Developing a Biomimetic 3D Neointimal Layer as a Prothrombotic Substrate for a Humanized In Vitro Model of Atherothrombosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
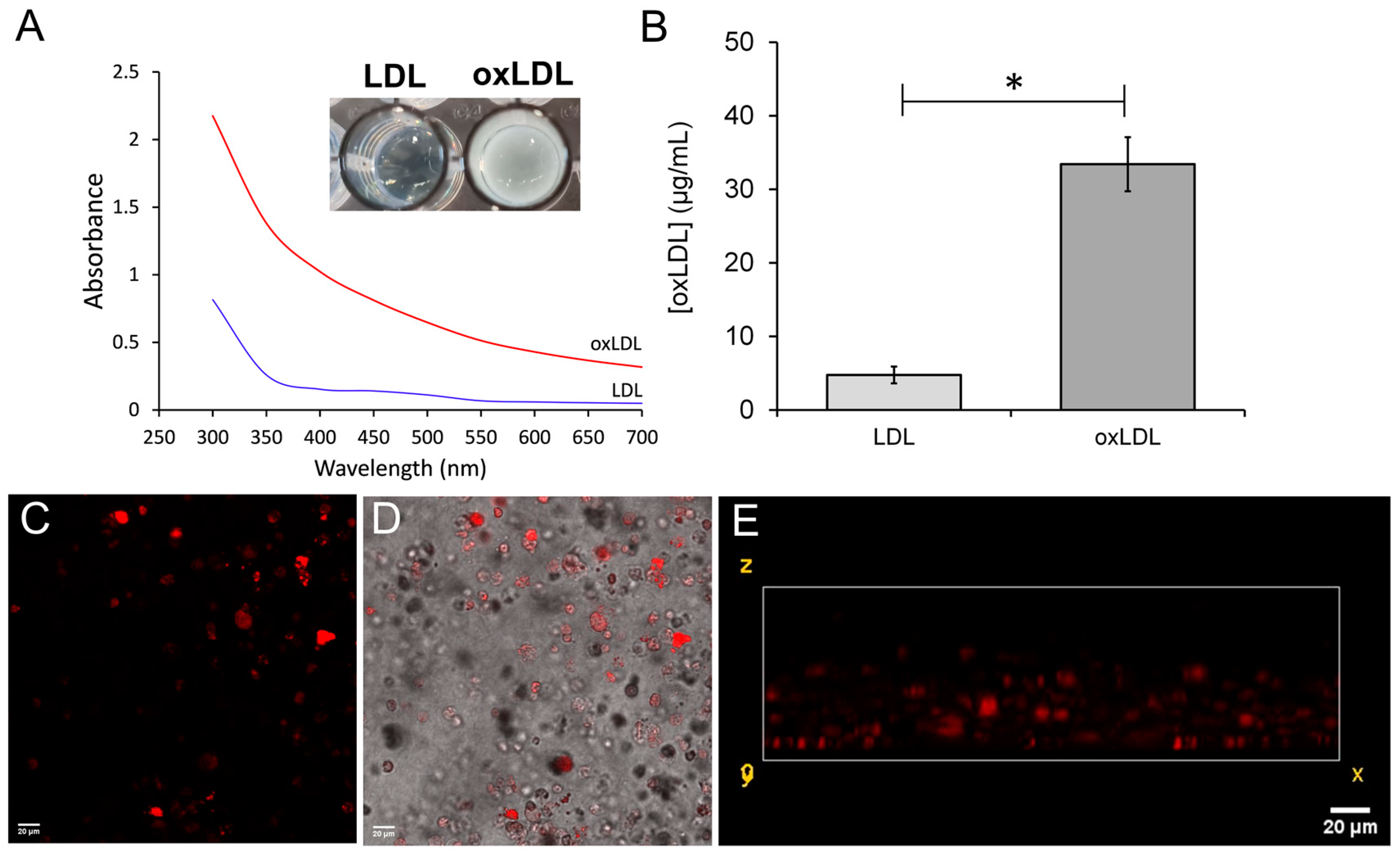
2.2. Preparation and Validation of Oxidized Low-Density Lipoproteins
2.3. Preparation of the 3D Human Neointimal Cell Culture Construct
2.3.1. To Trigger M0 Differentiation
2.3.2. To Trigger M1 Differentiation
2.3.3. To Generate THP-1-Derived Foam Cells
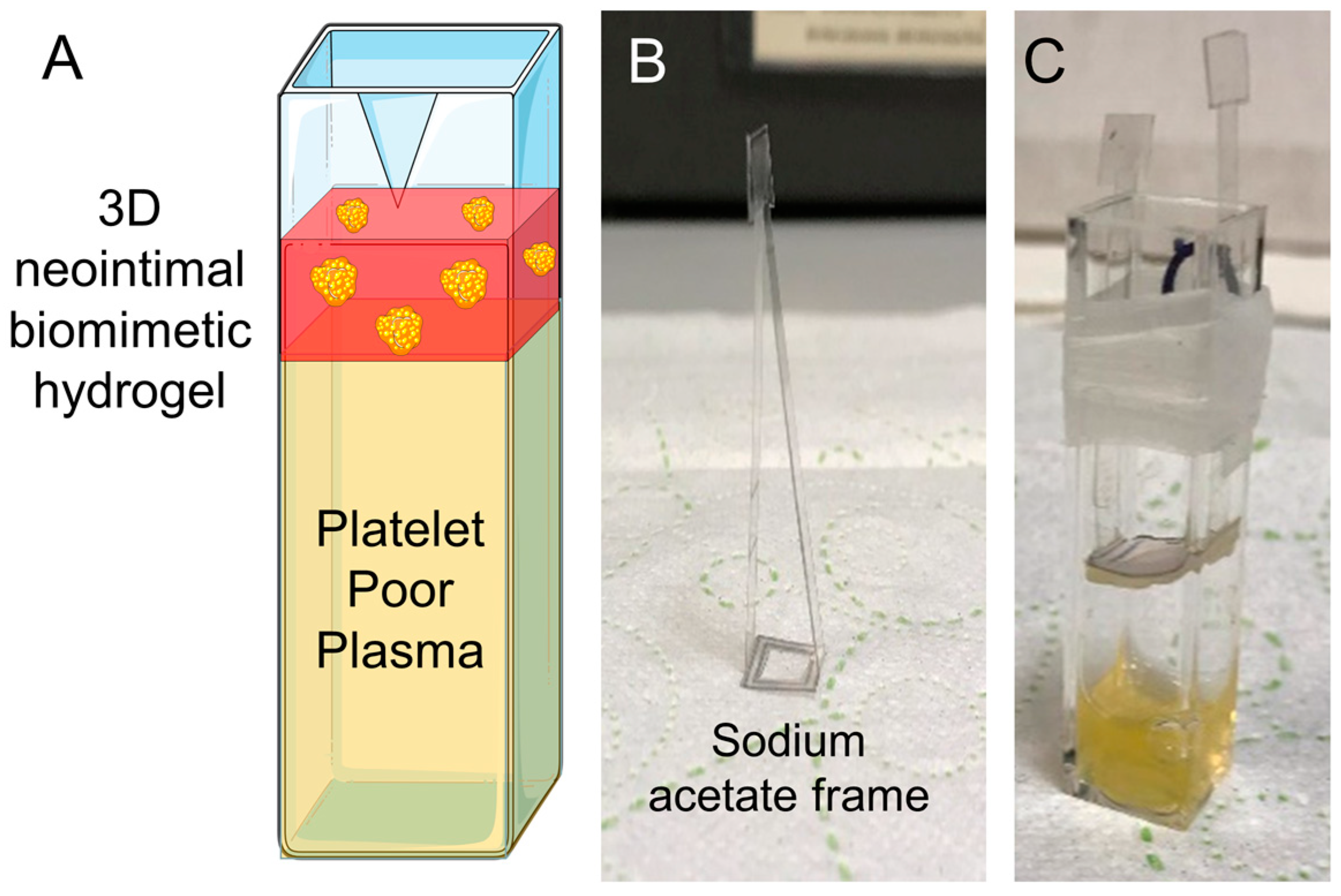
2.3.4. Plastic Compression of 3D Neointimal Biomimetic Hydrogels
2.4. Assessment of Differentiation of THP-1 Cells within 3D Collagen Hydrogels
2.4.1. Assessing Cell Viability of 3D Culture of THP-1-Derived Cells
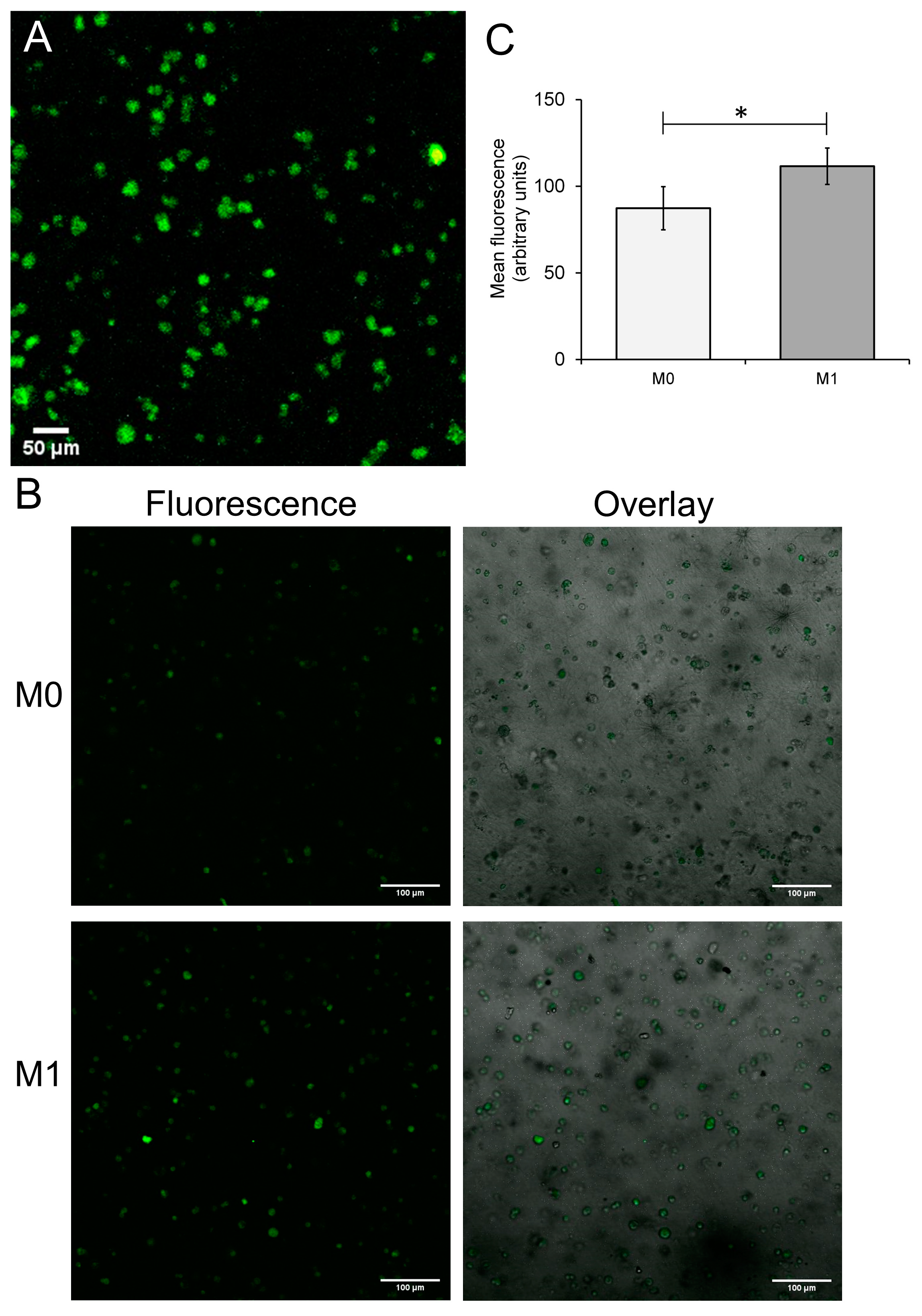
2.4.2. Immunofluorescent Staining of the M1-Containing Hydrogels
2.4.3. Fluorescent Imaging of THP-1 Derived Foam Cells in the 3D Neointimal Biomimetic Hydrogels
2.4.4. Measuring oxLDL Uptake into 3D Neointimal Cell Cultures
2.5. Assessment of the Hemostatic Effects of the 3D Neointimal Biomimetic Hydrogel
2.5.1. Blood Donations
2.5.2. Preparation of Human Platelet-Rich Plasma (PRP) and Platelet-Poor Plasma (PPP)
2.5.3. Preparation of Washed Human Platelet Suspensions
2.5.4. Prothrombin Time Measurement
2.5.5. Extrinsic Pathway Clotting Factor Assay
2.5.6. Light Transmission Aggregometry
2.5.7. ATP Secretion Assay
2.6. Statistical Analysis
3. Results
3.1. Development of a 3D Neointimal Biomimetic Hydrogel
3.2. Differentiation of THP-1-Derived Foam Cells in the 3D Collagen Hydrogel
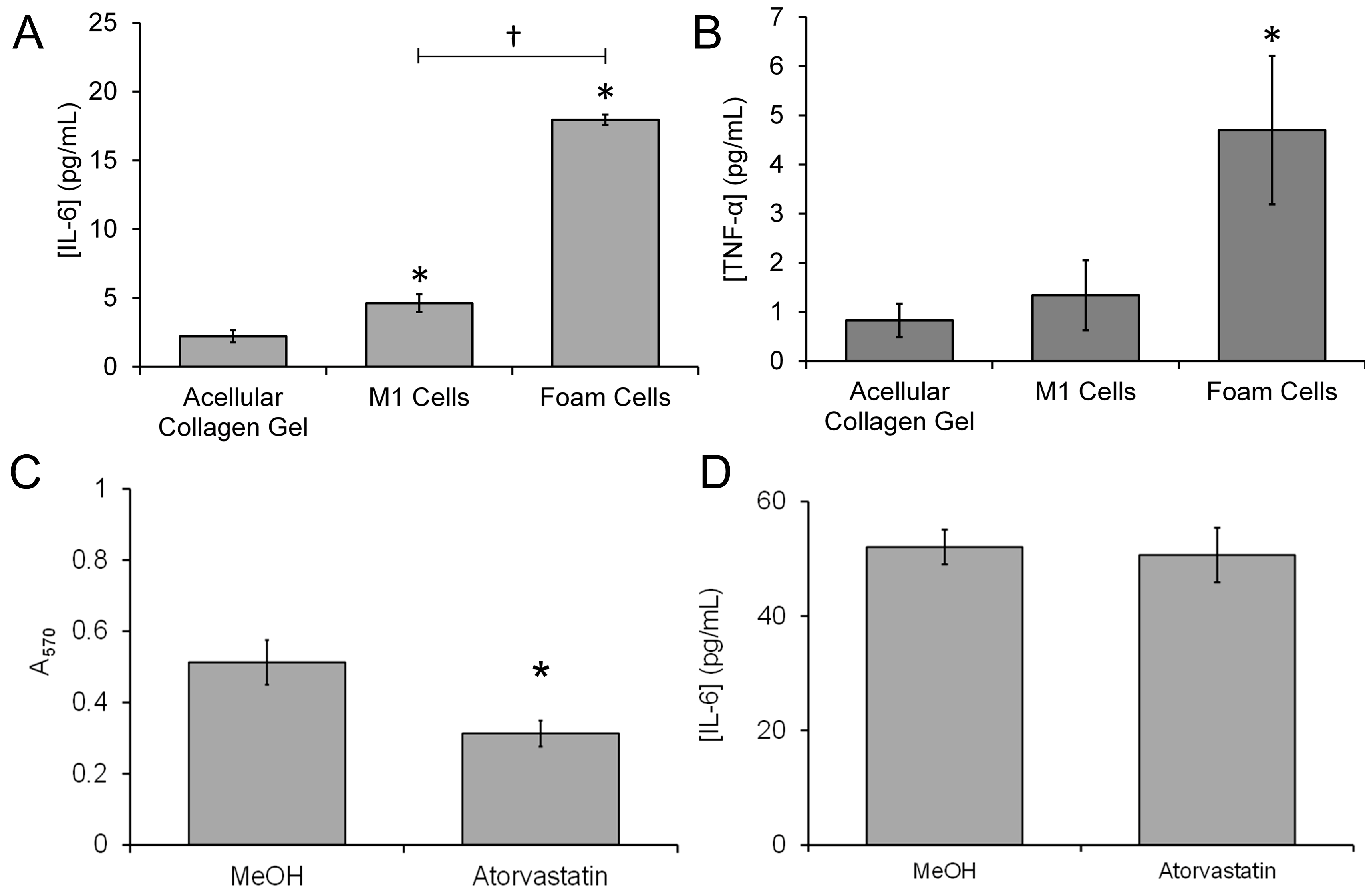
3.3. Treatment with Atorvastatin Reduces the Accumulation of Lipids into the 3D Neointimal Biomimetic Hydrogel
3.4. Treatment with Atorvastatin Reduces the Accumulation of Lipids but Not the Proinflammatory Properties of the 3D Neointimal Biomimetic Hydrogel
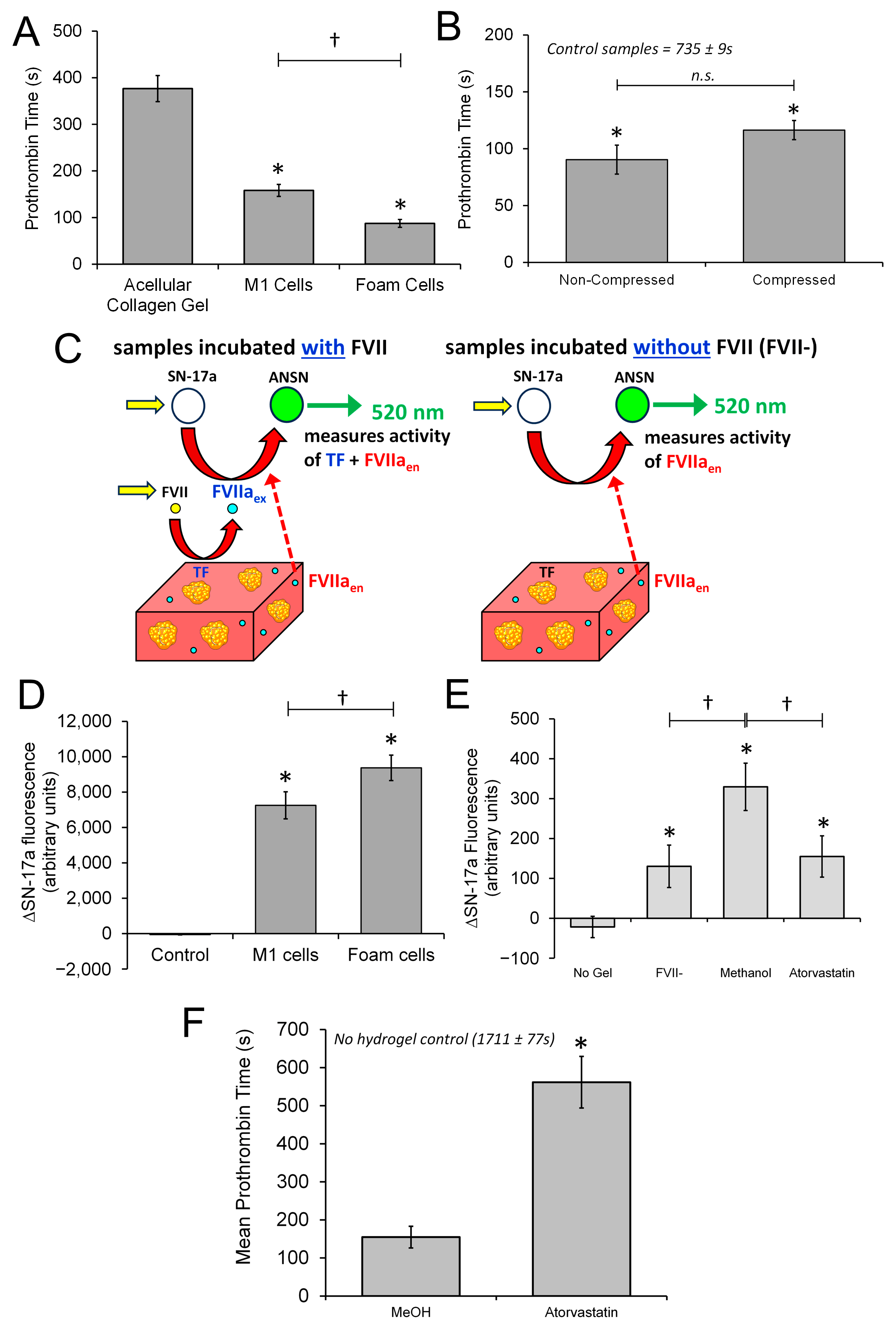
3.5. The 3D Neointimal Cell Culture Model Containing THP-1-Derived Foam Cells Is Able to Trigger Coagulation of Human Platelet Poor Plasma
3.6. The 3D Neointimal Culture Model Possesses Measurable Extrinsic Factor Activity That Is Enhanced by Incubation with oxLDL
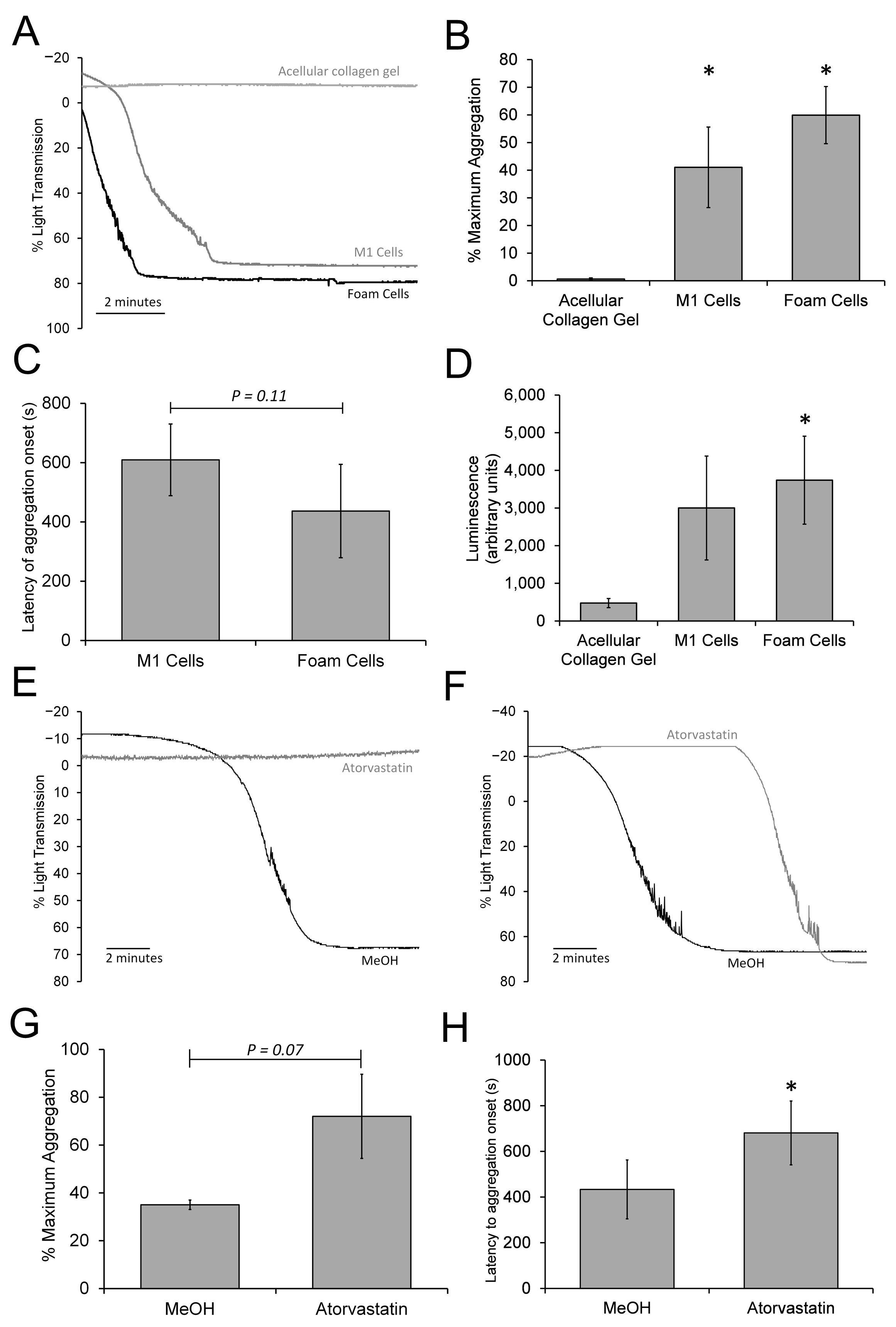
3.7. The 3D Neointimal Model Can Trigger a Slow Platelet Activation and Aggregation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C. Natural history and histological classification of atherosclerotic lesions: An update. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1177–1178. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Witztum, J.L. Atherosclerosis. the road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Emini Veseli, B.; Perrotta, P.; De Meyer, G.R.A.; Roth, L.; Van der Donckt, C.; Martinet, W.; De Meyer, G.R.Y. Animal models of atherosclerosis. Eur. J. Pharmacol. 2017, 816, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Tall, A.R.; Daemen, M.J.A.P.; Falk, E.; Fisher, E.A.; García-Cardeña, G.; Lusis, A.J.; Owens, A.P., 3rd; Rosenfeld, M.E.; Virmani, R. American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; and Council on Basic Cardiovascular Sciences. Recommendation on Design, Execution, and Reporting of Animal Atherosclerosis Studies: A Scientific Statement From the American Heart Association. Circ. Res. 2017, 121, e53–e79. [Google Scholar] [PubMed]
- Oppi, S.; Lüscher, T.F.; Stein, S. Mouse Models for Atherosclerosis Research-Which Is My Line? Front. Cardiovasc. Med. 2019, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Falk, E. Atherosclerotic lesions in mouse and man: Is it the same disease? Curr. Opin. Lipidol. 2010, 21, 434–440. [Google Scholar] [CrossRef]
- Van der Donckt, C.; Van Herck, J.L.; Schrijvers, D.M.; Vanhoutte, G.; Verhoye, M.; Blockx, I.; Van Der Linden, A.; Bauters, D.; Lijnen, H.R.; Sluimer, J.C.; et al. Elastin fragmentation in atherosclerotic mice leads to intraplaque neovascularization, plaque rupture, myocardial infarction, stroke, and sudden death. Eur. Heart J. 2015, 36, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Karel, M.; Hechler, B.; Kuijpers, M.; Cosemans, J. Atherosclerotic plaque injury-mediated murine thrombosis models: Advantages and limitations. Platelets 2020, 31, 439–446. [Google Scholar] [CrossRef]
- Hechler, B.; Gachet, C. Comparison of two murine models of thrombosis induced by atherosclerotic plaque injury. Thromb. Haemost. 2011, 105 (Suppl. S1), S3–S12. [Google Scholar] [CrossRef]
- van Zanten, G.H.; de Graaf, S.; Slootweg, P.J.; Heijnen, H.F.; Connolly, T.M.; de Groot, P.G.; Sixma, J.J. Increased platelet deposition on atherosclerotic coronary arteries. J. Clin. Investig. 1994, 93, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Penz, S.; Reininger, A.J.; Brandl, R.; Goyal, P.; Rabie, T.; Bernlochner, I.; Rother, E.; Goetz, C.; Engelmann, B.; Smethurst, P.A.; et al. Human atheromatous plaques stimulate thrombus formation by activating platelet glycoprotein VI. FASEB J. 2005, 19, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Karel, M.F.A.; Lemmens, T.P.; Tullemans, B.M.E.; Wielders, S.J.H.; Gubbins, E.; van Beurden, D.; van Rijt, S.; Cosemans, J.M.E.M. Characterization of Atherosclerotic Plaque Coating for Thrombosis Microfluidics Assays. Cell Mol. Bioeng. 2021, 15, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, S.; Kaji, T. Atherosclerosis and extracellular matrix. J. Atheroscler. Thromb. 2003, 10, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Weber, B.; Frese, L.; Emmert, M.Y.; Schmidt, D.; von Eckardstein, A.; Rohrer, L.; Hoerstrup, S.P. A three-dimensional engineered artery model for in vitro atherosclerosis research. PLoS ONE 2013, 8, e79821. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Karagiannis, A.; Sis, M.; Rask, E.; Kidambi, S.; Chatzizisis, Y. THree-dimensional co-cultures of human coronary artery endothelial and smooth muscle cells: A novel in-vitro experimental model of atherosclerosis. Atherosclerosis 2017, 263, E77–E78. [Google Scholar] [CrossRef]
- Gu, X.; Xie, S.; Hong, D.; Ding, Y. An in vitro model of foam cell formation induced by a stretchable microfluidic device. Sci. Rep. 2019, 9, 7461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bishawi, M.; Zhang, G.; Prasad, V.; Salmon, E.; Breithaupt, J.J.; Zhang, Q.; Truskey, G.A. Modeling early stage atherosclerosis in a primary human vascular microphysiological system. Nat. Commun. 2020, 11, 5426. [Google Scholar] [CrossRef]
- Mallone, A.; Gericke, C.; Hosseini, V.; Chahbi, K.; Haenseler, W.; Emmert, M.Y.; von Eckardstein, A.; Walther, J.H.; Vogel, V.; Weber, B.; et al. Human induced pluripotent stem cell-derived vessels as dynamic atherosclerosis model on a chip. bioRxiv 2020. [CrossRef]
- Stary, H.C.; Chandler, A.B.; Glagov, S.; Guyton, J.R.; Insull, W., Jr.; Rosenfeld, M.E.; Schaffer, S.A.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. 1994, 14, 840–856. [Google Scholar] [CrossRef]
- Mallone, A.; Stenger, C.; Von Eckardstein, A.; Hoerstrup, S.P.; Weber, B. Biofabricating atherosclerotic plaques: In vitro engineering of a three-dimensional human fibroatheroma model. Biomaterials 2018, 150, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Musa, F.I.; Harper, A.G.S.; Yang, Y. A real-time monitoring system to assess the platelet aggregatory capacity of components of a tissue-engineered blood vessel wall. Tissue Eng. Part. C Methods 2016, 22, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, W.; Hernández, A.C.H.; Musa, F.I.; Butler, R.; Harper, A.G.S.; Yang, Y. The Combination of Tissue-Engineered Blood Vessel Constructs and Parallel Flow Chamber Provides a Potential Alternative to In Vivo Drug Testing Models. Pharmaceutics 2021, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, J.; Njoroge, W.; Gibbins, J.M.; Roach, P.; Yang, Y.; Harper, A.G.S. Developing Biomimetic Hydrogels of the Arterial Wall as a Prothrombotic Substrate for In Vitro Human Thrombosis Models. Gels 2023, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Wraith, K.S.; Magwenzi, S.; Aburima, A.; Wen, Y.; Leake, D.; Naseem, K.M. Oxidized low-density lipoproteins induce rapid platelet activation and shape change through tyrosine kinase and Rho kinase-signaling pathways. Blood 2013, 122, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Wiseman, M.; Chuo, C.B.; Cheema, U.; Nazhat, S.N. Ultrarapid engineering of biomimetic materials and tissues: Fabrication of nano-and microstructures by plastic compression. Adv. Funct. Mat. 2005, 15, 1762–1770. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Silaeva, Y.Y.; Orekhov, A.N.; Deykin, A.V. Animal models of human atherosclerosis: Current progress. Braz. J. Med. Biol. Res. 2020, 53, e9557. [Google Scholar] [CrossRef] [PubMed]
- Abujua, P.M.; Albertini, R. Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin. Chim. Acta 2001, 306, 1–17. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef]
- Braziulis, E.; Diezi, M.; Biedermann, T.; Pontiggia, L.; Schmucki, M.; Hartmann-Fritsch, F.; Luginbühl, J.; Schiestl, C.; Meuli, M.; Reichmann, E. Modified plastic compression of collagen hydrogels provides an ideal matrix for clinically applicable skin substitutes. Tissue Eng. Part. C Methods 2012, 18, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Hu, Y.W.; Sha, Y.H.; Gao, J.J.; Ma, X.; Li, S.F.; Zhao, J.Y.; Qiu, Y.R.; Lu, J.B.; Huang, C.; et al. Ox-LDL Upregulates IL-6 Expression by Enhancing NF-κB in an IGF2-Dependent Manner in THP-1 Macrophages. Inflammation 2015, 38, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, C.; Feng, J.; Li, J.F.; Fan, Z.C. Curcumin affects ox-LDL-induced IL-6, TNF-α, MCP-1 secretion and cholesterol efflux in THP-1 cells by suppressing the TLR4/NF-κB/miR33a signaling pathway. Exp. Ther. Med. 2020, 20, 1856–1870. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, Y.; Zhou, Z.; He, M.; Dai, Y. OxLDL-induced IL-1 beta secretion promoting foam cells formation was mainly via CD36 mediated ROS production leading to NLRP3 inflammasome activation. Inflamm. Res. 2014, 63, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Hofnagel, O.; Luechtenborg, B.; Weissen-Plenz, G.; Robenek, H. Statins and foam cell formation: Impact on LDL oxidation and uptake of oxidized lipoproteins via scavenger receptors. Biochim. Biophys. Acta 2007, 1771, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Owens, A.P., 3rd; Mackman, N. Sources of tissue factor that contribute to thrombosis after rupture of an atherosclerotic plaque. Thromb. Res. 2012, 129 (Suppl. S2), S30–S33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilcox, J.N.; Smith, K.M.; Schwartz, S.M.; Gordon, D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc. Natl. Acad. Sci. USA 1989, 86, 2839–2843. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell Signal 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Owens, A.P., 3rd; Byrnes, J.R.; Mackman, N. Hyperlipidemia, tissue factor, coagulation, and simvastatin. Trends Cardiovasc. Med. 2014, 24, 95–98. [Google Scholar] [CrossRef]
- van der Meijden, P.E.; Munnix, I.C.; Auger, J.M.; Govers-Riemslag, J.W.; Cosemans, J.M.; Kuijpers, M.J.; Spronk, H.M.; Watson, S.P.; Renné, T.; Heemskerk, J.W. Dual role of collagen in factor XII-dependent thrombus formation. Blood 2009, 114, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M. Introduction to cell-hydrogel mechanosensing. Interface Focus. 2014, 4, 20130038. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Cavanagh, B.; Cameron, A.R.; Kelly, D.J.; O’Brien, F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019, 89, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Mastenbroek, T.G.; van Geffen, J.P.; Heemskerk, J.W.; Cosemans, J.M. Acute and persistent platelet and coagulant activities in atherothrombosis. J. Thromb. Haemost. 2015, 13 (Suppl. S1), S272–S280. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J.N.; Noguchi, S.; Casanova, J. Extrahepatic synthesis of factor VII in human atherosclerotic vessels. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 136–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hilgendorff, A.; Muth, H.; Parviz, B.; Staubitz, A.; Haberbosch, W.; Tillmanns, H.; Hölschermann, H. Statins differ in their ability to block NF-kappaB activation in human blood monocytes. Int. J. Clin. Pharmacol. Ther. 2003, 41, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Huilcaman, R.; Venturini, W.; Fuenzalida, L.; Cayo, A.; Segovia, R.; Valenzuela, C.; Brown, N.; Moore-Carrasco, R. Platelets, a Key Cell in Inflammation and Atherosclerosis Progression. Cells 2022, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Tekten, T.; Ceyhan, C.; Ercan, E.; Onbasili, A.O.; Turkoglu, C. The effect of atorvastatin on platelet function in patients with coronary artery disease. Acta Cardiol. 2004, 59, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Sanguigni, V.; Pignatelli, P.; Lenti, L.; Ferro, D.; Bellia, A.; Carnevale, R.; Tesauro, M.; Sorge, R.; Lauro, R.; Violi, F. Short-term treatment with atorvastatin reduces platelet CD40 ligand and thrombin generation in hypercholesterolemic patients. Circulation 2005, 111, 412–419. [Google Scholar] [CrossRef]
- Garcia-Sabaté, A.; Mohamed, W.K.E.; Sapudom, J.; Alatoom, A.; Al Safadi, L.; Teo, J.C.M. Biomimetic 3D Models for Investigating the Role of Monocytes and Macrophages in Atherosclerosis. Bioengineering 2020, 7, 113. [Google Scholar] [CrossRef]
- Smith, E.B. The influence of age and atherosclerosis on the chemistry of aortic intima. J. Atheroscler. Res. 1965, 5, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W., Jr.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef]
- Rekhter, M.D. Collagen synthesis in atherosclerosis: Too much and not enough. Cardiovasc. Res. 1999, 41, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Zhang, Y.; Sun, S.; Li, Q.; Chen, K.; An, C.; Wang, L.; van den Beucken, J.J.J.P.; Wang, H. Control of Matrix Stiffness Using Methacrylate-Gelatin Hydrogels for a Macrophage-Mediated Inflammatory Response. ACS Biomater. Sci. Eng. 2020, 6, 3091–3102. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Shami, A.; Gonçalves, I. Extracellular matrix: Paving the way to the newest trends in atherosclerosis. Curr. Opin. Lipidol. 2021, 32, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Annex, B.H.; Denning, S.M.; Channon, K.M.; Sketch, M.H., Jr.; Stack, R.S.; Morrissey, J.H.; Peters, K.G. Differential expression of tissue factor protein in directional atherectomy specimens from patients with stable and unstable coronary syndromes. Circulation 1995, 91, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Marmur, J.D.; Thiruvikraman, S.V.; Fyfe, B.S.; Guha, A.; Sharma, S.K.; Ambrose, J.A.; Fallon, J.T.; Nemerson, Y.; Taubman, M.B. Identification of active tissue factor in human coronary atheroma. Circulation 1996, 94, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Ardissino, D.; Merlini, P.A.; Ariëns, R.; Coppola, R.; Bramucci, E.; Mannucci, P.M. Tissue-factor antigen and activity in human coronary atherosclerotic plaques. Lancet 1997, 349, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, K.; Asada, Y.; Marutsuka, K.; Sato, Y.; Kamikubo, Y.; Sumiyoshi, A. Localization and activity of tissue factor in human aortic atherosclerotic lesions. Atherosclerosis 1997, 133, 213–219. [Google Scholar] [CrossRef]
- Kaikita, K.; Ogawa, H.; Yasue, H.; Takeya, M.; Takahashi, K.; Saito, T.; Hayasaki, K.; Horiuchi, K.; Takizawa, A.; Kamikubo, Y.; et al. Tissue factor expression on macrophages in coronary plaques in patients with unstable angina. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2232–2237. [Google Scholar] [CrossRef]
- Landers, S.C.; Gupta, M.; Lewis, J.C. Ultrastructural localization of tissue factor on monocyte-derived macrophages and macrophage foam cells associated with atherosclerotic lesions. Virchows Arch. 1994, 425, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Colli, S.; Eligini, S.; Lalli, M.; Camera, M.; Paoletti, R.; Tremoli, E. Vastatins inhibit tissue factor in cultured human macrophages. A novel mechanism of protection against atherothrombosis. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Lesnik, P.; Rouis, M.; Skarlatos, S.; Kruth, H.S.; Chapman, M.J. Uptake of exogenous free cholesterol induces upregulation of tissue factor expression in human monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA 1992, 89, 10370–10374. [Google Scholar] [CrossRef] [PubMed]
- Meisel, S.R.; Xu, X.-P.; Edgington, T.S.; Cercek, B.; Ong, J.; Kaul, S.; Shah, P.K. Dose-dependent modulation of tissue factor protein and procoagulant activity in human monocyte-derived macrophages by oxidized low density lipoprotein. J. Atheroscler. Thromb. 2011, 18, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N.; Brand, K.; Edgington, T.S. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THP-1 monocytic cells requires both activator protein 1 and nuclear factor kappa B binding sites. J. Exp. Med. 1991, 174, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; De Biase, L.; Lenti, L.; Tocci, G.; Brunelli, A.; Cangemi, R.; Riondino, S.; Grego, S.; Volpe, M.; Violi, F. Tumor necrosis factor-alpha as trigger of platelet activation in patients with heart failure. Blood 2005, 106, 1992–1994. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, M.; Cullen, P.; Lorkowski, J.; Stolle, K.; Robenek, H.; Troyer, D.; Rauterberg, J.; Lorkowski, S. Production of type VI collagen by human macrophages: A new dimension in macrophage functional heterogeneity. J. Immunol. 2008, 180, 5707–5719. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.M.; McIntire, L.V.; Moake, J.L.; Rand, J.H. Platelet adhesion and aggregation on human type VI collagen surfaces under physiological flow conditions. Blood 1995, 85, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Davì, G.; Patrono, C. Platelet activation and atherothrombosis. N. Engl. J. Med. 2007, 357, 2482–2494. [Google Scholar] [CrossRef]
- Gallone, G.; Baldetti, L.; Pagnesi, M.; Latib, A.; Colombo, A.; Libby, P.; Giannini, F. Medical Therapy for Long-Term Prevention of Atherothrombosis Following an Acute Coronary Syndrome: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2886–2903. [Google Scholar] [CrossRef]
- Argmann, C.A.; Edwards, J.Y.; Sawyez, C.G.; O’Neil, C.H.; Hegele, R.A.; Pickering, J.G.; Huff, M.W. Regulation of macrophage cholesterol efflux through hydroxymethylglutaryl-CoA reductase inhibition: A role for RhoA in ABCA1-mediated cholesterol efflux. J. Biol. Chem. 2005, 280, 22212–22221. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Du, Y.; Ye, Q.; Zha, K.; Feng, J. Atorvastatin Enhances Foam Cell Lipophagy and Promotes Cholesterol Efflux Through the AMP-Activated Protein Kinase/Mammalian Target of Rapamycin Pathway. J. Cardiovasc. Pharmacol. 2021, 77, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S.; Quintin, J.; Joosten, L.A.; van der Meer, J.W.; Netea, M.G.; Riksen, N.P. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echrish, J.; Pasca, M.-I.; Cabrera, D.; Yang, Y.; Harper, A.G.S. Developing a Biomimetic 3D Neointimal Layer as a Prothrombotic Substrate for a Humanized In Vitro Model of Atherothrombosis. Biomimetics 2024, 9, 372. https://doi.org/10.3390/biomimetics9060372
Echrish J, Pasca M-I, Cabrera D, Yang Y, Harper AGS. Developing a Biomimetic 3D Neointimal Layer as a Prothrombotic Substrate for a Humanized In Vitro Model of Atherothrombosis. Biomimetics. 2024; 9(6):372. https://doi.org/10.3390/biomimetics9060372
Chicago/Turabian StyleEchrish, Jassim, Madalina-Ioana Pasca, David Cabrera, Ying Yang, and Alan G. S. Harper. 2024. "Developing a Biomimetic 3D Neointimal Layer as a Prothrombotic Substrate for a Humanized In Vitro Model of Atherothrombosis" Biomimetics 9, no. 6: 372. https://doi.org/10.3390/biomimetics9060372
APA StyleEchrish, J., Pasca, M.-I., Cabrera, D., Yang, Y., & Harper, A. G. S. (2024). Developing a Biomimetic 3D Neointimal Layer as a Prothrombotic Substrate for a Humanized In Vitro Model of Atherothrombosis. Biomimetics, 9(6), 372. https://doi.org/10.3390/biomimetics9060372






