Replace or Regenerate? Diverse Approaches to Biomaterials for Treating Corneal Lesions
Abstract
:1. Introduction
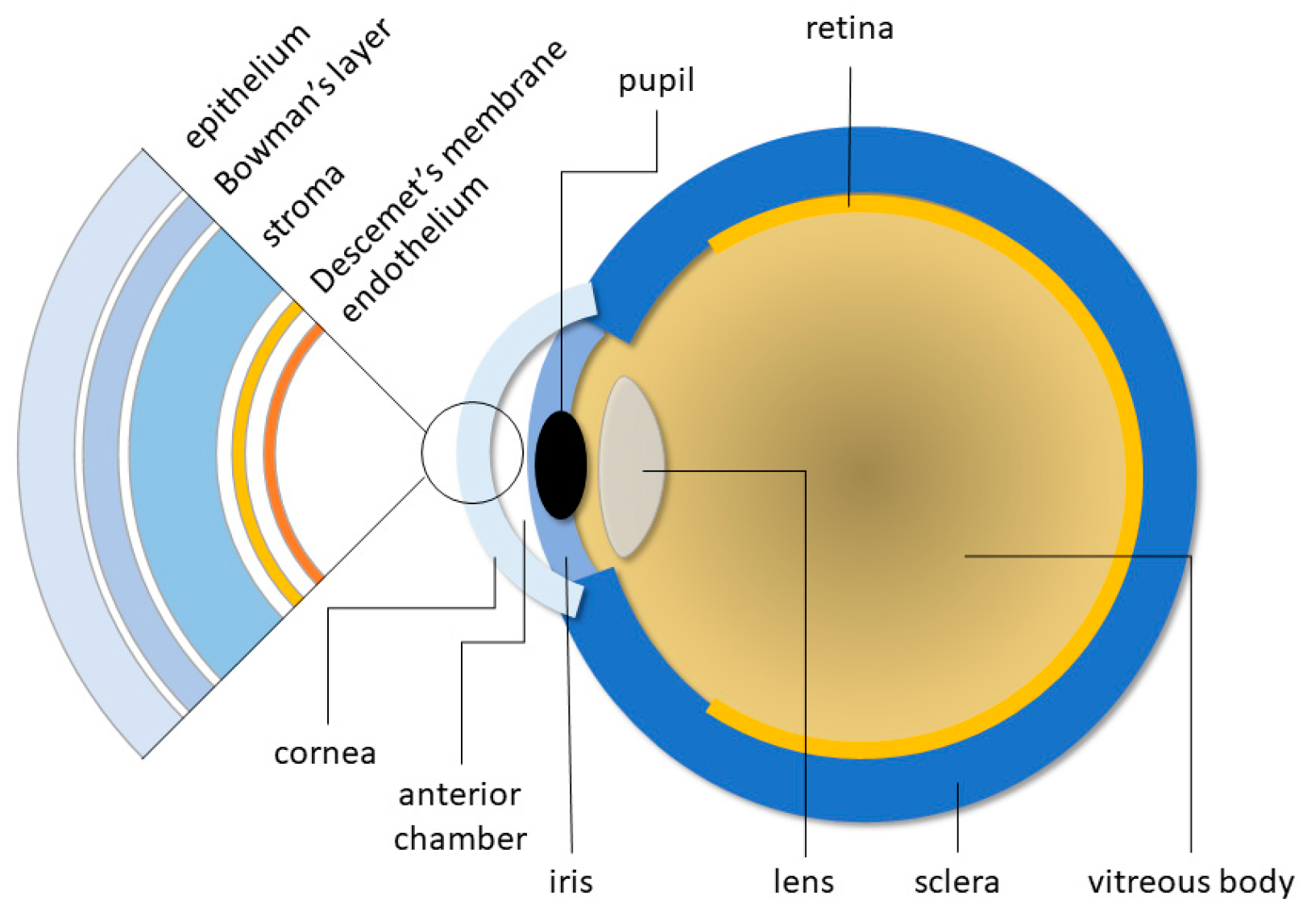
2. Lesions and Diseases Affecting the Cornea
3. Current Therapeutic Approaches
3.1. Keratoplasty
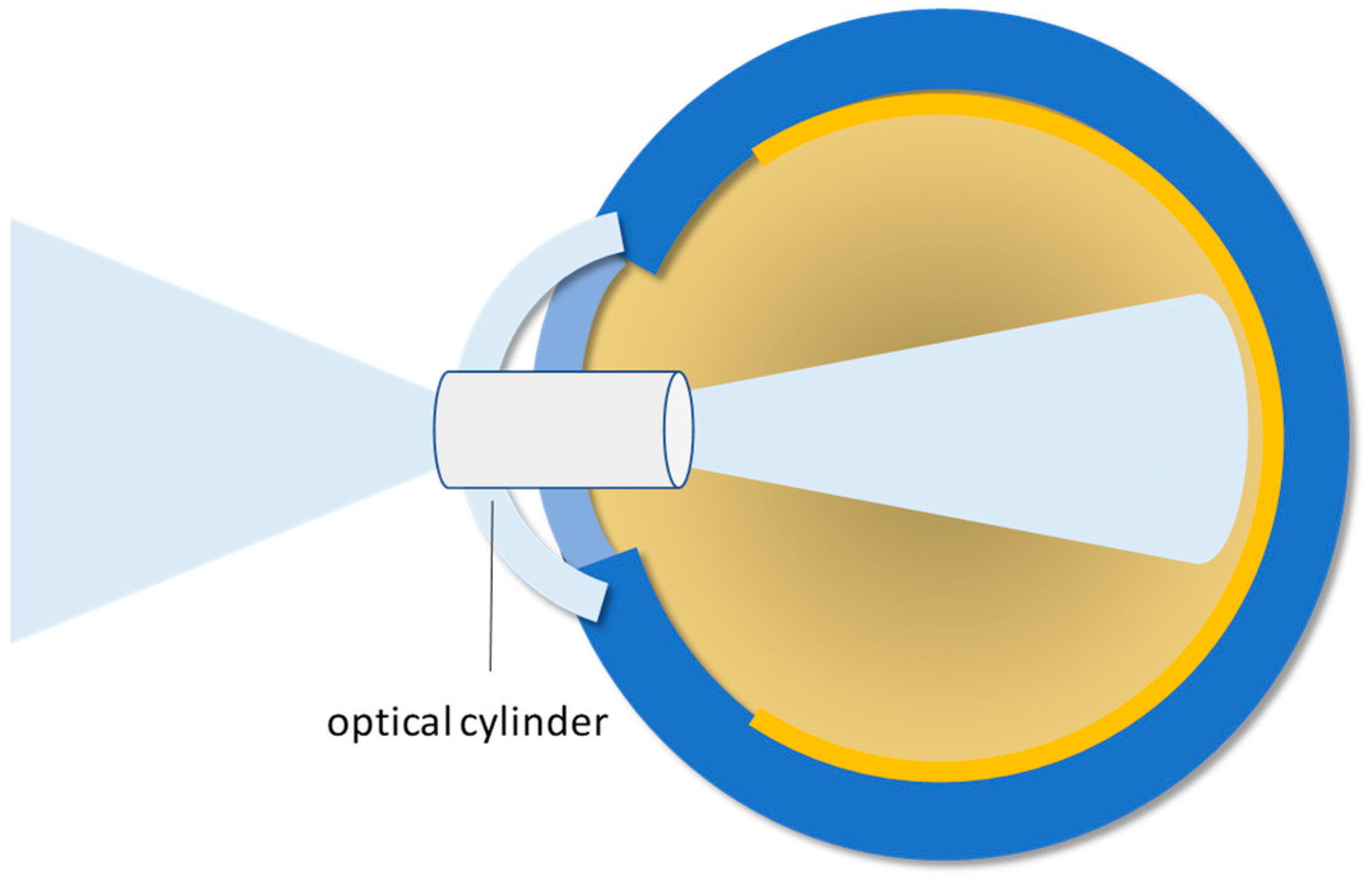
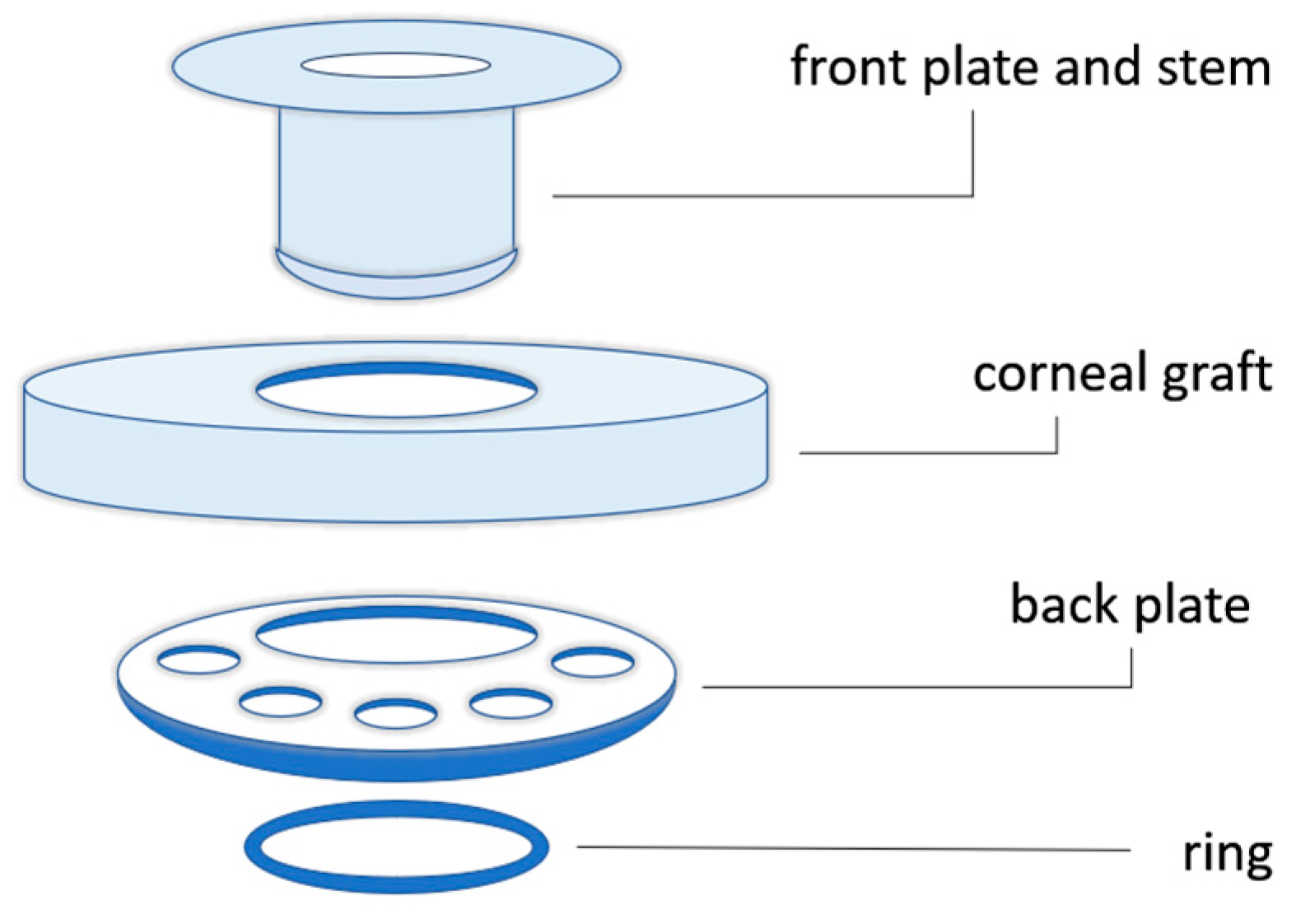
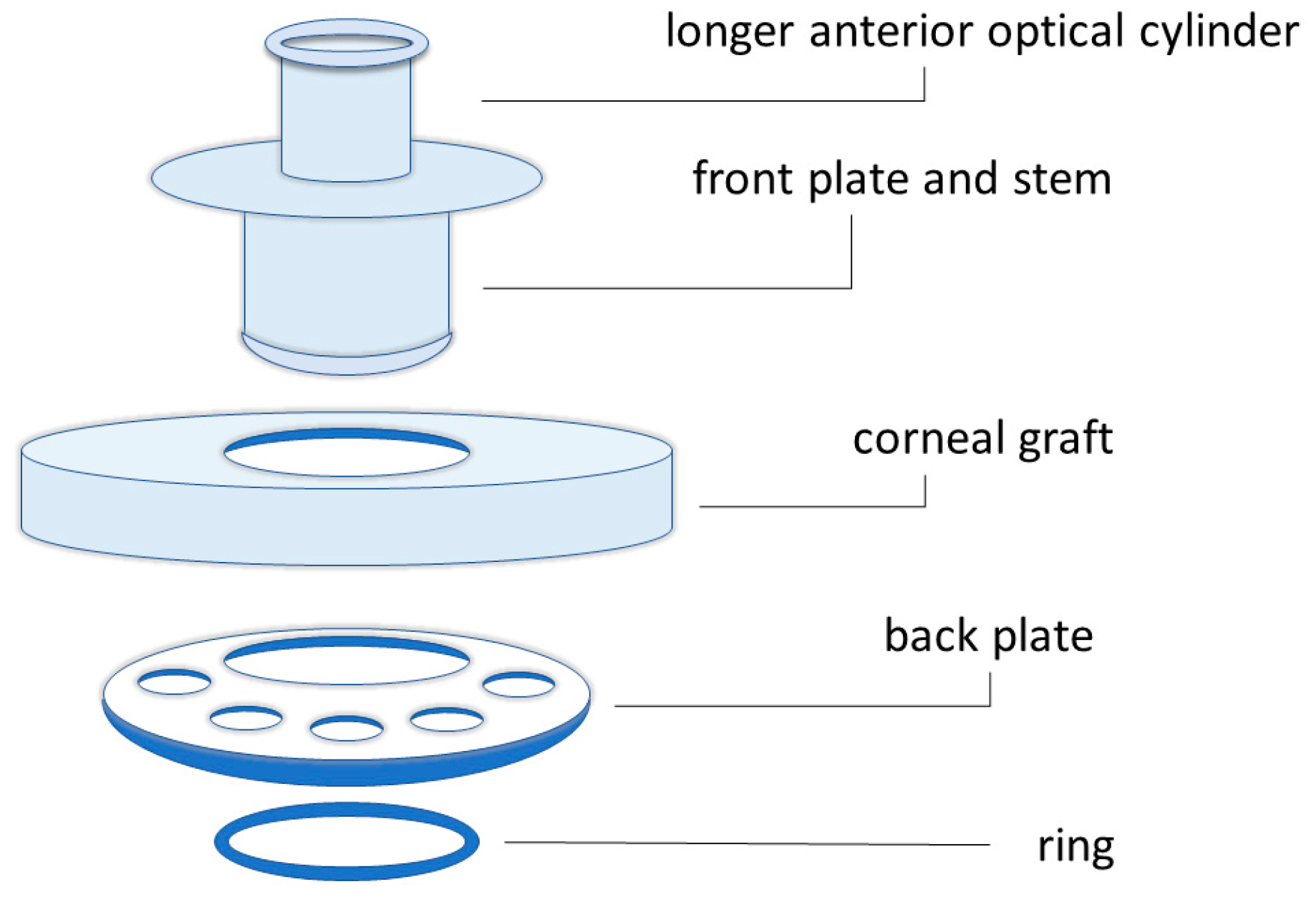
3.2. Keratoprosthesis
4. Replace or Regenerate?
4.1. Biomaterials for Cornea Replacement and Restoration
4.2. Cells and Biomaterials for Cornea Regeneration
4.2.1. Scaffold-Free Approach
4.2.2. Cell-Free Scaffold
4.2.3. Cell-Based Scaffold
5. Future Perspectives
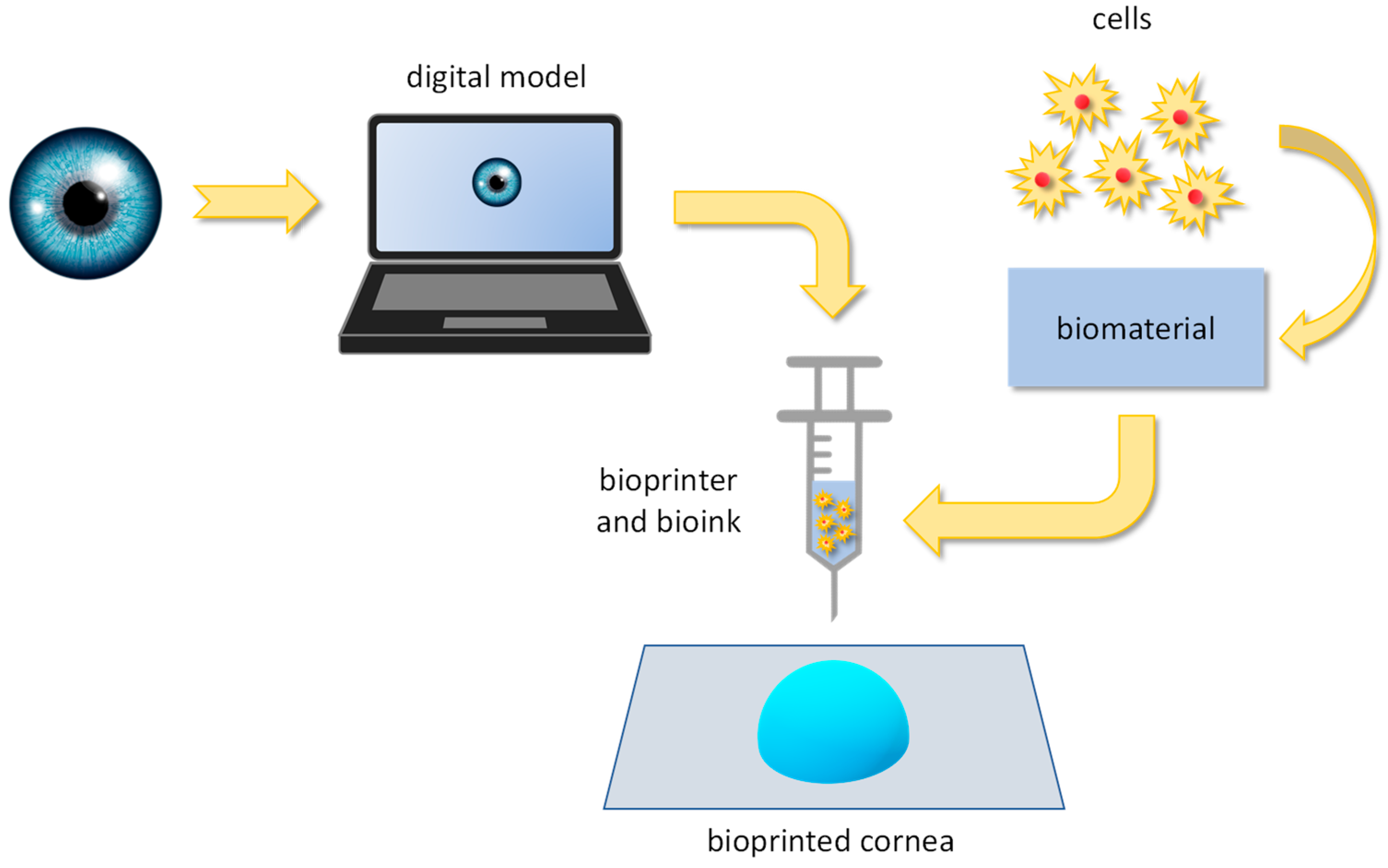
5.1. Bioprinting
5.2. Xenoimplants
6. Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sridhar, M. Anatomy of Cornea and Ocular Surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Guérin, L.-P.; Le-Bel, G.; Desjardins, P.; Couture, C.; Gillard, E.; Boisselier, É.; Bazin, R.; Germain, L.; Guérin, S.L. The Human Tissue-Engineered Cornea (hTEC): Recent Progress. Int. J. Mol. Sci. 2021, 22, 1291. [Google Scholar] [CrossRef] [PubMed]
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the Human Corneal Innervation. Exp. Eye Res. 2010, 90, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Alizadeh, Z.; Nosrati, A.; Ashrafi-Dehkordi, K.; Banitalebi-Dehkordi, M.; Sanami, S.; Khodaei, M. Stem Cell-Based Therapeutic Strategies for Corneal Epithelium Regeneration. Tissue Cell 2021, 68, 101470. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M. Multifunctional Biomimetic Materials for Corneal Regeneration; Karolinska Institutet: Stockholm, Sweden, 2016. [Google Scholar]
- Palchesko, R.N.; Carrasquilla, S.D.; Feinberg, A.W. Natural Biomaterials for Corneal Tissue Engineering, Repair, and Regeneration. Adv. Healthc. Mater. 2018, 7, 1701434. [Google Scholar] [CrossRef] [PubMed]
- Koujah, L.; Suryawanshi, R.K.; Shukla, D. Pathological Processes Activated by Herpes Simplex Virus-1 (HSV-1) Infection in the Cornea. Cell. Mol. Life Sci. 2019, 76, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Sibley, D.; Larkin, D.F.P. Update on Herpes Simplex Keratitis Management. Eye 2020, 34, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Valerio, G.S.; Lin, C.C. Ocular Manifestations of Herpes Simplex Virus. Curr. Opin. Ophthalmol. 2019, 30, 525–531. [Google Scholar] [CrossRef]
- Rowe, A.M.; Leger, A.S.; Jeon, S.; Dhaliwal, D.K.; Knickelbein, J.E.; Hendricks, R.L. Herpes Keratitis. Prog. Retin. Eye Res. 2013, 32, 88–101. [Google Scholar] [CrossRef]
- Kennedy, D.P.; Clement, C.; Arceneaux, R.L.; Bhattacharjee, P.S.; Huq, T.S.; Hill, J.M. Ocular Herpes Simplex Virus Type 1: Is the Cornea a Reservoir for Viral Latency or a Fast Pit Stop? Cornea 2011, 30, 251–259. [Google Scholar] [CrossRef]
- Lobo, A.-M.; Agelidis, A.M.; Shukla, D. Pathogenesis of Herpes Simplex Keratitis: The Host Cell Response and Ocular Surface Sequelae to Infection and Inflammation. Ocul. Surf. 2019, 17, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Wallang, B.S.; Das, S. Keratoglobus. Eye 2013, 27, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Abad, A.; Piñero, D.P. Pellucid Marginal Degeneration: Detection, Discrimination from Other Corneal Ectatic Disorders and Progression. Contact Lens Anterior Eye 2019, 42, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Murri, M.S.; Birdsong, O.C.; Ronquillo, Y.; Moshirfar, M. Terrien Marginal Degeneration. Surv. Ophthalmol. 2019, 64, 162–174. [Google Scholar] [CrossRef]
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An Updated Review. Contact Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef]
- Morishige, N.; Wahlert, A.J.; Kenney, M.C.; Brown, D.J.; Kawamoto, K.; Chikama, T.; Nishida, T.; Jester, J.V. Second-Harmonic Imaging Microscopy of Normal Human and Keratoconus Cornea. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1087–1094. [Google Scholar] [CrossRef]
- Romero-Jiménez, M.; Santodomingo-Rubido, J.; González-Méijome, J.-M. The Thinnest, Steepest, and Maximum Elevation Corneal Locations in Noncontact and Contact Lens Wearers in Keratoconus. Cornea 2013, 32, 332–337. [Google Scholar] [CrossRef]
- Schrems-Hoesl, L.M.; Schrems, W.A.; Cruzat, A.; Shahatit, B.M.; Bayhan, H.A.; Jurkunas, U.V.; Hamrah, P. Cellular and Subbasal Nerve Alterations in Early Stage Fuchs’ Endothelial Corneal Dystrophy: An In Vivo Confocal Microscopy Study. Eye 2013, 27, 42–49. [Google Scholar] [CrossRef]
- Parekh, M.; Romano, V.; Hassanin, K.; Testa, V.; Wongvisavavit, R.; Ferrari, S.; Haneef, A.; Willoughby, C.; Ponzin, D.; Jhanji, V.; et al. Biomaterials for Corneal Endothelial Cell Culture and Tissue Engineering. J. Tissue Eng 2021, 12, 2041731421990536. [Google Scholar] [CrossRef]
- Ong Tone, S.; Kocaba, V.; Böhm, M.; Wylegala, A.; White, T.L.; Jurkunas, U.V. Fuchs Endothelial Corneal Dystrophy: The Vicious Cycle of Fuchs Pathogenesis. Prog. Retin. Eye Res. 2021, 80, 100863. [Google Scholar] [CrossRef] [PubMed]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.T.; Dart, J.K.; Holland, E.J.; Kinoshita, S. Corneal Transplantation. Lancet 2012, 379, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Moramarco, A.; Gardini, L.; Iannetta, D.; Versura, P.; Fontana, L. Post Penetrating Keratoplasty Ectasia: Incidence, Risk Factors, Clinical Features, and Treatment Options. J. Clin. Med. 2022, 11, 2678. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Chaudhary, M.; Sitaula, S. Penetrating Keratoplasty—Indications in a Tertiary Care Center in Nepal. Nepal. J. Ophthalmol. 2020, 12, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gupta, N.; Vanathi, M.; Tandon, R. Corneal Transplantation in the Modern Era. Indian J. Med. Res. 2019, 150, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Shimmura, S.; Tsubota, K. Deep Anterior Lamellar Keratoplasty. Curr. Opin. Ophthalmol. 2006, 17, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Fogla, R. Deep Anterior Lamellar Keratoplasty in the Management of Keratoconus. Indian J. Ophthalmol. 2013, 61, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Grottone, G.T.; Pereira, N.C.; Gomes, J.Á.P. Endothelial Keratoplasty: Evolution and Horizons. Arq. Bras. Oftalmol. 2012, 75, 439–446. [Google Scholar] [CrossRef]
- Price, M.O.; Mehta, J.S.; Jurkunas, U.V.; Price, F.W. Corneal Endothelial Dysfunction: Evolving Understanding and Treatment Options. Prog. Retin. Eye Res. 2021, 82, 100904. [Google Scholar] [CrossRef]
- Singh, N.; Said, D.; Dua, H. Lamellar Keratoplasty Techniques. Indian J. Ophthalmol. 2018, 66, 1239–1250. [Google Scholar] [CrossRef]
- Stuart, A.J.; Romano, V.; Virgili, G.; Shortt, A.J. Descemet’s Membrane Endothelial Keratoplasty (DMEK) versus Descemet’s Stripping Automated Endothelial Keratoplasty (DSAEK) for Corneal Endothelial Failure. Cochrane Database Syst. Rev. 2018, 2018, CD012097. [Google Scholar] [CrossRef]
- Park, C.Y.; Lee, J.K.; Gore, P.K.; Lim, C.-Y.; Chuck, R.S. Keratoplasty in the United States. Ophthalmology 2015, 122, 2432–2442. [Google Scholar] [CrossRef] [PubMed]
- Kruse, F.E.; Laaser, K.; Cursiefen, C.; Heindl, L.M.; Schlötzer-Schrehardt, U.; Riss, S.; Bachmann, B.O. A Stepwise Approach to Donor Preparation and Insertion Increases Safety and Outcome of Descemet Membrane Endothelial Keratoplasty. Cornea 2011, 30, 580–587. [Google Scholar] [CrossRef]
- Holland, G.; Pandit, A.; Sánchez-Abella, L.; Haiek, A.; Loinaz, I.; Dupin, D.; Gonzalez, M.; Larra, E.; Bidaguren, A.; Lagali, N.; et al. Artificial Cornea: Past, Current, and Future Directions. Front. Med. 2021, 8, 770780. [Google Scholar] [CrossRef]
- Basak, S.; Basak, S. Recurrent Cystoid Macular Edema Following Boston Keratoprosthesis Type-II Implantation: A Treatment Option. Indian J. Ophthalmol. 2020, 68, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H.N.; Shanbhag, S.; Chodosh, J. The Boston Keratoprosthesis. Curr. Opin. Ophthalmol. 2017, 28, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Nonpassopon, M.; Niparugs, M.; Cortina, M.S. Boston Type 1 Keratoprosthesis: Updated Perspectives. Clin. Ophthalmol. 2020, 14, 1189–1200. [Google Scholar] [CrossRef]
- Dohlman, C. The Boston Keratoprosthesis—The First 50 Years: Some Reminiscences. Annu. Rev. Vis. Sci. 2022, 8, 1–32. [Google Scholar] [CrossRef]
- Bakshi, S.K.; Graney, J.; Paschalis, E.I.; Agarwal, S.; Basu, S.; Iyer, G.; Liu, C.; Srinivasan, B.; Chodosh, J. Design and Outcomes of a Novel Keratoprosthesis: Addressing Unmet Needs in End-Stage Cicatricial Corneal Blindness. Cornea 2020, 39, 484–490. [Google Scholar] [CrossRef]
- Salvador-Culla, B.; Jeong, K.J.; Kolovou, P.E.; Chiang, H.H.; Chodosh, J.; Dohlman, C.H.; Kohane, D.S. Titanium Coating of the Boston Keratoprosthesis. Trans. Vis. Sci. Technol. 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.S.; Chodosh, J. Expanding Application of the Boston Type I Keratoprosthesis Due to Advances in Design and Improved Post-Operative Therapeutic Strategies. Semin. Ophthalmol. 2010, 25, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.F.; Harissi-Dagher, M.; Khan, D.M.; Dohlman, C.H. Advances in Boston Keratoprosthesis: Enhancing Retention and Prevention of Infection and Inflammation. Int. Ophthalmol. Clin. 2007, 47, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Dudenhoefer, E.J.; Dohlman, C.H. Keratoprosthesis: An Update. Curr. Opin. Ophthalmol. 2001, 12, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, E.I.; Taniguchi, E.V.; Chodosh, J.; Pasquale, L.R.; Colby, K.; Dohlman, C.H.; Shen, L.Q. Blood Levels of Tumor Necrosis Factor Alpha and Its Type 2 Receptor Are Elevated in Patients with Boston Type I Keratoprosthesis. Curr. Eye Res. 2019, 44, 599–606. [Google Scholar] [CrossRef]
- Todani, A.; Ciolino, J.B.; Ament, J.D.; Colby, K.A.; Pineda, R.; Belin, M.W.; Aquavella, J.V.; Chodosh, J.; Dohlman, C.H. Titanium Back Plate for a PMMA Keratoprosthesis: Clinical Outcomes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Ebrahimi, Z.; Ebrahimi, K.S.; Farhadian, N.; Shahlaei, M.; Cheraqpour, K.; Ghasemi, H.; Moradi, S.; Chang, A.Y.; Sharifi, S.; et al. Application of Biomaterials and Nanotechnology in Corneal Tissue Engineering. J. Int. Med. Res. 2023, 51, 03000605231190473. [Google Scholar] [CrossRef] [PubMed]
- Zarei-Ghanavati, M.; Avadhanam, V.; Vasquez Perez, A.; Liu, C. The Osteo-Odonto-Keratoprosthesis. Curr. Opin. Ophthalmol. 2017, 28, 397–402. [Google Scholar] [CrossRef]
- Goossen, C.; Stempels, N.; Colpaert, C.; Tassignon, M.J. Strampelli Osteo-Odonto-Keratoprosthesis: Case Report. Bull. Soc. Belg. Ophtalmol. 1998, 268, 129–133. [Google Scholar] [PubMed]
- Avadhanam, V.; Smith, H.; Liu, C. Keratoprostheses for Corneal Blindness: A Review of Contemporary Devices. Clin. Ophthalmol. 2015, 9, 697–720. [Google Scholar] [CrossRef]
- Chirila, T.V. An Overview of the Development of Artificial Corneas with Porous Skirts and the Use of PHEMA for Such an Application. Biomaterials 2001, 22, 3311–3317. [Google Scholar] [CrossRef] [PubMed]
- Hollick, E.J. Legeais BioKpro III Keratoprosthesis Implantation: Long Term Results in Seven Patients. Br. J. Ophthalmol. 2006, 90, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Moody, J.J.; Barke, M.R.; Martheswaran, T.; Thomson, A.C.; Thomson, R.J.; Somani, S.N.; Shmunes, K.M.; Ronquillo, Y.C.; Hoopes, P. The Historical Development and an Overview of Contemporary Keratoprostheses. Surv. Ophthalmol. 2022, 67, 1175–1199. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Desai, N.; Salave, S.; Karunakaran, B.; Giri, J.; Benival, D.; Gorantla, S.; Kommineni, N. Collagen-Based Hydrogels for the Eye: A Comprehensive Review. Gels 2023, 9, 643. [Google Scholar] [CrossRef] [PubMed]
- Xeroudaki, M.; Thangavelu, M.; Lennikov, A.; Ratnayake, A.; Bisevac, J.; Petrovski, G.; Fagerholm, P.; Rafat, M.; Lagali, N. A Porous Collagen-Based Hydrogel and Implantation Method for Corneal Stromal Regeneration and Sustained Local Drug Delivery. Sci. Rep. 2020, 10, 16936. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.M.; Lagali, N.; Merrett, K.; Edelhauser, H.; Sun, Y.; Gan, L.; Griffith, M.; Fagerholm, P. Biosynthetic Corneal Implants for Replacement of Pathologic Corneal Tissue: Performance in a Controlled Rabbit Alkali Burn Model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 651–657. [Google Scholar] [CrossRef]
- Islam, M.M.; Buznyk, O.; Reddy, J.C.; Pasyechnikova, N.; Alarcon, E.I.; Hayes, S.; Lewis, P.; Fagerholm, P.; He, C.; Iakymenko, S.; et al. Biomaterials-Enabled Cornea Regeneration in Patients at High Risk for Rejection of Donor Tissue Transplantation. npj Regen. Med. 2018, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Le, P.; Fernandes-Cunha, G.M.; Heilshorn, S.C.; Myung, D. Bio-Orthogonally Crosslinked Hyaluronate-Collagen Hydrogel for Suture-Free Corneal Defect Repair. Biomaterials 2020, 255, 120176. [Google Scholar] [CrossRef]
- Chen, F.; Mundy, D.C.; Le, P.; Seo, Y.A.; Logan, C.M.; Fernandes-Cunha, G.M.; Basco, C.A.; Myung, D. In Situ-Forming Collagen-Hyaluronate Semi-Interpenetrating Network Hydrogel Enhances Corneal Defect Repair. Transl. Vis. Sci. Technol. 2022, 11, 22. [Google Scholar] [CrossRef]
- Rafat, M.; Xeroudaki, M.; Koulikovska, M.; Sherrell, P.; Groth, F.; Fagerholm, P.; Lagali, N. Composite Core-and-Skirt Collagen Hydrogels with Differential Degradation for Corneal Therapeutic Applications. Biomaterials 2016, 83, 142–155. [Google Scholar] [CrossRef]
- Rosenquist, J.; Folkesson, M.; Höglund, L.; Pupkaite, J.; Hilborn, J.; Samanta, A. An Injectable, Shape-Retaining Collagen Hydrogel Cross-Linked Using Thiol-Maleimide Click Chemistry for Sealing Corneal Perforations. ACS Appl. Mater. Interfaces 2023, 15, 34407–34418. [Google Scholar] [CrossRef] [PubMed]
- Khosravimelal, S.; Mobaraki, M.; Eftekhari, S.; Ahearne, M.; Seifalian, A.M.; Gholipourmalekabadi, M. Hydrogels as Emerging Materials for Cornea Wound Healing. Small 2021, 17, 2006335. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-S.; Kim, B.K.; Truong, M.-D.; Yang, H.S.; Park, S.-H.; Park, H.S.; Choi, B.H.; Won, B.H.; Min, B.-H. Corneal Repair with Adhesive Cell Sheets of Fetal Cartilage-Derived Stem Cells. Tissue Eng. Regen. Med. 2021, 18, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, J.H.; Hong, S.; Sunwoo, J.H.; Kim, H.T.; Kim, E.; Kim, J.Y.; Hwang, C.; Tchah, H. Transplantation of Human Corneal Limbal Epithelial Cell Sheet Harvested on Synthesized Carboxymethyl Cellulose and Dopamine in a Limbal Stem Cell Deficiency. J. Tissue Eng. Regen. Med. 2021, 15, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Gramm, S.; Götze, T.; Valtink, M.; Drichel, J.; Voit, B.; Engelmann, K.; Werner, C. Thermo-Responsive Poly(NiPAAm-Co-DEGMA) Substrates for Gentle Harvest of Human Corneal Endothelial Cell Sheets. J. Biomed. Mater. Res. 2007, 80A, 1003–1010. [Google Scholar] [CrossRef]
- Khalili, M.; Zarebkohan, A.; Dianat-Moghadam, H.; Panahi, M.; Andre, H.; Alizadeh, E. Corneal Endothelial Cell Sheet Bioengineering from Neural Crest Cell-Derived Adipose Stem Cells on Novel Thermo-Responsive Elastin-Mimetic Dendrimers Decorated with RGD. Chem. Eng. J. 2022, 429, 132523. [Google Scholar] [CrossRef]
- Koizumi, N. Ocular Surface Reconstruction, Amniotic Membrane, and Cultivated Epithelial Cells from the Limbus. Br. J. Ophthalmol. 2003, 87, 1437–1439. [Google Scholar] [CrossRef]
- Gomes, J.Á.P.; Geraldes Monteiro, B.; Melo, G.B.; Smith, R.L.; Cavenaghi Pereira Da Silva, M.; Lizier, N.F.; Kerkis, A.; Cerruti, H.; Kerkis, I. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Human Immature Dental Pulp Stem Cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1408–1414. [Google Scholar] [CrossRef]
- Bandeira, F.; Goh, T.-W.; Setiawan, M.; Yam, G.H.-F.; Mehta, J.S. Cellular Therapy of Corneal Epithelial Defect by Adipose Mesenchymal Stem Cell-Derived Epithelial Progenitors. Stem Cell Res. Ther. 2020, 11, 14. [Google Scholar] [CrossRef]
- Davis, A.B.; Schnabel, L.V.; Gilger, B.C. Subconjunctival Bone Marrow-Derived Mesenchymal Stem Cell Therapy as a Novel Treatment Alternative for Equine Immune-Mediated Keratitis: A Case Series. Vet. Ophthalmol. 2019, 22, 674–682. [Google Scholar] [CrossRef]
- Nieto-Nicolau, N.; Martínez-Conesa, E.M.; Velasco-García, A.M.; Aloy-Reverté, C.; Vilarrodona, A.; Casaroli-Marano, R.P. Xenofree Generation of Limbal Stem Cells for Ocular Surface Advanced Cell Therapy. Stem Cell Res Ther. 2019, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Griffith, M.; Alarcon, E.I.; Brunette, I. Regenerative Approaches for the Cornea. J. Intern. Med. 2016, 280, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.H.; Choi, H.J.; Kim, M.K. Corneal Xenotransplantation: Where Are We Standing? Prog. Retin. Eye Res. 2021, 80, 100876. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An Overview of Tissue and Whole Organ Decellularization Processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Isidan, A.; Liu, S.; Li, P.; Lashmet, M.; Smith, L.J.; Hara, H.; Cooper, D.K.C.; Ekser, B. Decellularization Methods for Developing Porcine Corneal Xenografts and Future Perspectives. Xenotransplantation 2019, 26, e12564. [Google Scholar] [CrossRef] [PubMed]
- Polisetti, N.; Roschinski, B.; Schlötzer-Schrehardt, U.; Maier, P.; Schlunck, G.; Reinhard, T. A Decellularized Human Limbal Scaffold for Limbal Stem Cell Niche Reconstruction. Int. J. Mol. Sci. 2021, 22, 10067. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Funamoto, S.; Hashimoto, Y.; Kimura, T.; Honda, T.; Hattori, S.; Kobayashi, H.; Kishida, A.; Mochizuki, M. In Vivo Evaluation of a Novel Scaffold for Artificial Corneas Prepared by Using Ultrahigh Hydrostatic Pressure to Decellularize Porcine Corneas. Mol. Vis. 2009, 15, 2022–2028. [Google Scholar] [PubMed]
- Yoeruek, E.; Bayyoud, T.; Maurus, C.; Hofmann, J.; Spitzer, M.S.; Bartz-Schmidt, K.; Szurman, P. Decellularization of Porcine Corneas and Repopulation with Human Corneal Cells for Tissue-Engineered Xenografts. Acta Ophthalmol. 2012, 90, e125–e131. [Google Scholar] [CrossRef] [PubMed]
- Isidan, A.; Liu, S.; Chen, A.M.; Zhang, W.; Li, P.; Smith, L.J.; Hara, H.; Cooper, D.K.C.; Ekser, B. Comparison of Porcine Corneal Decellularization Methods and Importance of Preserving Corneal Limbus through Decellularization. PLoS ONE 2021, 16, e0243682. [Google Scholar] [CrossRef]
- Fu, Y.; Fan, X.; Chen, P.; Shao, C.; Lu, W. Reconstruction of a Tissue-Engineered Cornea with Porcine Corneal Acellular Matrix as the Scaffold. Cells Tissues Organs 2010, 191, 193–202. [Google Scholar] [CrossRef]
- Tang, Z.; Akiyama, Y.; Okano, T. Recent Development of Temperature-Responsive Cell Culture Surface Using Poly(N-isopropylacrylamide). J. Polym. Sci. B Polym. Phys. 2014, 52, 917–926. [Google Scholar] [CrossRef]
- Wu, J.; Du, Y.; Mann, M.M.; Yang, E.; Funderburgh, J.L.; Wagner, W.R. Bioengineering Organized, Multilamellar Human Corneal Stromal Tissue by Growth Factor Supplementation on Highly Aligned Synthetic Substrates. Tissue Eng. Part A 2013, 19, 2063–2075. [Google Scholar] [CrossRef] [PubMed]
- Momenzadeh, D.; Baradaran-Rafii, A.; Keshel, S.H.; Ebrahimi, M.; Biazar, E. Electrospun Mat with Eyelid Fat-Derived Stem Cells as a Scaffold for Ocular Epithelial Regeneration. Artif. Cells Nanomed. Biotechnol. 2017, 45, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Darshan, G.H.; Kong, D.; Gautrot, J.; Vootla, S. Physico-Chemical Characterization of Antheraea Mylitta Silk Mats for Wound Healing Applications. Sci. Rep. 2017, 7, 10344. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Ghezzi, C.E.; Kaplan, D.L. Optimization of Silk Films as Substrate for Functional Corneal Epithelium Growth. J. Biomed. Mater. Res. 2016, 104, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Sim, B.R.; Khang, G. Nature-Derived Aloe Vera Gel Blended Silk Fibroin Film Scaffolds for Cornea Endothelial Cell Regeneration and Transplantation. ACS Appl. Mater. Interfaces 2016, 8, 15160–15168. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Sim, B.R.; Kim, J.I.; Khang, G. Functionalized Silk Fibroin Film Scaffold Using β-Carotene for Cornea Endothelial Cell Regeneration. Colloids Surf. B Biointerfaces 2018, 164, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jeon, H.; Song, J.; Oliveira, J.; Reis, R.; Khang, G. Biofunctionalized Lysophosphatidic Acid/Silk Fibroin Film for Cornea Endothelial Cell Regeneration. Nanomaterials 2018, 8, 290. [Google Scholar] [CrossRef]
- Guan, L.; Ge, H.; Tang, X.; Su, S.; Tian, P.; Xiao, N.; Zhang, H.; Zhang, L.; Liu, P. Use of a Silk Fibroin-Chitosan Scaffold to Construct a Tissue-Engineered Corneal Stroma. Cells Tissues Organs 2013, 198, 190–197. [Google Scholar] [CrossRef]
- Navaratnam, J.; Utheim, T.; Rajasekhar, V.; Shahdadfar, A. Substrates for Expansion of Corneal Endothelial Cells towards Bioengineering of Human Corneal Endothelium. J. Funct. Biomater. 2015, 6, 917–945. [Google Scholar] [CrossRef]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D Bioprinting of a Corneal Stroma Equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef]
- Yilmaz, B.; Tahmasebifar, A.; Baran, E.T. Bioprinting Technologies in Tissue Engineering. In Current Applications of Pharmaceutical Biotechnology; Silva, A.C., Moreira, J.N., Lobo, J.M.S., Almeida, H., Eds.; Advances in Biochemical Engineering/Biotechnology; pringer International Publishing: Cham, Switzerland, 2019; Volume 171, pp. 279–319. ISBN 978-3-030-40463-5. [Google Scholar]
- Jia, S.; Bu, Y.; Lau, D.-S.A.; Lin, Z.; Sun, T.; Lu, W.W.; Lu, S.; Ruan, C.; Chan, C.-H.J. Advances in 3D Bioprinting Technology for Functional Corneal Reconstruction and Regeneration. Front. Bioeng. Biotechnol. 2023, 10, 1065460. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, B.; Liu, Y.; Liu, F.; Lee, I.-S. Functional Engineering Strategies of 3D Printed Implants for Hard Tissue Replacement. Regen. Biomater. 2023, 10, rbac094. [Google Scholar] [CrossRef]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.L.; Chen, X. 3D Bioprinted Scaffolds for Bone Tissue Engineering: State-Of-The-Art and Emerging Technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef]
- Tharakan, S.; Khondkar, S.; Ilyas, A. Bioprinting of Stem Cells in Multimaterial Scaffolds and Their Applications in Bone Tissue Engineering. Sensors 2021, 21, 7477. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Rizzo, R.; Surman, F.; Zenobi-Wong, M. Guiding Lights: Tissue Bioprinting Using Photoactivated Materials. Chem. Rev. 2020, 120, 10950–11027. [Google Scholar] [CrossRef] [PubMed]
- Jessop, Z.M.; Al-Sabah, A.; Gardiner, M.D.; Combellack, E.; Hawkins, K.; Whitaker, I.S. 3D Bioprinting for Reconstructive Surgery: Principles, Applications and Challenges. J. Plast. Reconstr. Aesthetic Surg. 2017, 70, 1155–1170. [Google Scholar] [CrossRef]
- Tanveer, W.; Ridwan-Pramana, A.; Molinero-Mourelle, P.; Forouzanfar, T. Systematic Review of Clinical Applications of CAD/CAM Technology for Craniofacial Implants Placement and Manufacturing of Orbital Prostheses. Int. J. Environ. Res. Public Health 2021, 18, 11349. [Google Scholar] [CrossRef]
- de Silva, L.; Bernal, P.N.; Rosenberg, A.; Malda, J.; Levato, R.; Gawlitta, D. Biofabricating the Vascular Tree in Engineered Bone Tissue. Acta Biomater. 2023, 156, 250–268. [Google Scholar] [CrossRef]
- Rengier, F.; Mehndiratta, A.; Von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.-U.; Giesel, F.L. 3D Printing Based on Imaging Data: Review of Medical Applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.T.; Wei, Y.; Wan, J.; Zhu, J.; Peng, Y.; Abdul Kadir, S.Y.; Zainol, J.; Oglah, Z.; Cheng, L.; Shi, Z. Bone Tissue Engineering through 3D Bioprinting of Bioceramic Scaffolds: A Review and Update. Life 2022, 12, 903. [Google Scholar] [CrossRef] [PubMed]
- Ostaș, D.; Almășan, O.; Ileșan, R.R.; Andrei, V.; Thieringer, F.M.; Hedeșiu, M.; Rotar, H. Point-of-Care Virtual Surgical Planning and 3D Printing in Oral and Cranio-Maxillofacial Surgery: A Narrative Review. J. Clin. Med. 2022, 11, 6625. [Google Scholar] [CrossRef] [PubMed]
- Fuest, M.; Yam, G.H.-F.; Mehta, J.S.; Duarte Campos, D.F. Prospects and Challenges of Translational Corneal Bioprinting. Bioengineering 2020, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Aghamirsalim, M.; Mobaraki, M.; Soltani, M.; Kiani Shahvandi, M.; Jabbarvand, M.; Afzali, E.; Raahemifar, K. 3D Printed Hydrogels for Ocular Wound Healing. Biomedicines 2022, 10, 1562. [Google Scholar] [CrossRef] [PubMed]
- Mancha Sánchez, E.; Gómez-Blanco, J.C.; López Nieto, E.; Casado, J.G.; Macías-García, A.; Díaz Díez, M.A.; Carrasco-Amador, J.P.; Torrejón Martín, D.; Sánchez-Margallo, F.M.; Pagador, J.B. Hydrogels for Bioprinting: A Systematic Review of Hydrogels Synthesis, Bioprinting Parameters, and Bioprinted Structures Behavior. Front. Bioeng. Biotechnol. 2020, 8, 776. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hua, S.; Sayyar, S.; Chen, Z.; Chung, J.; Liu, X.; Yue, Z.; Angus, C.; Filippi, B.; Beirne, S.; et al. Corneal Bioprinting Using a High Concentration Pure Collagen I Transparent Bioink. Bioprinting 2022, 28, e00235. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Rohde, M.; Ross, M.; Anvari, P.; Blaeser, A.; Vogt, M.; Panfil, C.; Yam, G.H.; Mehta, J.S.; Fischer, H.; et al. Corneal Bioprinting Utilizing Collagen-Based Bioinks and Primary Human Keratocytes. J. Biomed. Mater. Res. 2019, 107, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, Y.; Wang, C. Investigation of Collagen-Incorporated Sodium Alginate Bioprinting Hydrogel for Tissue Engineering. J. Compos. Sci. 2022, 6, 227. [Google Scholar] [CrossRef]
- Tarsitano, M.; Cristiano, M.C.; Fresta, M.; Paolino, D.; Rafaniello, C. Alginate-Based Composites for Corneal Regeneration: The Optimization of a Biomaterial to Overcome Its Limits. Gels 2022, 8, 431. [Google Scholar] [CrossRef]
- Gong, C.; Kong, Z.; Wang, X. The Effect of Agarose on 3D Bioprinting. Polymers 2021, 13, 4028. [Google Scholar] [CrossRef] [PubMed]
- Gingras, A.A.; Jansen, P.A.; Smith, C.; Zhang, X.; Niu, Y.; Zhao, Y.; Roberts, C.J.; Herderick, E.D.; Swindle-Reilly, K.E. 3D Bioprinting of Acellular Corneal Stromal Scaffolds with a Low Cost Modified 3D Printer: A Feasibility Study. Curr. Eye Res. 2023, 48, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Mörö, A.; Samanta, S.; Honkamäki, L.; Rangasami, V.K.; Puistola, P.; Kauppila, M.; Narkilahti, S.; Miettinen, S.; Oommen, O.; Skottman, H. Hyaluronic Acid Based next Generation Bioink for 3D Bioprinting of Human Stem Cell Derived Corneal Stromal Model with Innervation. Biofabrication 2023, 15, 015020. [Google Scholar] [CrossRef]
- Tayebi, T.; Baradaran-Rafii, A.; Hajifathali, A.; Rahimpour, A.; Zali, H.; Shaabani, A.; Niknejad, H. Biofabrication of Chitosan/Chitosan Nanoparticles/Polycaprolactone Transparent Membrane for Corneal Endothelial Tissue Engineering. Sci. Rep. 2021, 11, 7060. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tharayil, A.; Thomas, S. 3D Bioprinting of Nature-Inspired Hydrogel Inks Based on Synthetic Polymers. ACS Appl. Polym. Mater. 2021, 3, 3685–3701. [Google Scholar] [CrossRef]
- Balters, L.; Reichl, S. 3D Bioprinting of Corneal Models: A Review of the Current State and Future Outlook. J. Tissue Eng. 2023, 14, 20417314231197793. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.A.; Liu, W.; Jimenez, A.; Yang, J.; Akpek, A.; Liu, X.; Pi, Q.; Mu, X.; Hu, N.; Schiffelers, R.M.; et al. 3D Bioprinting: From Benches to Translational Applications. Small 2019, 15, 1805510. [Google Scholar] [CrossRef]
- Niu, D.; Ma, X.; Yuan, T.; Niu, Y.; Xu, Y.; Sun, Z.; Ping, Y.; Li, W.; Zhang, J.; Wang, T.; et al. Porcine Genome Engineering for Xenotransplantation. Adv. Drug Deliv. Rev. 2021, 168, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Arabi, T.Z.; Sabbah, B.N.; Lerman, A.; Zhu, X.-Y.; Lerman, L.O. Xenotransplantation: Current Challenges and Emerging Solutions. Cell Transpl. 2023, 32, 09636897221148771. [Google Scholar] [CrossRef]
- Thomas, A.; Hawthorne, W.J.; Burlak, C. Xenotransplantation Literature Update, November/December 2019. Xenotransplantation 2020, 27, e12582. [Google Scholar] [CrossRef]
- Jiang, F.; Doudna, J.A. CRISPR–Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Geng, Z.; Gonelle-Gispert, C.; Hawthrone, W.J.; Deng, S.; Buhler, L. International Human Xenotransplantation Inventory: A 10-Year Follow-Up. Transplantation 2022, 106, 1713–1716. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, A. Account of the Use of an Instrument for Cutting the Cornea, in the Operation of Extracting a Cataract. Med. Phys. J. 1799, 1, 332–333. [Google Scholar] [PubMed]
- Shimmura, S.; Inagaki, E.; Hirayama, M.; Hatou, S. The Cornea: An Ideal Tissue for Regenerative Medicine. Keio J. Med. 2024, 73, 1–7. [Google Scholar] [CrossRef] [PubMed]


| Corneal Stem Cells | Non Corneal Stem Cells | |
|---|---|---|
| Limbal epithelial | Pluripotent | Embryonic |
| Limbal mesenchymal | iPS | |
| Epithelial | Oral mucosa | |
| Hair follicle | ||
| Epidermis | ||
| Amniotic membrane | ||
| Mesenchymal | Bone marrow | |
| Adipose derived | ||
| Amniotic membrane | ||
| Placenta | ||
| Umbilical cord | ||
| Neural crest | Dental pulp | |
| Droplet-Based (DBB) | Extrusion-Based (EBB) | Vat Photopolymerization (VP) | ||||||
|---|---|---|---|---|---|---|---|---|
| Typology | Thermal | Piezoelectric | Micro-Valves | EBB | SLA | DLP | CLIP | Volumetric Printing |
| Print rate | Fast (1–104 droplets/s) [95,96,97] 105 droplets/s [98] 30 kHz [99] | Fast (1–104 droplets/s) [95,96,97] 105 droplets/s [98] 30 kHz [99] | kHz [99] | Slow [95,100] 10–700 mm/s [98] | Fast [95,96,101] | Fast [96], Very fast [99] | Very fast (25–100 mm/h) [99] | Extremely fast (seconds) [102] |
| % Cell survival | >85% [95,96,97,98] | NT [99] | >85% [95,96,97,98] | 80–90% [96,98] 40–80% [95,97] | >90% [96], >85% [95] | >SLA [96] | NT [99] | |
| Cellular density (cells/mL) | <106 [95,96,99] | NT [99] | <106 [95,96,99] | >108 [95,96,98] | <108 [95,96] | <108 [96] | NT [99] | |
| Range of viscosity | 3.5–12 mPa/s [95,96,97] <10 mPa/s [98] <15 mPa/s [99] | 3.5–12 mPa/s [95,96,97] <10 mPa/s [98] <15 mPa/s [99] | 3.5–12 mPa/s [95,97] <10 mPa/s [98] <200 mPa/s [99] | 30–6·107 mPa/s [95,96,97,98] | - [95,96] | 10–5000 mPa/s [99] | 10–5000 mPa/s [99] | <90,000 mPa/s [99] |
| Resolution | 75 µm [96] High [95,100] 10–50 µm [98] 50–500 µm [99] | 75 µm [96] High [95,100] 10–50 µm [98] 50–500 µm [99] | 75 µm [96] High [95,100] 10–50 µm [98] 50–500 µm [99] | 200–1000 µm [98] Good [95,97] Low [100] 100–600 µm [99] | 60–150 µm [96] Very high [100,101,103,104] >5 µm [102] | 25–50 µm [96] >5 µm [102] 25–150 µm [99] | 25–150 µm [99] | >100 µm [102] 25–150 µm [99] |
| Vertical resolution | Low [95] | Low [95] | Low [95] | High [95] | 50–150 µm [101] | 25–100 µm [99] | 10 µm [99] | |
| Mechanical properties | Low [99,104] Moderate [103] | Low [99,104] Moderate [103] | Low [99,104] Moderate [103] | Low [100] | Low [100,101] Moderate [103] | Very good [99] | Very good [99] | Very good [99] |
| Scaffold dimension | cm [99] | cm [99] | cm [99] | cm [99] | Wide range k | cm [99] | cm [99] | cm [99] |
| Cost | Low [95,97,98,104] 5000 $ [99] | >Thermal [99] Low [95,98] | 5000 $ [99] | 30 k–250 k $ [99] | Medium [101,103] 3.5 k–5 k $ [105] | 30 k–50 k $ [99] | 30 k–50 k $ [99] | 30 k–50 k $ [99] |
| Risks and drawbacks | Nozzle obstruction; cellular damage at 15–25 kHz [97]; limited pore size and dissolution if organic solvents are used [100]; thermal technology possibly oncogenic [99] | Mechanical or thermal stress at cellular level [98,100] | Require long post-printing treatments [104] | Risk of cellular damage due to UV rays or photo-initiators [99] | Risk of cellular damage due to UV rays or photo-initiators [99]; High density require a lot of software changes [102] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonato, P.; Bagno, A. Replace or Regenerate? Diverse Approaches to Biomaterials for Treating Corneal Lesions. Biomimetics 2024, 9, 202. https://doi.org/10.3390/biomimetics9040202
Bonato P, Bagno A. Replace or Regenerate? Diverse Approaches to Biomaterials for Treating Corneal Lesions. Biomimetics. 2024; 9(4):202. https://doi.org/10.3390/biomimetics9040202
Chicago/Turabian StyleBonato, Pietro, and Andrea Bagno. 2024. "Replace or Regenerate? Diverse Approaches to Biomaterials for Treating Corneal Lesions" Biomimetics 9, no. 4: 202. https://doi.org/10.3390/biomimetics9040202
APA StyleBonato, P., & Bagno, A. (2024). Replace or Regenerate? Diverse Approaches to Biomaterials for Treating Corneal Lesions. Biomimetics, 9(4), 202. https://doi.org/10.3390/biomimetics9040202







