Region-Specific Decellularization of Porcine Uterine Tube Extracellular Matrix: A New Approach for Reproductive Tissue-Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
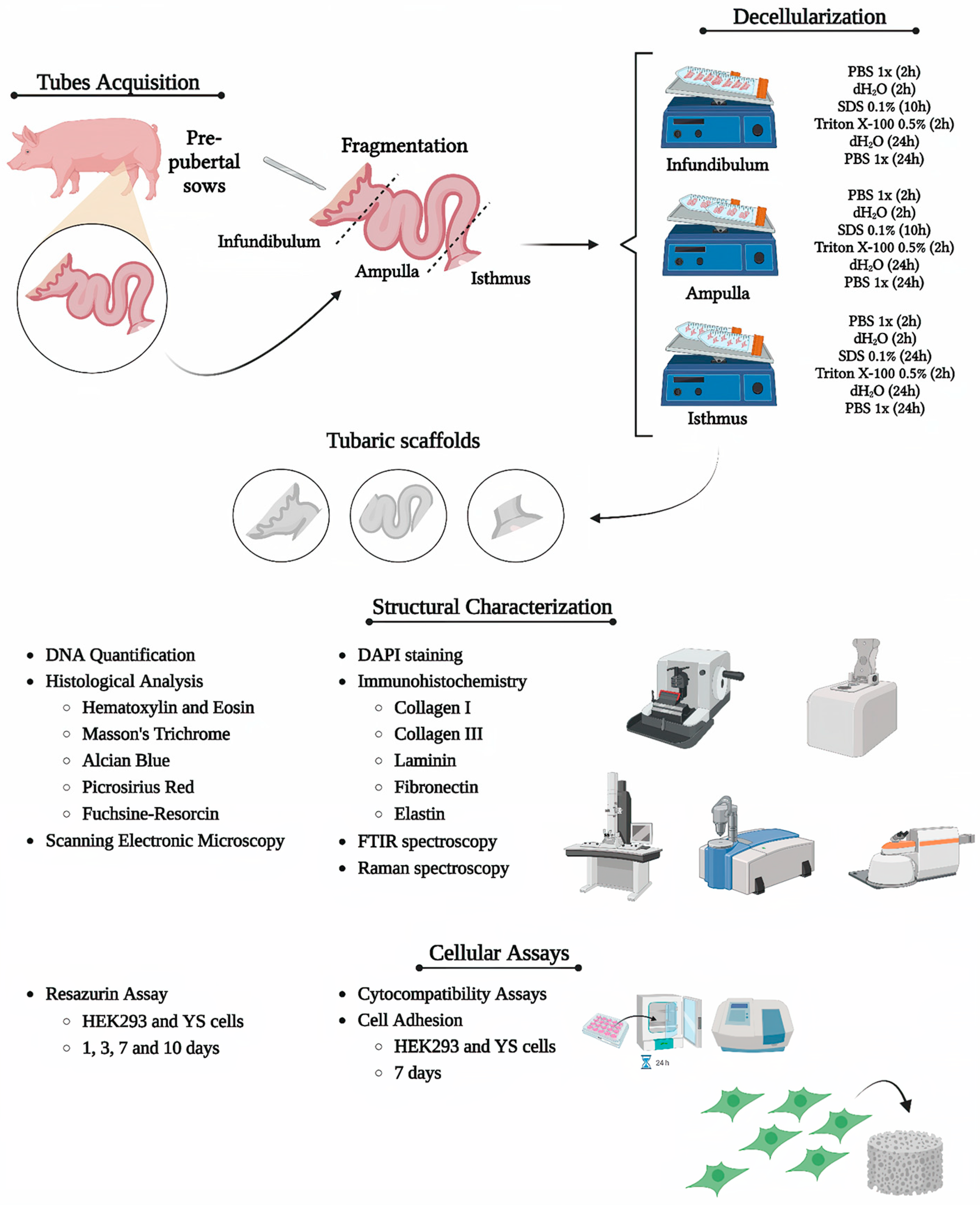
2.1. Sample Acquisition
2.2. Uterine Tube Segment Decellularization
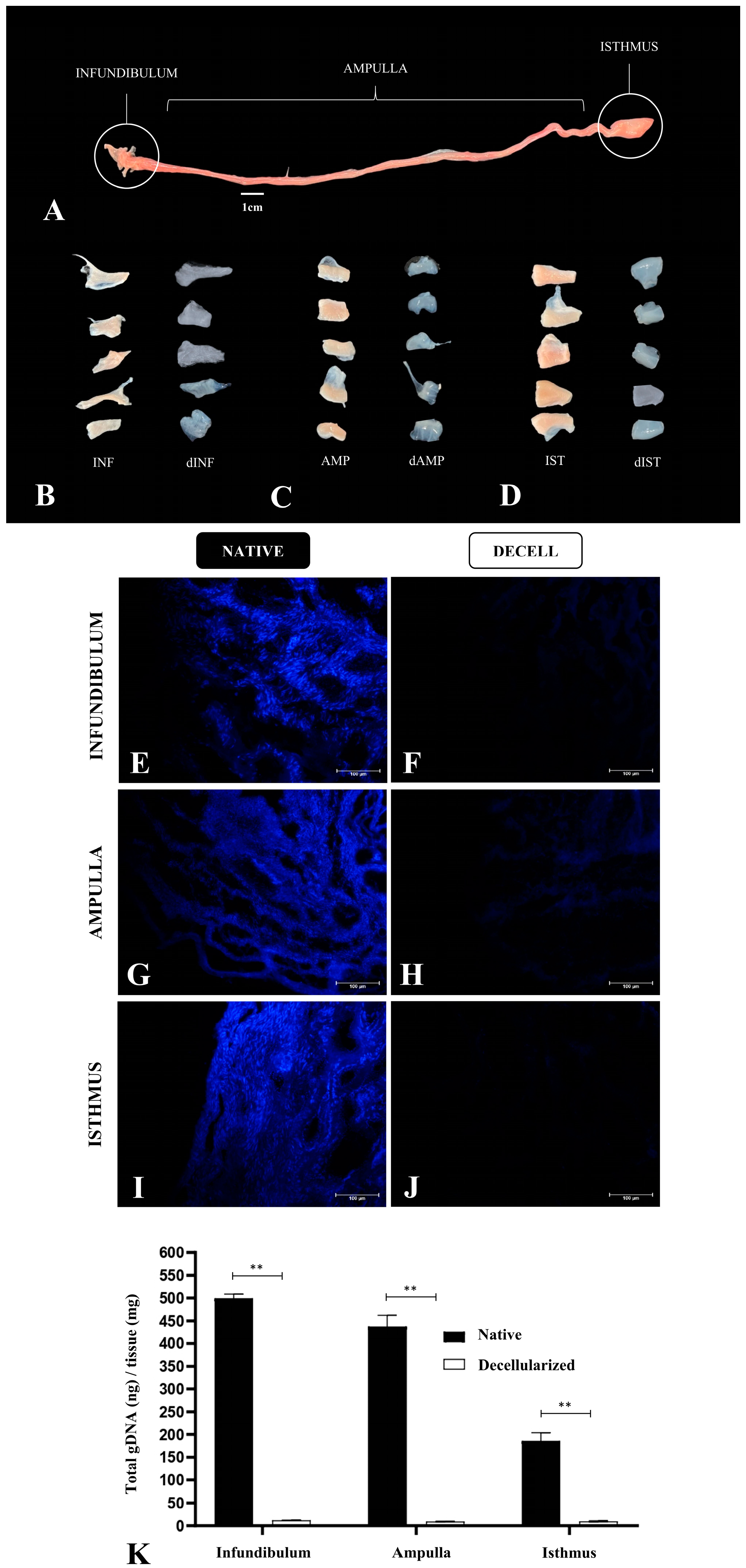
2.3. 4,6-Diamidino-2-Fenilindole (DAPI) Staining
2.4. Genomic DNA Quantification
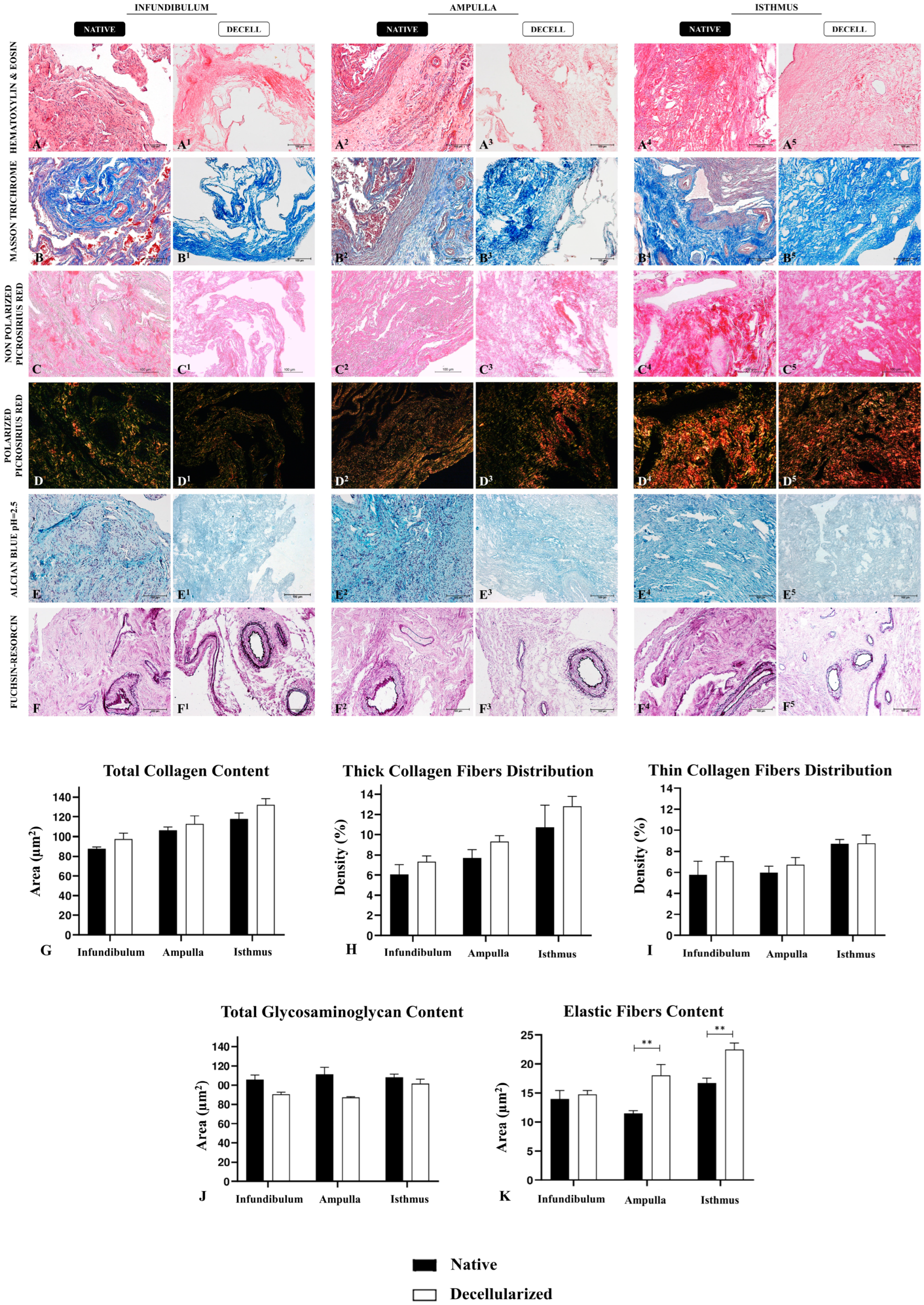
2.5. Histological Analysis
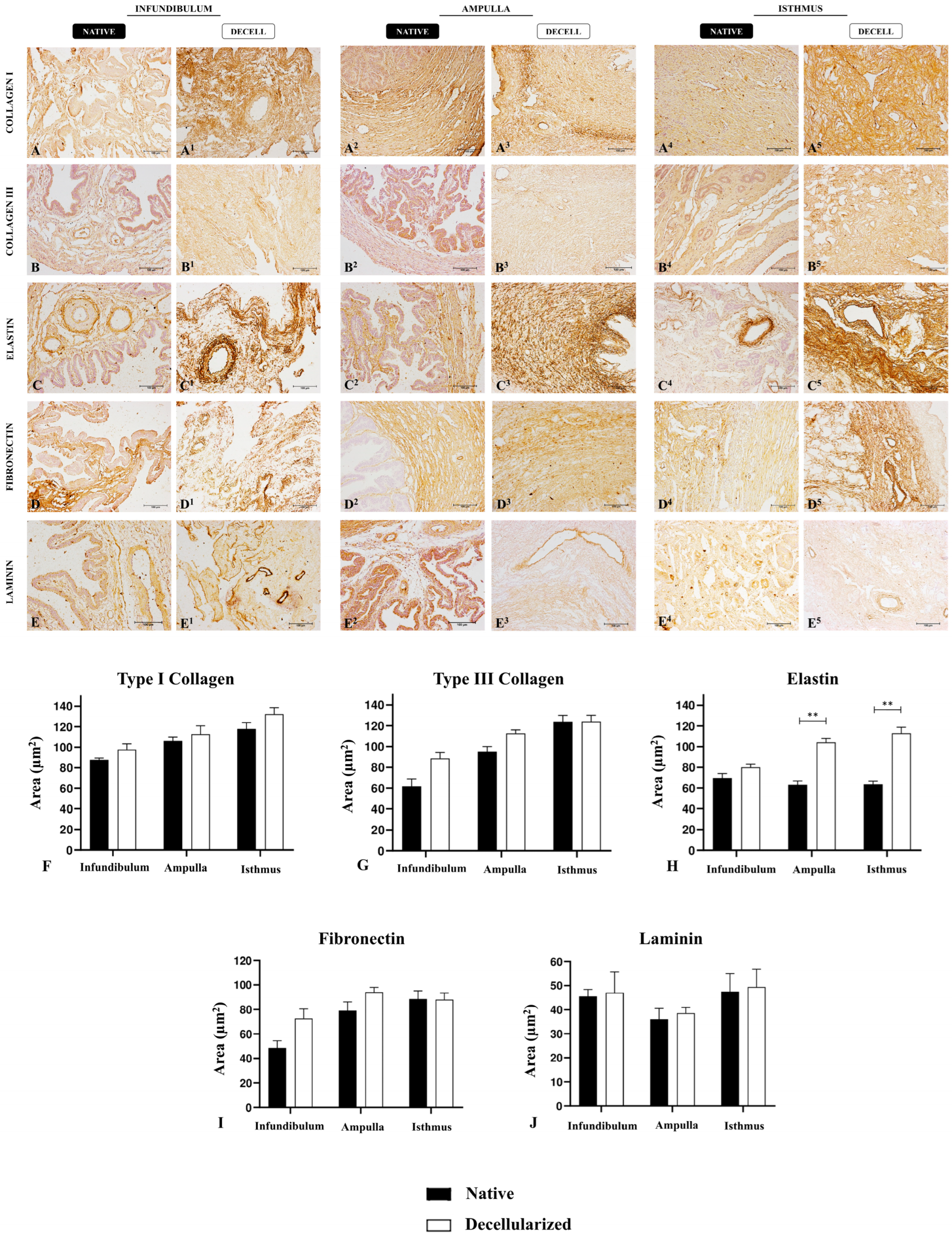
2.6. Immunohistochemistry Analysis
2.7. Scanning Electronic Microscopy (SEM)
2.8. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.9. Raman Spectroscopy Analysis
2.10. Scaffold Sterilization
2.11. Cytotoxicity Assay
2.12. Cytocompatibility Assay
2.13. Statistical Analysis
3. Results
3.1. Tubal Segment Decellularization: Protocol Standardization
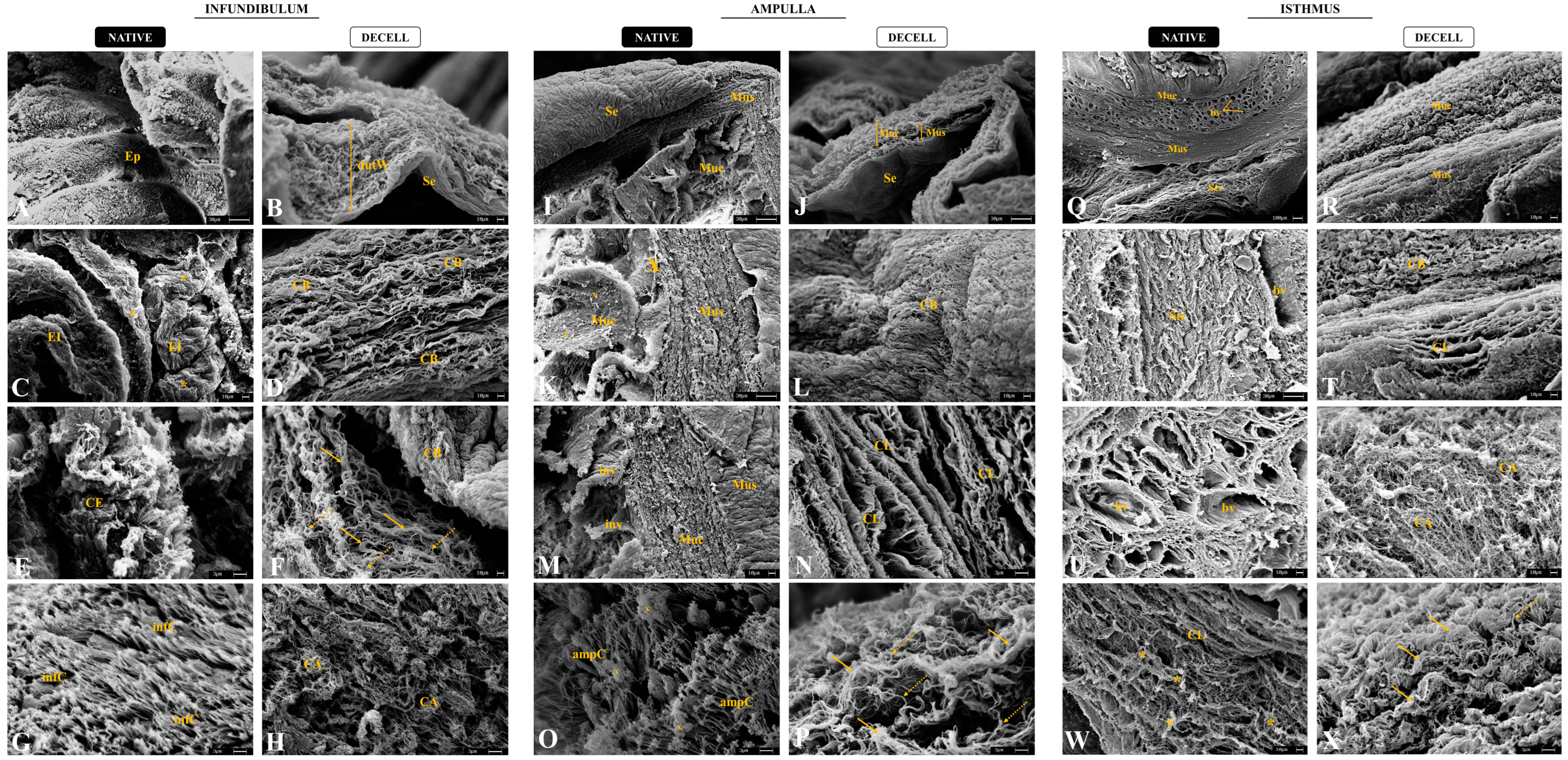
3.2. Morphological and Ultrastructural Characterization
3.3. ECM Physico–Chemical Composition Characterization
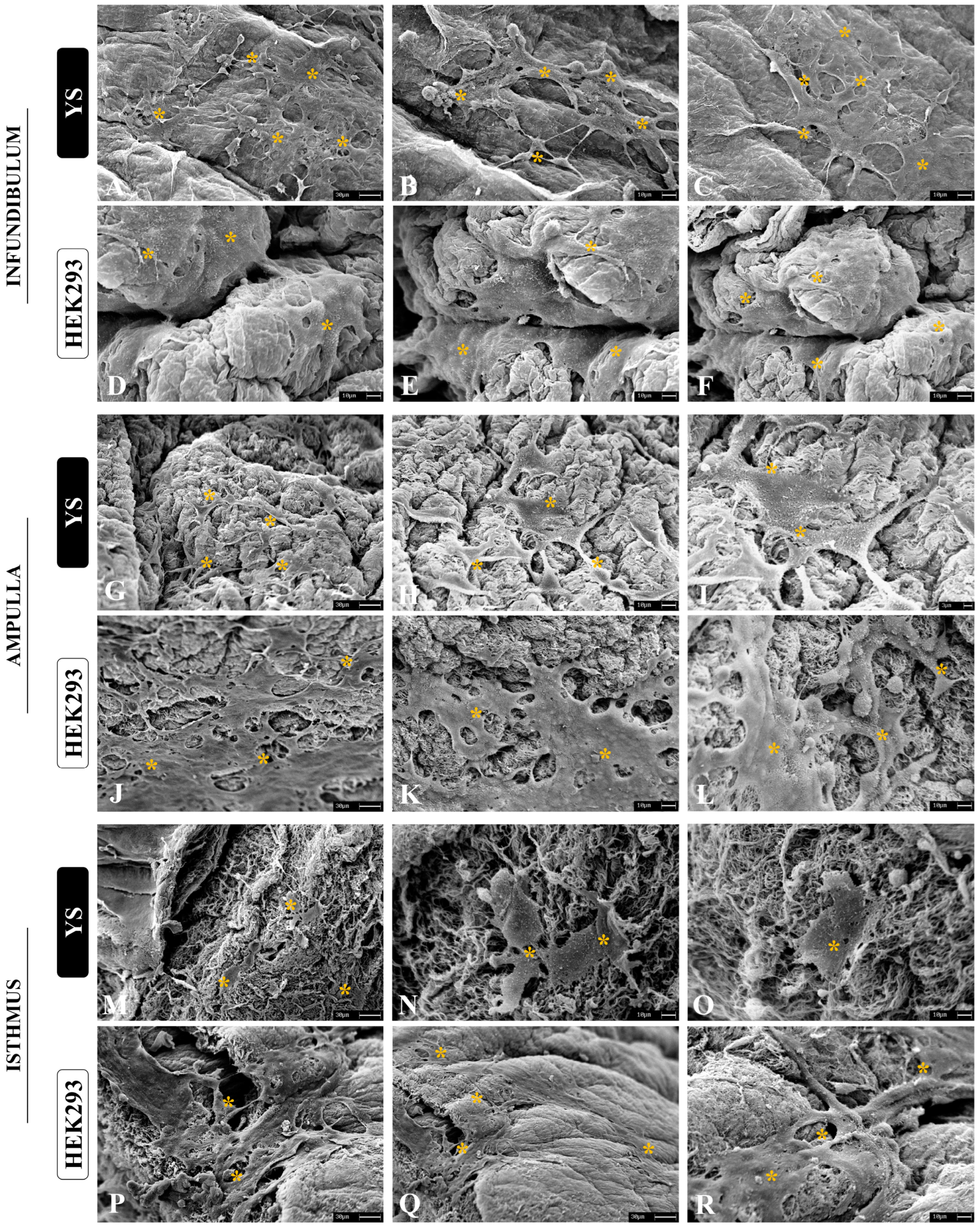
3.4. Cytocompatibility Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klohonatz, K.M.; Nulton, L.C.; Hess, A.M.; Bouma, G.J.; Bruemmer, J.E. The role of embryo contact and focal adhesions during maternal recognition of pregnancy. PLoS ONE 2019, 14, e0213322. [Google Scholar] [CrossRef] [PubMed]
- Kölle, S.; Hughes, B.; Steele, H. Early embryo-maternal communication in the oviduct: A review. Mol. Reprod. Dev. 2020, 87, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.A.; Stephens, K.K.; Winuthayanon, W. Extracellular Vesicles and the Oviduct Function. Int. J. Mol. Sci. 2020, 21, 8280. [Google Scholar] [CrossRef] [PubMed]
- Csöbönyeiová, M.; Varga, I.; Lapides, L.; Pavlíková, L.; Feitscherová, C.; Klein, M. From a Passive Conduit to Highly Dynamic Organ. What are the Roles of Uterine Tube Epithelium in Reproduction? Physiol. Res. 2022, 71, S11–S20. [Google Scholar] [CrossRef] [PubMed]
- Kleijkers, S.H.M.; Eijssen, L.M.T.; Coonen, E.; Derhaag, J.G.; Mantikou, E.; Jonker, M.J.; Mastenbroek, S.; Repping, S.; Evers, J.L.H.; Dumoulin, J.C.M.; et al. Differences in gene expression profiles between human preimplantation embryos cultured in two different IVF culture media. Hum. Reprod. 2015, 30, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Krisher, R.L.; Schlenker, T. Culture of Human Preimplantation Embryos in a Clinical ART Setting. Methods Mol. Biol. 2019, 2006, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Shaulov, T.; Sierra, S.; Sylvestre, C. Recurrent implantation failure in IVF: A Canadian Fertility and Andrology Society Clinical Practice Guideline. Reprod. Biomed. Online 2020, 41, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Romanski, P.A.; Gelvin, B.; Bortoletto, P.; Rosenwaks, Z.; Kang, H.J. Live-Birth Outcomes among Women with Infertility and Anti-Müllerian Hormone Levels of 0.3 ng/mL or Lower. Obstet. Gynecol. 2022, 140, 743–750. [Google Scholar] [CrossRef]
- Demetrio, D.G.B.; Benedetti, E.; Demetrio, C.G.B.; Fonseca, J.; Oliveira, M.; Magalhaes, A.; dos Santos, R.M. How can we improve embryo production and pregnancy outcomes of Holstein embryos produced in vitro? (12 years of practical results at a California dairy farm). Anim. Reprod. 2020, 17, e20200053. [Google Scholar] [CrossRef]
- Ranjbaran, A.; Latifi, Z.; Nejabati, H.R.; Abroon, S.; Mihanfar, A.; Sadigh, A.R.; Fattahi, A.; Nouri, M.; Raffel, N. Exosome-based intercellular communication in female reproductive microenvironments. J. Cell. Physiol. 2019, 234, 19212–19222. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Lin, H.; Kong, S.; Wang, S.; Wang, H.; Armant, D.R. Physiological and molecular determinants of embryo implantation. Mol. Asp. Med. 2013, 34, 939–980. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, L.; Guo, N.; Liu, C.; Wang, M.; Zhu, L.; Li, F.; Jin, L.; Sui, C. Oviductal extracellular vesicles from women with endometriosis impair embryo development. Front. Endocrinol. (Lausanne) 2023, 14, 1171778. [Google Scholar] [CrossRef] [PubMed]
- Saint-Dizier, M.; Schoen, J.; Chen, S.; Banliat, C.; Mermillod, P. Composing the Early Embryonic Microenvironment: Physiology and Regulation of Oviductal Secretions. Int. J. Mol. Sci. 2020, 21, 223. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.H.D.R.; Iglesia, R.P.; Rinaldi, J.d.C.; Murai, M.K.; Calomeno, C.V.A.Q.; da Silva Junior, L.N.; Horvath-Pereira, B.d.O.; Pinho, L.B.M.; Miglino, M.A.; Carreira, A.C.O. Current Trends on Bioengineering Approaches for Ovarian Microenvironment Reconstruction. Tissue Eng. Part B Rev. 2022, 29, 260–298. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.H.D.; Iglesia, R.P.; Araujo, M.S.; Carreira, A.C.O.; Dos Santos, E.X.; Calomeno, C.V.A.Q.; Miglino, M.A. Uterine Tissue Engineering: Where We Stand and the Challenges Ahead. Tissue Eng. Part B Rev. 2022, 28, 861–890. [Google Scholar] [CrossRef]
- Godoy-Guzmán, C.; Nuñez, C.; Orihuela, P.; Campos, A.; Carriel, V. Distribution of extracellular matrix molecules in human uterine tubes during the menstrual cycle: A histological and immunohistochemical analysis. J. Anat. 2018, 233, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Elad, D.; Jaffa, A.J.; Grisaru, D. Biomechanics of early life in the female reproductive tract. Physiology 2020, 35, 134–143. [Google Scholar] [CrossRef]
- Matsuzaki, S. Mechanobiology of the female reproductive system. Reprod. Med. Biol. 2021, 20, 371–401. [Google Scholar] [CrossRef]
- Choucair, F.; Avella, M. Basic, translational and clinical studies in reproductive medicine and clinical reproductive sciences. J. Transl. Med. 2023, 21, 534. [Google Scholar] [CrossRef]
- Jiao, S.Y.; Yang, Y.H.; Chen, S.R. Molecular genetics of infertility: Loss-of-function mutations in humans and corresponding knockout/mutated mice. Hum. Reprod. Update 2021, 27, 154–189. [Google Scholar] [CrossRef]
- Rugg-Gunn, P.J.; Moris, N.; Tam, P.P.L. Technical challenges of studying early human development. Development 2023, 150, dev201797. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.A.M.M.; Henning, H.H.W.; Stout, T.A.E.; Vos, P.L.A.M.; Gadella, B.M. Designing 3-Dimensional In Vitro Oviduct Culture Systems to Study Mammalian Fertilization and Embryo Production. Ann. Biomed. Eng. 2016, 45, 1731–1744. [Google Scholar] [CrossRef] [PubMed]
- Alzamil, L.; Nikolakopoulou, K.; Turco, M.Y. Organoid systems to study the human female reproductive tract and pregnancy. Cell Death Differ. 2021, 28, 35. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Nishida-Aoki, N.; Gujral, T.S. Emerging approaches to study cell–cell interactions in tumor microenvironment. Oncotarget 2019, 10, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Cramer, M.C.; Badylak, S.F. Extracellular Matrix-Based Biomaterials and Their Influence upon Cell Behavior. Ann. Biomed. Eng. 2020, 48, 2132–2153. [Google Scholar] [CrossRef] [PubMed]
- Francés-Herrero, E.; Rodríguez-Eguren, A.; Gómez-álvarez, M.; de Miguel-Gómez, L.; Ferrero, H.; Cervelló, I. Future Challenges and Opportunities of Extracellular Matrix Hydrogels in Female Reproductive Medicine. Int. J. Mol. Sci. 2022, 23, 3765. [Google Scholar] [CrossRef] [PubMed]
- Francés-Herrero, E.; De Miguel-Gómez, L.; López-Martínez, S.; Campo, H.; Garcia-Dominguez, X.; Diretto, G.; Faus, A.; Vicente, J.S.; Marco-Jiménez, F.; Cervelló, I. Development of Decellularized Oviductal Hydrogels as a Support for Rabbit Embryo Culture. Reprod. Sci. 2021, 28, 1644–1658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, Y.; Long, X.; Hu, L.; Wu, Y.; Wu, J.; Shi, X.; Xie, R.; Bi, Y.; Yu, F.; et al. Preparation and Application of Decellularized ECM-Based Biological Scaffolds for Articular Cartilage Repair: A Review. Front. Bioeng. Biotechnol. 2022, 10, 908082. [Google Scholar] [CrossRef]
- McInnes, A.D.; Moser, M.A.J.; Chen, X. Preparation and Use of Decellularized Extracellular Matrix for Tissue Engineering. J. Funct. Biomater. 2022, 13, 240. [Google Scholar] [CrossRef]
- Fratini, P.; Carreira, A.C.O.; Alcântara, D.; de Oliveira e Silva, F.M.; Rodrigues, M.N.; Miglino, M.A. Endothelial differentiation of canine yolk sac cells transduced with VEGF. Res. Vet. Sci. 2016, 104, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pei, M.; Li, Q.; Zhang, Y. Decellularized extracellular matrix mediates tissue construction and regeneration. Front. Med. 2022, 16, 56–82. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.H.D.R.; da Silva-Júnior, L.N.; Gibin, M.S.; dos Santos, H.; de Oliveira Horvath-Pereira, B.; Pinho, L.B.M.; Baesso, M.L.; Sato, F.; Hernandes, L.; Long, C.R.; et al. Perfusion and Ultrasonication Produce a Decellularized Porcine Whole-Ovary Scaffold with a Preserved Microarchitecture. Cells 2023, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; ur Rehman, I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Sohutskay, D.O.; Buno, K.P.; Tholpady, S.S.; Nier, S.J.; Voytik-Harbin, S.L. Design and biofabrication of dermal regeneration scaffolds: Role of oligomeric collagen fibril density and architecture. Regen. Med. 2020, 15, 1295–1312. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Lin, S.; Liang, Q.; Shi, Z.; Xu, J.; Ma, H. Isolation and characterization of collagen from the muscle of Amur sturgeon (Acipenser schrenckii). Biotechnol. Bioprocess Eng. 2014, 19, 935–941. [Google Scholar] [CrossRef]
- Nara, S.; Chameettachal, S.; Midha, S.; Murab, S.; Ghosh, S. Preservation of biomacromolecular composition and ultrastructure of a decellularized cornea using a perfusion bioreactor. RSC Adv. 2015, 6, 2225–2240. [Google Scholar] [CrossRef]
- Arezoo, N.; Mohammad, H.; Malihezaman, M. Tissue engineering of mouse uterus using menstrual blood stem cells (MenSCs) and decellularized uterine scaffold. Stem Cell Res. Ther. 2021, 12, 475. [Google Scholar] [CrossRef]
- Bergholt, M.S.; Serio, A.; Albro, M.B. Raman Spectroscopy: Guiding Light for the Extracellular Matrix. Front. Bioeng. Biotechnol. 2019, 7, 303. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Happillon, T.; Feru, J.; Brassart-Passco, S.; Angiboust, J.F.; Manfait, M.; Piot, O. Raman comparison of skin dermis of different ages: Focus on spectral markers of collagen hydration. J. Raman Spectrosc. 2013, 44, 1230–1237. [Google Scholar] [CrossRef]
- Zhang, Q.; Andrew Chan, K.L.; Zhang, G.; Gillece, T.; Senak, L.; Moore, D.J.; Mendelsohn, R.; Flach, C.R. Raman microspectroscopic and dynamic vapor sorption characterization of hydration in collagen and dermal tissue. Biopolymers 2011, 95, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Timchenko, E.V.; Timchenko, P.E.; Lichtenberg, A.; Assmann, A.; Aubin, H.; Akhyari, P.; Volova, L.T.; Pershutkina, S.V. Assessment of decellularization of heart bioimplants using a Raman spectroscopy method. J. Biomed. Opt. 2017, 22, 091511. [Google Scholar] [CrossRef] [PubMed]
- Villaret, A.; Ipinazar, C.; Satar, T.; Gravier, E.; Mias, C.; Questel, E.; Schmitt, A.M.; Samouillan, V.; Nadal, F.; Josse, G. Raman characterization of human skin aging. Skin Res. Technol. 2019, 25, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Naeem, E.M.; Sajad, D.; Talaei-Khozani, T.; Khajeh, S.; Azarpira, N.; Alaei, S.; Tanideh, N.; Reza, T.M.; Razban, V. Decellularized liver transplant could be recellularized in rat partial hepatectomy model. J. Biomed. Mater. Res. A 2019, 107, 2576–2588. [Google Scholar] [CrossRef] [PubMed]
- Emami, A.; Talaei-Khozani, T.; Vojdani, Z.; Zarei Fard, N. Comparative assessment of the efficiency of various decellularization agents for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 19–32. [Google Scholar] [CrossRef]
- Huang, G.; Li, F.; Zhao, X.; Ma, Y.; Li, Y.; Lin, M.; Jin, G.; Lu, T.J.; Genin, G.M.; Xu, F. Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef]
- Vieira-da-Silva, B.; Castanho, M.A.R.B. Resazurin Reduction-Based Assays Revisited: Guidelines for Accurate Reporting of Relative Differences on Metabolic Status. Molecules 2023, 28, 2283. [Google Scholar] [CrossRef]
- Liu, R.; Dai, M.; Gong, G.; Chen, M.; Cao, C.; Wang, T.; Hou, Z.; Shi, Y.; Guo, J.; Zhang, Y.; et al. The role of extracellular matrix on unfavorable maternal–fetal interface: Focusing on the function of collagen in human fertility. J. Leather Sci. Eng. 2022, 4, 13–17. [Google Scholar] [CrossRef]
- Idelevich, A.; Vilella, F. Mother and Embryo Cross-Communication. Genes 2020, 11, 376. [Google Scholar] [CrossRef]
- Slayden, O.D.; Luo, F.; Bishop, C.V. Physiological Action of Progesterone in the Nonhuman Primate Oviduct. Cells 2022, 11, 1534. [Google Scholar] [CrossRef]
- Fujii, D.T.; Yohannes, E.; Por, E.D.; Gillette, L.; Beesley, R.D.; Heitmann, R.J.; Chow, G.E.; Burney, R.O. The proteome of human Fallopian tube lavages during the phase of embryo transit reveals candidate proteins for the optimization of preimplantation embryo culture. Hum. Reprod. 2021, 36, 367–380. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular mechanotransduction: From tension to function. Front. Physiol. 2018, 9, 378185. [Google Scholar] [CrossRef]
- Venkata, V.D.; Fairuz, M.; Jamaluddin, B.; Goad, J.; Drury, H.R.; Tadros, M.A.; Lim, R.; Karakoti, A.; O’sullivan, R.; Ius, Y.; et al. Development and characterization of human fetal female reproductive tract organoids to understand Müllerian duct anomalies. Proc. Natl. Acad. Sci. USA 2022, 119, e2118054119. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Urciuolo, F.; Imparato, G.; Netti, P.A. In vitro strategies for mimicking dynamic cell–ECM reciprocity in 3D culture models. Front. Bioeng. Biotechnol. 2023, 11, 1197075. [Google Scholar] [CrossRef] [PubMed]
- Moffat, D.; Ye, K.; Jin, S. Decellularization for the retention of tissue niches. J. Tissue Eng. 2022, 13, 20417314221101151. [Google Scholar] [CrossRef]
- Kasturi, M.; Vasanthan, K.S. Effect of decellularization using sodium dodecyl sulfate on glycosaminoglycans content in the liver. Regen. Med. 2023, 18, 527–530. [Google Scholar] [CrossRef]
- White, L.J.; Taylor, A.J.; Faulk, D.M.; Keane, T.J.; Saldin, L.T.; Reing, J.E.; Swinehart, I.T.; Turner, N.J.; Ratner, B.D.; Badylak, S.F. The impact of detergents on the tissue decellularization process: A ToF-SIMS study. Acta Biomater. 2017, 50, 207–219. [Google Scholar] [CrossRef]
- Ahsan, T.; Nerem, R.M. Bioengineered tissues: The science, the technology, and the industry. Orthod. Craniofac. Res. 2005, 8, 134–140. [Google Scholar] [CrossRef]
- de Jongh, D.; Massey, E.K.; Cronin, A.J.; Schermer, M.H.N.; Bunnik, E.M. Early-Phase Clinical Trials of Bio-Artificial Organ Technology: A Systematic Review of Ethical Issues. Transpl. Int. 2022, 35, 10751. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.L.; Khraibi, A.A.; Dumée, L.F.; Corridon, P.R. From waste to wealth: Repurposing slaughterhouse waste for xenotransplantation. Front. Bioeng. Biotechnol. 2023, 11, 1091554. [Google Scholar] [CrossRef]
- Mozhiarasi, V.; Natarajan, T.S. Slaughterhouse and poultry wastes: Management practices, feedstocks for renewable energy production, and recovery of value added products. Biomass Convers. Biorefinery 2022, 1, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.S.; Solar, P.A.; Juica, N.E.; Orihuela, P.A.; Cardenas, H.; Christodoulides, M.; Vargas, R.; Velasquez, L.A. Differential expression of extracellular matrix components in the Fallopian tubes throughout the menstrual cycle. Reprod. Biol. Endocrinol. 2012, 10, 56. [Google Scholar] [CrossRef]
- Varga, I.; Csöbönyeiová, M.; Visnyaiová, K.; Záhumenský, J.; Pavlíková, L.; Feitscherová, C.; Klein, M. Functional Morphology of the Human Uterine Tubes in the 21st Century: Anatomical Novelties and Their Possible Clinical Applications. Physiol. Res. 2022, 71, S151–S159. [Google Scholar] [CrossRef]
- O’Connor, B.B.; Pope, B.D.; Peters, M.M.; Ris-Stalpers, C.; Parker, K.K. The role of extracellular matrix in normal and pathological pregnancy: Future applications of microphysiological systems in reproductive medicine. Exp. Biol. Med. 2020, 245, 1163–1174. [Google Scholar] [CrossRef]
- Jorge, S.; Chang, S.; Barzilai, J.J.; Leppert, P.; Segars, J.H. Mechanical Signaling in Reproductive Tissues: Mechanisms and Importance. Reprod. Sci. 2014, 21, 1093. [Google Scholar] [CrossRef] [PubMed]
- Ouni, E.; Bouzin, C.; Dolmans, M.M.; Marbaix, E.; Pyr Dit Ruys, S.; Vertommen, D.; Amorim, C.A. Spatiotemporal changes in mechanical matrisome components of the human ovary from prepuberty to menopause. Hum. Reprod. 2020, 35, 1391–1410. [Google Scholar] [CrossRef] [PubMed]
- Karvas, R.M.; Zemke, J.E.; Ali, S.S.; Upton, E.; Sane, E.; Fischer, L.A.; Dong, C.; mi Park, K.; Wang, F.; Park, K.; et al. 3D-cultured blastoids model human embryogenesis from pre-implantation to early gastrulation stages. Cell Stem Cell 2023, 30, 1148–1165.e7. [Google Scholar] [CrossRef]
- Tu, Z.; Bi, Y.; Zhu, X.; Liu, W.; Hu, J.; Wu, L.; Mao, T.; Zhou, J.; Wang, H.; Wang, H.; et al. Modeling human pregastrulation development by 3D culture of blastoids generated from primed-to-naïve transitioning intermediates. Protein Cell 2023, 14, 337–349. [Google Scholar] [CrossRef]
- Fontoura, J.C.; Viezzer, C.; dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110264. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Miao, X.; Cui, W.; Sun, Y. Biophysical optimization of preimplantation embryo culture: What mechanics can offer ART. Mol. Hum. Reprod. 2021, 27, gaaa087. [Google Scholar] [CrossRef] [PubMed]
- Besenfelder, U.; Havlicek, V. The interaction between the environment and embryo development in assisted reproduction. Anim. Reprod. 2023, 20, e20230034. [Google Scholar] [CrossRef] [PubMed]
- Jafarbeglou, F.; Nazari, M.A.; Keikha, F.; Amanpour, S.; Azadi, M. Visco-hyperelastic characterization of the mechanical properties of human fallopian tube tissue using atomic force microscopy. Materialia 2021, 16, 101074. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, F.; Zhong, W.; Puscheck, E.; Shen, H.; Rappolee, D.A. Shear stress induces preimplantation embryo death that is delayed by the zona pellucida and associated with stress-activated protein kinase-mediated apoptosis. Biol. Reprod. 2006, 75, 45–55. [Google Scholar] [CrossRef]
- Adil, A.; Xu, M.; Haykal, S. Recellularization of Bioengineered Scaffolds for Vascular Composite Allotransplantation. Front. Surg. 2022, 9, 843677. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Saleh, T.; Xu, M. Recellularization of Native Tissue Derived Acellular Scaffolds with Mesenchymal Stem Cells. Cells 2021, 10, 1787. [Google Scholar] [CrossRef] [PubMed]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive potential of natural biomaterials: Identification, retention and assessment of biological properties. Signal Transduct. Target. Ther. 2021, 6, 122. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Sowamber, R.; Nelson, O.; Dodds, L.; Decastro, V.; Paudel, I.; Milea, A.; Considine, M.; Cope, L.; Pinto, A.; Schlumbrecht, M.; et al. Integrative Transcriptome Analyses of the Human Fallopian Tube: Fimbria and Ampulla—Site of Origin of Serous Carcinoma of the Ovary. Cancers 2020, 12, 1090. [Google Scholar] [CrossRef]
- Cruz Walma, D.A.; Yamada, K.M. The extracellular matrix in development. Development 2020, 147, dev175596. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, G.H.D.R.; da Silva, R.S.; Gibin, M.S.; Gonzaga, V.H.d.S.; dos Santos, H.; Igleisa, R.P.; Fernandes, L.A.; Fernandes, I.C.; Nesiyama, T.N.G.; Sato, F.; et al. Region-Specific Decellularization of Porcine Uterine Tube Extracellular Matrix: A New Approach for Reproductive Tissue-Engineering Applications. Biomimetics 2024, 9, 382. https://doi.org/10.3390/biomimetics9070382
Almeida GHDR, da Silva RS, Gibin MS, Gonzaga VHdS, dos Santos H, Igleisa RP, Fernandes LA, Fernandes IC, Nesiyama TNG, Sato F, et al. Region-Specific Decellularization of Porcine Uterine Tube Extracellular Matrix: A New Approach for Reproductive Tissue-Engineering Applications. Biomimetics. 2024; 9(7):382. https://doi.org/10.3390/biomimetics9070382
Chicago/Turabian StyleAlmeida, Gustavo Henrique Doná Rodrigues, Raquel Souza da Silva, Mariana Sversut Gibin, Victória Hellen de Souza Gonzaga, Henrique dos Santos, Rebeca Piatniczka Igleisa, Leticia Alves Fernandes, Iorrane Couto Fernandes, Thais Naomi Gonçalves Nesiyama, Francielle Sato, and et al. 2024. "Region-Specific Decellularization of Porcine Uterine Tube Extracellular Matrix: A New Approach for Reproductive Tissue-Engineering Applications" Biomimetics 9, no. 7: 382. https://doi.org/10.3390/biomimetics9070382
APA StyleAlmeida, G. H. D. R., da Silva, R. S., Gibin, M. S., Gonzaga, V. H. d. S., dos Santos, H., Igleisa, R. P., Fernandes, L. A., Fernandes, I. C., Nesiyama, T. N. G., Sato, F., Baesso, M. L., Hernandes, L., Rinaldi, J. d. C., Meirelles, F. V., Astolfi-Ferreira, C. S., Ferreira, A. J. P., & Carreira, A. C. O. (2024). Region-Specific Decellularization of Porcine Uterine Tube Extracellular Matrix: A New Approach for Reproductive Tissue-Engineering Applications. Biomimetics, 9(7), 382. https://doi.org/10.3390/biomimetics9070382








