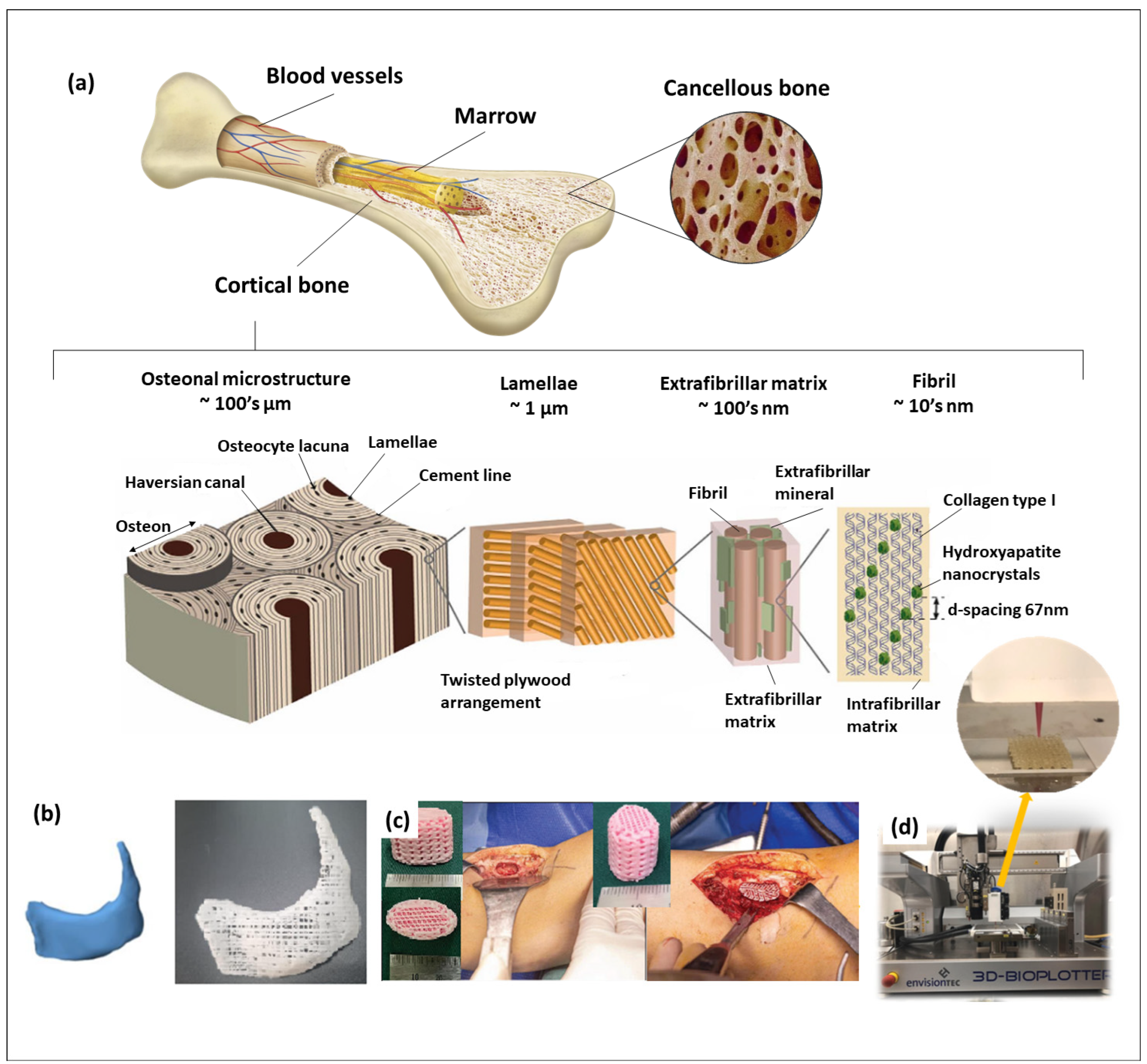
2. Calcium Phosphates Used in Tissue Engineering
2.1. Hydroxyapatite
2.2. Crystalline Tricalcium Phosphates
2.3. Monetite (Dicalcium Phosphate Anhydrous—DCPA) and Brushite (Dicalcium Phosphate Dihydrate—DCPD)
2.4. Amorphous Calcium Phosphate
2.5. Octacalcium Phosphate
3. Hydrogels Used with Calcium Phosphates in 3D Bioprinting
4. Status of Calcium Phosphate Biomaterials in 3D (Bio)Printing Applications
4.1. Hydroxyapatite (HAp)-Based 3D-Printed Scaffolds
4.2. Beta-Tricalcium Phosphate (β-TCP)-Based 3D-Printed Scaffolds
| Polymer | Filler Used | β-TCP Concentration/Particle Size | Highlights | Scaffold’s Pore Size (Porosity, %) | Ref. |
|---|---|---|---|---|---|
| Poly(hydroxyalkanoates) (PHAs) | 0 wt%, 5 wt%, 10 wt%, 20 wt% and 30 wt% of PHA/10–20 μm | The addition of β-TCP significantly improved the proliferation, adhesion and migration of MC3T3-E1 cells. The obtained scaffolds presented compressive strength compatible with natural bone. | ~400 μm | [61] | |
| Poly(lactic acid) (PLA) | 15 vol% β-TCP/(d50)—5 (±2) µm | Higher nozzle temperatures helped enhance the tensile strength of the printed parts. TCP–PLA printed samples experienced lower mechanical properties than PLA. | [66] | ||
| Polycaprolactone | 20% wt% | The PCL/β-TCP scaffold can provide durable support and enhance bone formation in complex zygomatic–maxillary defects. | 500 µm | [12] | |
| Poly(tri-methyl carbonate) (PTMC)/Poly(ε-caprolactone) (PCL) | 0–25% wt% | Provides good porous growth microenvironments and mechanical support for MC3T3-E1 cells and rBMSCs and enhances the proliferation of osteoblast cells. | PCL/25%TCP: (66.43 ± 2.56%) PTMC/25%TCP: (58.9 ± 2.81%) PTMC/PCL/25%TCP (48.0 ± 1.84%) | [63] | |
| Polycaprolactone (PCL) | Poly(ethylene glycol) | 10 wt%/9.3 μm | Compared with the PCL scaffolds, the PCL/TCP/PEG scaffolds exhibited good wettability (contact angle decreased from 85 °C to 0 °C, showing complete wettability). The alkaline phosphatase content of the PCL/TCP/PEG scaffold increased by 2.5 times after 14 days of co-culture compared with the PCL scaffold. | 550 μm | [64] |
| Polycaprolactone (PCL) | Carbon nanotubes (CNTs)/HAp | 10 and 20 wt% | Enhances gene expression. | 350 µm | [65] |
| Poly(lactic-co-glycol acid) (PLGA) | Elastic thermoplastic polyurethane | 20% w/v | Better mechanical properties compared to OsteoInk®. | Printing porosity: 100 nm–1 mm Surface porosity: 2–50 mm | [62] |
| Sodium acetate (SA) | Lignin | 70–80% | LG-containing scaffolds show a 15% increase in mechanical strength. The capacity to promote the osteoblasts’ adhesion and proliferation as well as their bioactivity (formation of hydroxyapatite crystals) was improved. | (37–41%) | [67] |
| Polyvinyl alcohol (PVA) | Dipyridamole | <100 μm | Improving the scaffold’s hydrophilicity and promoting cell proliferation and adhesion and significantly inducing osteogenic differentiation of stem cells were fixed. | ~500 μm | [68] |
4.3. Biphasic Calcium Phosphate (BCP)-Based 3D-Printed Scaffolds
4.4. Octacalcium Phosphate (OCP) as Biomaterial
4.5. Dicalcium Phosphate Dihydrate (DCPD) as Biomaterial
5. CaPs Incorporated Hydrogels for 3D Bioprinting
6. Discussion and Perspective
6.1. Issues of CaP/Hydrogels for 3D Bioprinting
6.2. Recent Trends and Future Direction of CaP/Hydrogels for 3D Bioprinting
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anada, T.; Pan, C.C.; Stahl, A.M.; Mori, S.; Fukuda, J.; Suzuki, O.; Yang, Y. Vascularized Bone-Mimetic Hydrogel Constructs by 3D Bioprinting to Promote Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2019, 20, 1096. [Google Scholar] [CrossRef]
- Taheri, S.; Ghazali, H.S.; Ghazali, Z.S.; Bhattacharyya, A.; Noh, I. Progress in biomechanical stimuli on the cell-encapsulated hydrogels for cartilage tissue regeneration. Biomater. Res. 2023, 27, 22. [Google Scholar] [CrossRef]
- Bedell, M.L.; Torres, A.L.; Hogan, K.J.; Wang, Z.; Wang, B.; Melchiorri, A.J.; Grande-Allen, K.J.; Mikos, A.G. Human gelatin-based composite hydrogels for osteochondral tissue engineering and their adaptation into bioinks for extrusion, inkjet, and digital light processing bioprinting. Biofabrication 2022, 14, 045012. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Janarthanan, G.; Noh, I. Nano-biomaterials for designing functional bioinks towards complex tissue and organ regeneration in 3D bioprinting. Addit. Manuf. 2021, 37, 101639. [Google Scholar] [CrossRef]
- Liu, S.; Bernhardt, A.; Wirsig, K.; Lode, A.; Hu, Q.; Gelinsky, M.; Kilian, D. Synergy of inorganic and organic inks in bioprinted tissue substitutes: Construct stability and cell response during long-term cultivation in vitro. Compos. Part B Eng. 2023, 261, 110804. [Google Scholar] [CrossRef]
- Budharaju, H.; Suresh, S.; Sekar, M.P.; De Vega, B.; Sethuraman, S.; Sundaramurthi, D.; Kalaskar, D.M. Ceramic materials for 3D printing of biomimetic bone scaffolds—Current state-of-the-art & future perspectives. Mater. Des. 2023, 231, 112064. [Google Scholar] [CrossRef]
- Gao, W.; Deng, J.; Ren, J.; Zhang, W.; Wang, Z.; He, R.; Wang, K.; Shi, X.; Liang, T. 3D-printed hydroxyapatite (HA) scaffolds combined with exos from BMSCs cultured in 3D HA scaffolds to repair bone defects. Compos. Part B Eng. 2022, 247, 110315. [Google Scholar] [CrossRef]
- Montelongo, S.A.; Chiou, G.; Ong, J.L.; Bizios, R.; Guda, T. Development of bioinks for 3D printing microporous, sintered calcium phosphate scaffolds. J. Mater. Sci. Mater. Med. 2021, 32, 94. [Google Scholar] [CrossRef] [PubMed]
- Herrada-Manchon, H.; Rodriguez-Gonzalez, D.; Fernandez, M.A.; Kucko, N.W.; Barrere-de Groot, F.; Aguilar, E. Effect on Rheological Properties and 3D Printability of Biphasic Calcium Phosphate Microporous Particles in Hydrocolloid-Based Hydrogels. Gels 2022, 8, 28. [Google Scholar] [CrossRef]
- Seo, Y.-W.; Park, J.-Y.; Lee, D.-N.; Jin, X.; Cha, J.-K.; Paik, J.-W.; Choi, S.-H. Three-dimensionally printed biphasic calcium phosphate blocks with different pore diameters for regeneration in rabbit calvarial defects. Biomater. Res. 2022, 26, 25. [Google Scholar] [CrossRef]
- Anderson, M.; Dubey, N.; Bogie, K.; Cao, C.; Li, J.; Lerchbacker, J.; Mendonca, G.; Kauffmann, F.; Bottino, M.C.; Kaigler, D. Three-dimensional printing of clinical scale and personalized calcium phosphate scaffolds for alveolar bone reconstruction. Dent. Mater. 2022, 38, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.S.; Kim, Y.C.; Min, J.C.; Park, H.J.; Lee, E.J.; Shim, J.H.; Choi, J.W. Clinical Application of 3D-Printed Patient-Specific Polycaprolactone/Beta Tricalcium Phosphate Scaffold for Complex Zygomatico-Maxillary Defects. Polymers 2022, 14, 740. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Cao, B.; Deng, L.; Li, J.; Ran, Z.; Wu, J.; Pang, B.; Tan, J.; Luo, D.; Wu, W. The first 3D-bioprinted personalized active bone to repair bone defects: A case report. Int. J. Bioprint 2023, 9, 654. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Naghieh, S.; Yazdanpanah, Z.; Alizadeh Sardroud, H.; Sharma, N.K.; Wilson, L.D.; Chen, X. Fabrication of chitosan/alginate/hydroxyapatite hybrid scaffolds using 3D printing and impregnating techniques for potential cartilage regeneration. Int. J. Biol. Macromol. 2022, 204, 62–75. [Google Scholar] [CrossRef]
- Filip, D.G.; Surdu, V.A.; Paduraru, A.V.; Andronescu, E. Current Development in Biomaterials-Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review. J. Funct. Biomater. 2022, 13, 248. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, K.; He, R.; Ding, G.; Xia, M.; Jin, X.; Xie, C. Additive manufacturing of hydroxyapatite bioceramic scaffolds: Dispersion, digital light processing, sintering, mechanical properties, and biocompatibility. J. Adv. Ceram. 2020, 9, 360–373. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, L.; Wu, T. Recent Advances in Bioengineering Bone Revascularization Based on Composite Materials Comprising Hydroxyapatite. Int. J. Mol. Sci. 2023, 24, 12492. [Google Scholar] [CrossRef]
- Trzaskowska, M.; Vivcharenko, V.; Przekora, A. The Impact of Hydroxyapatite Sintering Temperature on Its Microstructural, Mechanical, and Biological Properties. Int. J. Mol. Sci. 2023, 24, 5083. [Google Scholar] [CrossRef]
- Anita Lett, J.; Sagadevan, S.; Fatimah, I.; Hoque, M.E.; Lokanathan, Y.; Léonard, E.; Alshahateet, S.F.; Schirhagl, R.; Oh, W.C. Recent advances in natural polymer-based hydroxyapatite scaffolds: Properties and applications. Eur. Polym. J. 2021, 148, 110360. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Dobelin, N. beta-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, L.; Gbureck, U.; Bhaduri, S.B.; Sikder, P. Monetite, an important calcium phosphate compound-Its synthesis, properties and applications in orthopedics. Acta Biomater. 2021, 127, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Motameni, A.; Alshemary, A.Z.; Evis, Z. A review of synthesis methods, properties and use of monetite cements as filler for bone defects. Ceram. Int. 2021, 47, 13245–13256. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [PubMed]
- Vecstaudza, J.; Gasik, M.; Locs, J. Amorphous calcium phosphate materials: Formation, structure and thermal behaviour. J. Eur. Ceram. Soc. 2019, 39, 1642–1649. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Synthetic amorphous calcium phosphates (ACPs): Preparation, structure, properties, and biomedical applications. Biomater. Sci. 2021, 9, 7748–7798. [Google Scholar] [CrossRef] [PubMed]
- Davies, E.; Duer, M.J.; Ashbrook, S.E.; Griffin, J.M. Applications of NMR crystallography to problems in biomineralization: Refinement of the crystal structure and 31P solid-state NMR spectral assignment of octacalcium phosphate. J. Am. Chem. Soc. 2012, 134, 12508–12515. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Hu, J.; Fang, Y.; Hu, R.; Shi, W.; Ren, B.; Lin, C.; Tian, Z.Q. Surface Properties of Octacalcium Phosphate Nanocrystals Are Crucial for Their Bioactivities. ACS Omega 2021, 6, 25372–25380. [Google Scholar] [CrossRef]
- Shi, H.; Ye, X.; Zhang, J.; Wu, T.; Yu, T.; Zhou, C.; Ye, J. A thermostability perspective on enhancing physicochemical and cytological characteristics of octacalcium phosphate by doping iron and strontium. Bioact. Mater. 2021, 6, 1267–1282. [Google Scholar] [CrossRef]
- Suzuki, O.; Hamai, R.; Sakai, S. The material design of octacalcium phosphate bone substitute: Increased dissolution and osteogenecity. Acta Biomater. 2023, 158, 1–11. [Google Scholar] [CrossRef]
- Sai, Y.; Shiwaku, Y.; Anada, T.; Tsuchiya, K.; Takahashi, T.; Suzuki, O. Capacity of octacalcium phosphate to promote osteoblastic differentiation toward osteocytes in vitro. Acta Biomater. 2018, 69, 362–371. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, F.; Zou, Y.; Zhang, Z.; Wang, A.; Zhang, Y. Physicochemical and biological properties of composite bone cement based on octacalcium phosphate and tricalcium silicate. Ceram. Int. 2023, 49, 20315–20325. [Google Scholar] [CrossRef]
- Dong, Q.; Su, X.; Li, X.; Zhou, H.; Jian, H.; Bai, S.; Yin, J.; You, Q. In vitro construction of lung cancer organoids by 3D bioprinting for drug evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131288. [Google Scholar] [CrossRef]
- Tharakan, S.; Khondkar, S.; Lee, S.; Ahn, S.; Mathew, C.; Gresita, A.; Hadjiargyrou, M.; Ilyas, A. 3D Printed Osteoblast-Alginate/Collagen Hydrogels Promote Survival, Proliferation and Mineralization at Low Doses of Strontium Calcium Polyphosphate. Pharmaceutics 2022, 15, 11. [Google Scholar] [CrossRef]
- Jeon, O.; Lee, Y.B.; Lee, S.J.; Guliyeva, N.; Lee, J.; Alsberg, E. Stem cell-laden hydrogel bioink for generation of high resolution and fidelity engineered tissues with complex geometries. Bioact. Mater. 2022, 15, 185–193. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Khatun, M.R.; Narmatha, S.; Nagarajan, R.; Noh, I. Modulation of 3D Bioprintability in Polysaccharide Bioink by Bioglass Nanoparticles and Multiple Metal Ions for Tissue Engineering. Tissue Eng. Regen. Med. 2023, 21, 261–275. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Ham, H.W.; Sonh, J.; Gunbayar, M.; Jeffy, R.; Nagarajan, R.; Khatun, M.R.; Noh, I. 3D bioprinting of complex tissue scaffolds with in situ homogeneously mixed alginate-chitosan-kaolin bioink using advanced portable biopen. Carbohydr. Polym. 2023, 317, 121046. [Google Scholar] [CrossRef]
- Gharacheh, H.; Guvendiren, M. Cell-Laden Composite Hydrogel Bioinks with Human Bone Allograft Particles to Enhance Stem Cell Osteogenesis. Polymers 2022, 14, 3788. [Google Scholar] [CrossRef]
- Thanh, T.N.; Laowattanatham, N.; Ratanavaraporn, J.; Sereemaspun, A.; Yodmuang, S. Hyaluronic acid crosslinked with alginate hydrogel: A versatile and biocompatible bioink platform for tissue engineering. Eur. Polym. J. 2022, 166, 111027. [Google Scholar] [CrossRef]
- Khatun, M.R.; Bhattacharyya, A.; Taheri, S.; Ham, H.w.; Kim, H.; Chang, S.H.; Noh, I. High Molecular Weight Fucoidan Loading Into and Release from Hyaluronate-Based Prefabricated Hydrogel and its Nanogel Particles Controlled by Variable Pitch and Differential Extensional Shear Technology of Advanced Twin Screw-Based System. Adv. Mater. Technol. 2022, 8, 2201478. [Google Scholar] [CrossRef]
- Flegeau, K.; Puiggali-Jou, A.; Zenobi-Wong, M. Cartilage tissue engineering by extrusion bioprinting utilizing porous hyaluronic acid microgel bioinks. Biofabrication 2022, 14, 034105. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Wang, Z.; Yan, B.; Shi, A.; Xu, J.; Guan, J.; Zhang, L.; Zhou, P.; Mao, Y. Mechanically enhanced composite hydrogel scaffold for in situ bone repairs. Biomater. Adv. 2022, 134, 112700. [Google Scholar] [CrossRef]
- Chang, H.K.; Yang, D.H.; Ha, M.Y.; Kim, H.J.; Kim, C.H.; Kim, S.H.; Choi, J.W.; Chun, H.J. 3D printing of cell-laden visible light curable glycol chitosan bioink for bone tissue engineering. Carbohydr. Polym. 2022, 287, 119328. [Google Scholar] [CrossRef]
- Bharadwaj, T.; Chrungoo, S.; Verma, D. Self-assembled chitosan/gelatin nanofibrous aggregates incorporated thermosensitive nanocomposite bioink for bone tissue engineering. Carbohydr. Polym. 2024, 324, 121544. [Google Scholar] [CrossRef]
- Tran, H.N.; Kim, I.G.; Kim, J.H.; Bhattacharyya, A.; Chung, E.J.; Noh, I. Incorporation of Cell-Adhesive Proteins in 3D-Printed Lipoic Acid-Maleic Acid-Poly(Propylene Glycol)-Based Tough Gel Ink for Cell-Supportive Microenvironment. Macromol. Biosci. 2023, 23, e2300316. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Priya, V.N.K.; Kim, J.H.; Khatun, M.R.; Nagarajan, R.; Noh, I. Nanodiamond enhanced mechanical and biological properties of extrudable gelatin hydrogel cross-linked with tannic acid and ferrous sulphate. Biomater. Res. 2022, 26, 37. [Google Scholar] [CrossRef]
- Mirek, A.; Belaid, H.; Barranger, F.; Grzeczkowicz, M.; Bouden, Y.; Cavailles, V.; Lewinska, D.; Bechelany, M. Development of a new 3D bioprinted antibiotic delivery system based on a cross-linked gelatin-alginate hydrogel. J. Mater. Chem. B 2022, 10, 8862–8874. [Google Scholar] [CrossRef]
- Cui, Z.; Feng, Y.; Liu, F.; Jiang, L.; Yue, J. 3D Bioprinting of Living Materials for Structure-Dependent Production of Hyaluronic Acid. ACS Macro Lett. 2022, 11, 452–459. [Google Scholar] [CrossRef]
- Sathish, P.B.; Gayathri, S.; Priyanka, J.; Muthusamy, S.; Narmadha, R.; Krishnakumar, G.S.; Selvakumar, R. Tricomposite gelatin-carboxymethylcellulose-alginate bioink for direct and indirect 3D printing of human knee meniscal scaffold. Int. J. Biol. Macromol. 2022, 195, 179–189. [Google Scholar] [CrossRef]
- Khatun, M.R.; Bhattacharyya, A.; Gunbayar, M.; Jung, M.; Noh, I. Study on Bioresponsive Gelatin-Hyaluronic Acid-Genipin Hydrogel for High Cell-Density 3D Bioprinting. Gels 2023, 9, 601. [Google Scholar] [CrossRef]
- Sang, S.; Mao, X.; Cao, Y.; Liu, Z.; Shen, Z.; Li, M.; Jia, W.; Guo, Z.; Wang, Z.; Xiang, C.; et al. 3D Bioprinting Using Synovium-Derived MSC-Laden Photo-Cross-Linked ECM Bioink for Cartilage Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 8895–8913. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, C.; Zhang, B.; Song, P.; Xu, X.; Gui, X.; Chen, X.; Lu, G.; Li, X.; Liang, J.; et al. 3D printed calcium phosphate scaffolds with controlled release of osteogenic drugs for bone regeneration. Chem. Eng. J. 2022, 427, 130961. [Google Scholar] [CrossRef]
- Leu Alexa, R.; Cucuruz, A.; Ghitulica, C.D.; Voicu, G.; Stamat Balahura, L.R.; Dinescu, S.; Vlasceanu, G.M.; Stavarache, C.; Ianchis, R.; Iovu, H.; et al. 3D Printable Composite Biomaterials Based on GelMA and Hydroxyapatite Powders Doped with Cerium Ions for Bone Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 1841. [Google Scholar] [CrossRef]
- Leu Alexa, R.; Cucuruz, A.; Ghitulica, C.D.; Voicu, G.; Stamat Balahura, L.R.; Dinescu, S.; Vlasceanu, G.M.; Iovu, H.; Serafim, A.; Ianchis, R.; et al. 3D Printed Composite Scaffolds of GelMA and Hydroxyapatite Nanopowders Doped with Mg/Zn Ions to Evaluate the Expression of Genes and Proteins of Osteogenic Markers. Nanomaterials 2022, 12, 3420. [Google Scholar] [CrossRef]
- Pitrolino, K.A.; Felfel, R.M.; Pellizzeri, L.M.; McLaren, J.; Popov, A.A.; Sottile, V.; Scotchford, C.A.; Scammell, B.E.; Roberts, G.A.F.; Grant, D.M. Development and in vitro assessment of a bi-layered chitosan-nano-hydroxyapatite osteochondral scaffold. Carbohydr. Polym. 2022, 282, 119126. [Google Scholar] [CrossRef]
- Wei, J.; Yan, Y.; Gao, J.; Li, Y.; Wang, R.; Wang, J.; Zou, Q.; Zuo, Y.; Zhu, M.; Li, J. 3D-printed hydroxyapatite microspheres reinforced PLGA scaffolds for bone regeneration. Biomater. Adv. 2022, 133, 112618. [Google Scholar] [CrossRef]
- Lafuente-Merchan, M.; Ruiz-Alonso, S.; Garcia-Villen, F.; Zabala, A.; de Retana, A.M.O.; Gallego, I.; Saenz-Del-Burgo, L.; Pedraz, J.L. 3D Bioprinted Hydroxyapatite or Graphene Oxide Containing Nanocellulose-Based Scaffolds for Bone Regeneration. Macromol. Biosci. 2022, 22, e2200236. [Google Scholar] [CrossRef]
- Lee, H.; Yoo, J.M.; Ponnusamy, N.K.; Nam, S.Y. 3D-printed hydroxyapatite/gelatin bone scaffolds reinforced with graphene oxide: Optimized fabrication and mechanical characterization. Ceram. Int. 2022, 48, 10155–10163. [Google Scholar] [CrossRef]
- Pottathara, Y.B.; Kokol, V. Effect of nozzle diameter and cross-linking on the micro-structure, compressive and biodegradation properties of 3D printed gelatin/collagen/hydroxyapatite hydrogel. Bioprinting 2023, 31, e00266. [Google Scholar] [CrossRef]
- Yang, T.; Dong, Y.; Wan, J.; Liu, X.; Liu, Y.; Huang, J.; Zhou, J.; Xiao, H.; Tang, L.; Wang, Y.; et al. Sustained Release of BMSC-EVs from 3D Printing Gel/HA/nHAP Scaffolds for Promoting Bone Regeneration in Diabetic Rats. Adv. Healthc. Mater. 2023, 12, e2203131. [Google Scholar] [CrossRef]
- Fischetti, T.; Borciani, G.; Avnet, S.; Rubini, K.; Baldini, N.; Graziani, G.; Boanini, E. Incorporation/Enrichment of 3D Bioprinted Constructs by Biomimetic Nanoparticles: Tuning Printability and Cell Behavior in Bone Models. Nanomaterials 2023, 13, 2040. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, Y.; Liu, T.; Chen, Z.; Chen, W.; Wu, Z.; Wang, Y.; Li, J.; Li, C.; Jiang, T.; et al. Beta-tricalcium phosphate enhanced mechanical and biological properties of 3D-printed polyhydroxyalkanoates scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2022, 209, 1553–1561. [Google Scholar] [CrossRef]
- Hatt, L.P.; Wirth, S.; Ristaniemi, A.; Ciric, D.J.; Thompson, K.; Eglin, D.; Stoddart, M.J.; Armiento, A.R. Micro-porous PLGA/beta-TCP/TPU scaffolds prepared by solvent-based 3D printing for bone tissue engineering purposes. Regen. Biomater. 2023, 10, rbad084. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Liu, Z.W.; Kang, H.L.; Liu, F.; Yan, G.P.; Li, F. 3D-Printed scaffolds based on poly(Trimethylene carbonate), poly(epsilon-Caprolactone), and beta-Tricalcium phosphate. Int. J. Bioprint 2023, 9, 641. [Google Scholar] [CrossRef]
- Liu, K.; Sun, J.; Zhu, Q.; Jin, X.; Zhang, Z.; Zhao, Z.; Chen, G.; Wang, C.; Jiang, H.; Zhang, P. Microstructures and properties of polycaprolactone/tricalcium phosphate scaffolds containing polyethylene glycol fabricated by 3D printing. Ceram. Int. 2022, 48, 24032–24043. [Google Scholar] [CrossRef]
- Nalesso, P.R.L.; Vedovatto, M.; Gregorio, J.E.S.; Huang, B.; Vyas, C.; Santamaria, M., Jr.; Bartolo, P.; Caetano, G.F. Early In Vivo Osteogenic and Inflammatory Response of 3D Printed Polycaprolactone/Carbon Nanotube/Hydroxyapatite/Tricalcium Phosphate Composite Scaffolds. Polymers 2023, 15, 2952. [Google Scholar] [CrossRef]
- Elhattab, K.; Bhaduri, S.B.; Sikder, P. Influence of Fused Deposition Modelling Nozzle Temperature on the Rheology and Mechanical Properties of 3D Printed beta-Tricalcium Phosphate (TCP)/Polylactic Acid (PLA) Composite. Polymers 2022, 14, 1222. [Google Scholar] [CrossRef]
- Silva-Barroso, A.S.; Cabral, C.S.D.; Ferreira, P.; Moreira, A.F.; Correia, I.J. Lignin-enriched tricalcium phosphate/sodium alginate 3D scaffolds for application in bone tissue regeneration. Int. J. Biol. Macromol. 2023, 239, 124258. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, N.; Ma, Y.; Dai, H.; Han, B. Preparation and study of 3D printed dipyridamole/β-tricalcium phosphate/ polyvinyl alcohol composite scaffolds in bone tissue engineering. J. Drug Deliv. Sci. Technol. 2022, 68, 103053. [Google Scholar] [CrossRef]
- Lu, T.; Yuan, X.; Zhang, L.; He, F.; Wang, X.; Ye, J. Enhancing osteoinduction and bone regeneration of biphasic calcium phosphate scaffold thought modulating the balance between pro-osteogenesis and anti-osteoclastogenesis by zinc doping. Mater. Today Chem. 2023, 29, 101410. [Google Scholar] [CrossRef]
- Lu, T.; Miao, Y.; Yuan, X.; Zhang, Y.; Ye, J. Adjusting physicochemical and cytological properties of biphasic calcium phosphate by magnesium substitution: An in vitro study. Ceram. Int. 2023, 49, 15588–15598. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, B.; Wan, T.; Zhou, C.; Fan, Y.; Tian, W.; Jing, W. A 3D-printed biphasic calcium phosphate scaffold loaded with platelet lysate/gelatin methacrylate to promote vascularization. J. Mater. Chem. B 2022, 10, 3138–3151. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Zhang, Y.; Li, X.; Min, L.; Cao, Q.; Luo, Y.; Yang, X.; Lu, M.; Zhou, Y.; et al. Graphene oxide coated three-dimensional printed biphasic calcium phosphate scaffold for angiogenic and osteogenic synergy in repairing critical-size bone defect. J. Mater. Sci. Technol. 2023, 145, 25–39. [Google Scholar] [CrossRef]
- Torres, P.M.C.; Ribeiro, N.; Nunes, C.M.M.; Rodrigues, A.F.M.; Sousa, A.; Olhero, S.M. Toughening robocast chitosan/biphasic calcium phosphate composite scaffolds with silk fibroin: Tuning printable inks and scaffold structure for bone regeneration. Biomater. Adv. 2022, 134, 112690. [Google Scholar] [CrossRef]
- Park, S.S.; Park, M.; Lee, B.T. Autologous stromal vascular fraction-loaded hyaluronic acid/gelatin-biphasic calcium phosphate scaffold for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2022, 132, 112533. [Google Scholar] [CrossRef]
- Teterina, A.Y.; Smirnov, I.V.; Fadeeva, I.S.; Fadeev, R.S.; Smirnova, P.V.; Minaychev, V.V.; Kobyakova, M.I.; Fedotov, A.Y.; Barinov, S.M.; Komlev, V.S. Octacalcium Phosphate for Bone Tissue Engineering: Synthesis, Modification, and In Vitro Biocompatibility Assessment. Int. J. Mol. Sci. 2021, 22, 12747. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, B.; Zhao, C.; Lu, Y.; Huang, T.; Chen, Y.; Li, J.; Wu, R.; Liu, W.; Lin, J. Lanthanum doped octacalcium phosphate/polylactic acid scaffold fabricated by 3D printing for bone tissue engineering. J. Mater. Sci. Technol. 2022, 118, 229–242. [Google Scholar] [CrossRef]
- Jeong, C.H.; Kim, J.; Kim, H.S.; Lim, S.Y.; Han, D.; Huser, A.J.; Lee, S.B.; Gim, Y.; Ji, J.H.; Kim, D.; et al. Acceleration of bone formation by octacalcium phosphate composite in a rat tibia critical-sized defect. J. Orthop. Transl. 2022, 37, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Kamakura, S.; Matsui, K.; Fukuda, M.; Takano, H.; Iino, M.; Ishikawa, S.; Kawana, H.; Soma, T.; Imamura, E.; et al. Clinical study of octacalcium phosphate and collagen composite in oral and maxillofacial surgery. J. Tissue Eng. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kuvshinova, E.A.; Petrakova, N.V.; Nikitina, Y.O.; Sviridova, I.K.; Akhmedova, S.A.; Kirsanova, V.A.; Karalkin, P.A.; Komlev, V.S.; Sergeeva, N.S.; Kaprin, A.D. Functionalization of Octacalcium Phosphate Bone Graft with Cisplatin and Zoledronic Acid: Physicochemical and Bioactive Properties. Int. J. Mol. Sci. 2023, 24, 11633. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ma, M.; Yang, H.; He, W.; Xie, Y.; Li, J.; Li, J.; Zhao, F.; Zheng, Y. Chitosan/polydopamine/octacalcium phosphate composite microcarrier simulates natural bone components to induce osteogenic differentiation of stem cells. Biomater. Adv. 2023, 154, 213642. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, J.; Feng, Q.; Xie, E.; Meng, Q.; Shu, W.; Wu, J.; Bian, L.; Han, F.; Li, B. Building Osteogenic Microenvironments with a Double-Network Composite Hydrogel for Bone Repair. Research 2023, 6, 0021. [Google Scholar] [CrossRef] [PubMed]
- Bordbar-Khiabani, A.; Kovrlija, I.; Locs, J.; Loca, D.; Gasik, M. Octacalcium Phosphate-Laden Hydrogels on 3D-Printed Titanium Biomaterials Improve Corrosion Resistance in Simulated Biological Media. Int. J. Mol. Sci. 2023, 24, 13135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, T.; Meng, H.; Wang, X.; Peng, H.; Liu, G.; Wei, S.; Lu, Q.; Wang, Y.; Wang, A.; et al. 3D gel-printed porous magnesium scaffold coated with dibasic calcium phosphate dihydrate for bone repair in vivo. J. Orthop. Transl. 2022, 33, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Abu-Nada, L.; Al-Waeli, H.; Mezour, M.A.; Abdallah, M.N.; Kinsella, J.M.; Kort-Mascort, J.; Henderson, J.E.; Ramirez-Garcialuna, J.L.; Tran, S.D.; et al. Bone extracts immunomodulate and enhance the regenerative performance of dicalcium phosphates bioceramics. Acta Biomater. 2019, 89, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zhou, X.; Feng, Y.; Qian, K.; Liu, H.; Lu, M.; Chu, C.; Xue, F.; Bai, J. Insights into self-healing behavior and mechanism of dicalcium phosphate dihydrate coating on biomedical Mg. Bioact. Mater. 2021, 6, 158–168. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Zamparini, F.; Valente, S.; Parchi, G.; Pasquinelli, G.; Taddei, P.; Prati, C. Green Hydrogels Composed of Sodium Mannuronate/Guluronate, Gelatin and Biointeractive Calcium Silicates/Dicalcium Phosphate Dihydrate Designed for Oral Bone Defects Regeneration. Nanomaterials 2021, 11, 3439. [Google Scholar] [CrossRef]
- Guo, C.; Wu, J.; Zeng, Y.; Li, H. Construction of 3D bioprinting of HAP/collagen scaffold in gelation bath for bone tissue engineering. Regen. Biomater. 2023, 10, rbad067. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, S.; Meng, Y.; He, M.; Liu, C.; Wang, C.; Ni, X. Study on 3D-Printed Emodin/Nano-Hydroxyapatite Scaffolds Promoting Bone Regeneration by Supporting Osteoblast Proliferation and Macrophage M2 Polarization. ACS Appl. Polym. Mater. 2023, 5, 4069–4079. [Google Scholar] [CrossRef]
- Kang, Y.; Xu, J.; Meng, L.; Su, Y.; Fang, H.; Liu, J.; Cheng, Y.Y.; Jiang, D.; Nie, Y.; Song, K. 3D bioprinting of dECM/Gel/QCS/nHAp hybrid scaffolds laden with mesenchymal stem cell-derived exosomes to improve angiogenesis and osteogenesis. Biofabrication 2023, 15, 024103. [Google Scholar] [CrossRef]
- Koo, Y.; Kim, G.H. Bioprinted hASC-laden collagen/HA constructs with meringue-like macro/micropores. Bioeng. Transl. Med. 2022, 7, e10330. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Janarthanan, G.; Tran, H.N.; Ham, H.J.; Yoon, J.; Noh, I. Bioink homogeneity control during 3D bioprinting of multicomponent micro/nanocomposite hydrogel for even tissue regeneration using novel twin screw extrusion system. Chem. Eng. J. 2021, 415, 128971. [Google Scholar] [CrossRef]
- Ghahri, T.; Salehi, Z.; Aghajanpour, S.; Eslaminejad, M.B.; Kalantari, N.; Akrami, M.; Dinarvand, R.; Jang, H.L.; Esfandyari-Manesh, M. Development of osteon-like scaffold-cell construct by quadruple coaxial extrusion-based 3D bioprinting of nanocomposite hydrogel. Biomater. Adv. 2023, 145, 213254. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.B.; Abar, B.; Johnson, L.; Burbano, J.; Danilkowicz, R.M.; Adams, S.B. 3D-bioprinted GelMA-gelatin-hydroxyapatite osteoblast-laden composite hydrogels for bone tissue engineering. Bioprinting 2022, 26, e00196. [Google Scholar] [CrossRef]
- Vurat, M.T.; Seker, S.; Lalegul-Ulker, O.; Parmaksiz, M.; Elcin, A.E.; Elcin, Y.M. Development of a multicellular 3D-bioprinted microtissue model of human periodontal ligament-alveolar bone biointerface: Towards a pre-clinical model of periodontal diseases and personalized periodontal tissue engineering. Genes Dis. 2022, 9, 1008–1023. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Raja, N.; Choi, Y.J.; Gal, C.W.; Sung, A.; Park, H.; Yun, H.S. Enhancement of properties of a cell-laden GelMA hydrogel-based bioink via calcium phosphate phase transition. Biofabrication 2023, 16, 015010. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Janarthanan, G.; Kim, T.; Taheri, S.; Shin, J.; Kim, J.; Bae, H.C.; Han, H.S.; Noh, I. Modulation of bioactive calcium phosphate micro/nanoparticle size and shape during in situ synthesis of photo-crosslinkable gelatin methacryloyl based nanocomposite hydrogels for 3D bioprinting and tissue engineering. Biomater. Res. 2022, 26, 54. [Google Scholar] [CrossRef]
- Kim, W.; Lee, H.; Ji Roh, E.; Bae An, S.; Han, I.-B.; Hyung Kim, G. A multicellular bioprinted cell construct for vascularized bone tissue regeneration. Chem. Eng. J. 2022, 431, 133882. [Google Scholar] [CrossRef]
- Lee, D.N.; Park, J.Y.; Seo, Y.W.; Jin, X.; Hong, J.; Bhattacharyya, A.; Noh, I.; Choi, S.H. Photo-crosslinked gelatin methacryloyl hydrogel strengthened with calcium phosphate-based nanoparticles for early healing of rabbit calvarial defects. J. Periodontal Implant. Sci. 2023, 53, 321–335. [Google Scholar] [CrossRef]

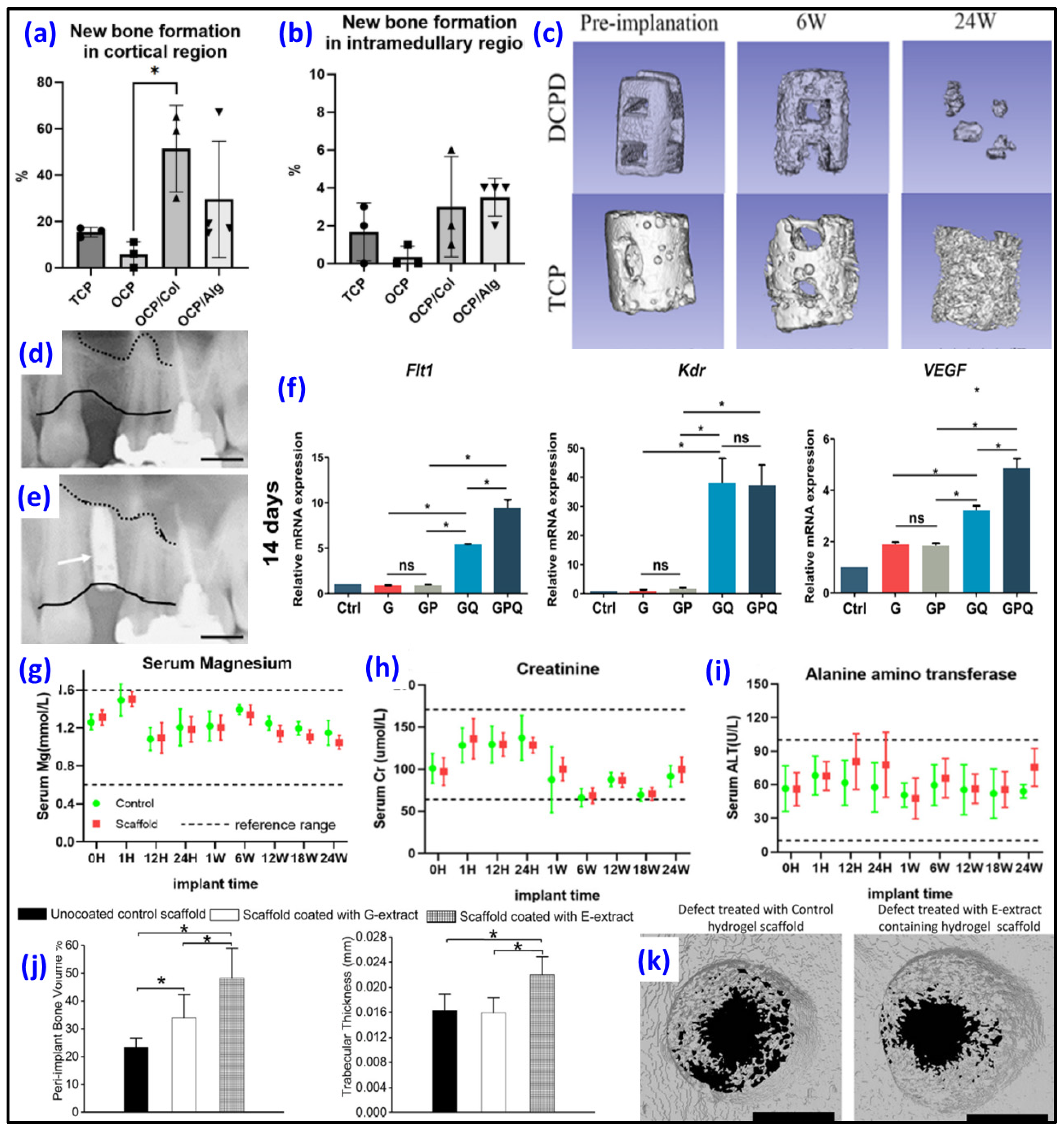
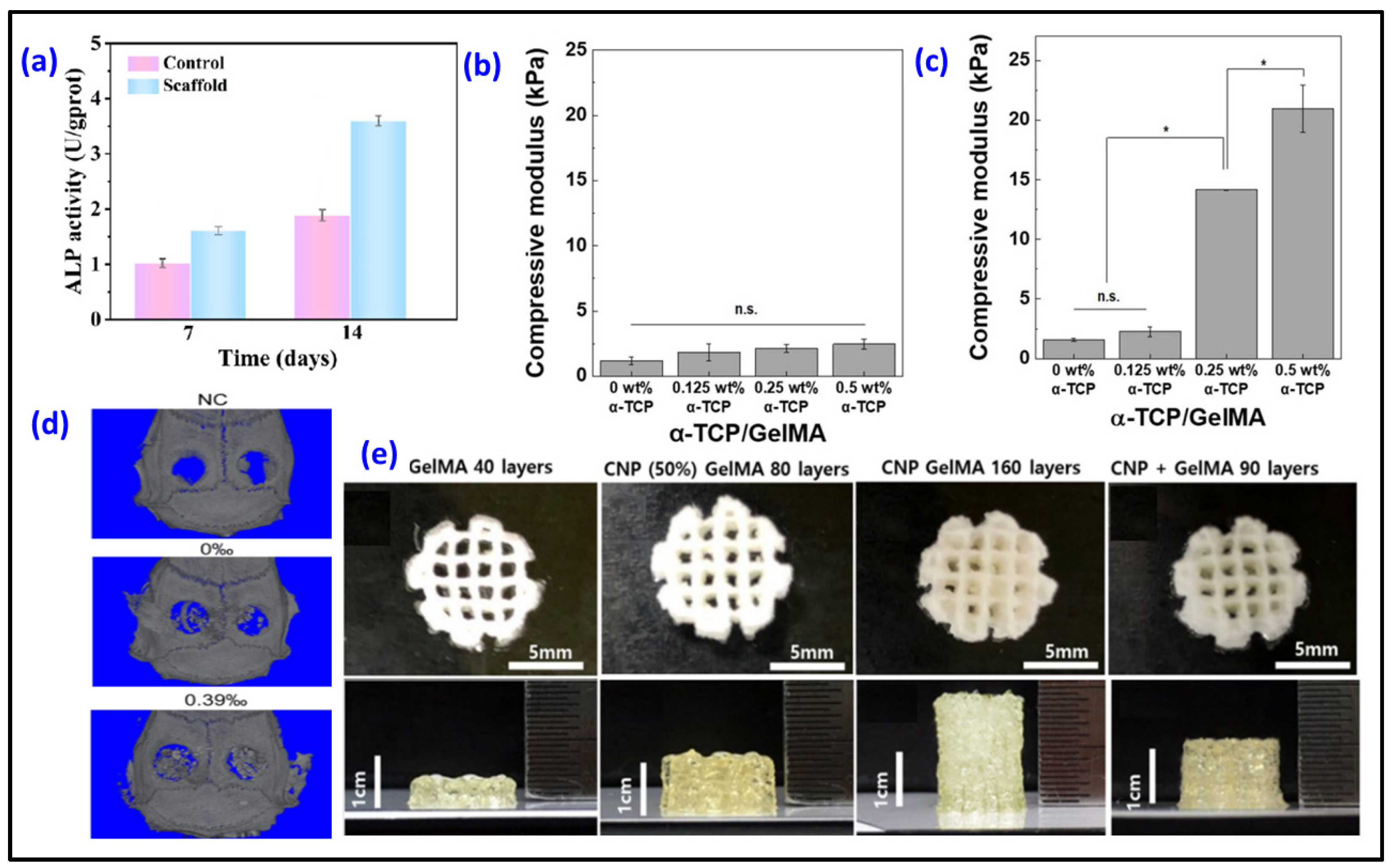
| Polymer | Additive Used | HAp’s Concentration/Particle Size | Highlights | Scaffold Pore Size (Porosity, %) | Ref. |
|---|---|---|---|---|---|
| Alginate | Icariin | 25% (w/v)/25–50 μm | Improved mechanical strength. Release of the icariin drug from the scaffold was slow and orderly sustained. | 400–500 μm | [51] |
| GelMA | Ce-HAp | 3% Ce-HAp (0.5%Ce mol.)/150–180 nm | Highly concentrated Ce-HAp (0.5 molar%)/GelMA composite shows the highest level of cell viability and proliferation potency along with a low cytotoxic potential. | 126–138 µm for 20%GelMA-3%HC5 and 30%GelMA-3%HC5 | [52] |
| GelMA | Mg/Zn-HAp | (Ca + Mg or Ca + Zn)/P of 1.67/ 70–140 nm (Mg) 50–120 nm (Zn) | Positive effect of magnesium and zinc on the osteogenic differentiation process. | 100–200 µm (45% for 25%GelMA3%HAp-Zn and 53.6% for 30%GelMA-3%HAp-Zn) | [53] |
| Chitosan | 70 wt%/nano size | Reinforces chitosan porous matrix. | 160–275 μm | [54] | |
| Poly (lactic-co-glycolic acid) | Polyvinyl alcohol | 0%, 15%, 30%, 45%, and 60% (wt%)/n-HA sphere diameter: 3–10 μm | 45 wt% HAp/PLGA had the highest compressive strength of more than 40 MPa, which was six times higher than that of the pure PLGA scaffold. | 359.4 ± 12 μm | [55] |
| Nanocellulose-alginate | Graphene oxide (GO)-containing scaffolds have a higher swelling capacity compared to HAp-containing ones and promote a higher expression of osteogenic markers than HA. Hence, osteoinductive properties could be higher than HAp’s. | HAP 200–300 μm GO 400–500 μm | [56] | ||
| Gelatin | Graphene oxide (GO) | 70% (w/v)/<200 nm | The 0.5% GO 3D-printed HA/gelatin scaffold shows increased compressive and flexural strength values by 15% and 22%, respectively. Additionally, GO’s reinforcer effects made the 3D-printed scaffolds compatible with cancellous bone. | [57] | |
| Gelatin/collagen | <200 nm | Adjusting the duration of crosslinking allows for control of the stiffness of printed scaffolds. | 430–550 μm | [58] | |
| Chitosan/alginate | 10% w/v/ <200 nm | The viability and attachment of the contracts on the scaffolds were enhanced using nHAp particles and alginate hydrogel. Chitosan-based scaffolds with nHAp particles had an improvement in elastic modulus and thermal stability behavior. | 2–3 mm | [14] | |
| Gelatin/hyaluronic acid | 10% nHAp solution/particle size < 100 nm | High glucose can block the proliferation, migration and osteogenic differentiation of bone marrow stem cells (BMSCs). Both NG-EVs and HG-EVs can stimulate proliferation and migration, prevent apoptosis and promote osteogenic differentiation, but HG-EVs have a lesser effect than NG-EVs. | 410–415 μm | [59] | |
| Alginate-RGD | Sr-HA | 0.5%, 1% and 2% (w/v) nHAp nanocrystals:
| The ink can still maintain optimal extrusion and biocompatibility by adding a 1% w/v concentration, regardless of the type of particle. | [60] |
| Calcium Phosphate | Hydrogel | Cells | Ref. |
|---|---|---|---|
| Hydroxyapatite | Collagen | Bone marrow mesenchymal stem cells (BMSCs) | [87] |
| Nanohydroxyapatite | Sodium alginate (SA)/gelatin (Gel) | Emodin-drug and mouse embryonic osteoblast precursor (MC3T3-E1) cells | [88] |
| Nanohydroxyapatite | Gelatin (Gel), quaternized chitosan (QCS) | Mesenchymal-stem-cell-derived exosomes | [89] |
| Hydroxyapatite | Collagen | MG63 cells and human adipose stem cells | [90] |
| α-TCP | Alginate | Mouse calvaria-derived preosteoblast cells (MC3T3) | [91] |
| Whitlockite and hydroxyapatite | Gelatin methacryloyl (GelMA) and alginate blended hydrogel (poly(ethylene glycol) as a hollowing agent in the first layer) | Human umbilical vein endothelial cells (HUVECs) and human mesenchymal stem cells (hMSCs) | [92] |
| Hydroxyapatite | Gelatin methacryloyl (GelMA), gelatin | MC3T3-E1 subclone 4 mouse calvaria osteoblast | [93] |
| Hydroxyapatite–magnetic iron oxide nanoparticles | Gelatin methacryloyl (GelMA) | Human PDLFs and human osteoblasts (hOBs) | [94] |
| α-TCP | Gelatin methacrylate (GelMA) | Mouse calvaria-derived preosteoblast cell line (MC3T3-E1) | [95] |
| Amorphous calcium phosphate micro/nanoparticles | Gelatin methacrylate (GelMA) | MC3T3, adipose-derived mesenchymal stem cells (AdMSC) | [96] |
| β-tricalcium phosphate | Polycaprolactone (PCL) | The patient’s autologous platelet-rich plasma (PRP) | [13] |
| β-tricalcium phosphate | Collagen | Human adipose stem cells (hASCs) and human umbilical vein endothelial cells (HUVECs) | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolmacheva, N.; Bhattacharyya, A.; Noh, I. Calcium Phosphate Biomaterials for 3D Bioprinting in Bone Tissue Engineering. Biomimetics 2024, 9, 95. https://doi.org/10.3390/biomimetics9020095
Tolmacheva N, Bhattacharyya A, Noh I. Calcium Phosphate Biomaterials for 3D Bioprinting in Bone Tissue Engineering. Biomimetics. 2024; 9(2):95. https://doi.org/10.3390/biomimetics9020095
Chicago/Turabian StyleTolmacheva, Nelli, Amitava Bhattacharyya, and Insup Noh. 2024. "Calcium Phosphate Biomaterials for 3D Bioprinting in Bone Tissue Engineering" Biomimetics 9, no. 2: 95. https://doi.org/10.3390/biomimetics9020095
APA StyleTolmacheva, N., Bhattacharyya, A., & Noh, I. (2024). Calcium Phosphate Biomaterials for 3D Bioprinting in Bone Tissue Engineering. Biomimetics, 9(2), 95. https://doi.org/10.3390/biomimetics9020095









