Silactins and Structural Diversity of Biosilica in Sponges
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Origins
- Freshwater sponges:
- Marine sponges:
2.2. Sample Preparation and Phalloidin Staining
2.3. Digital Microscopy
2.4. Scanning Electron Microscopy (SEM)
2.5. Fluorescence Microscopy
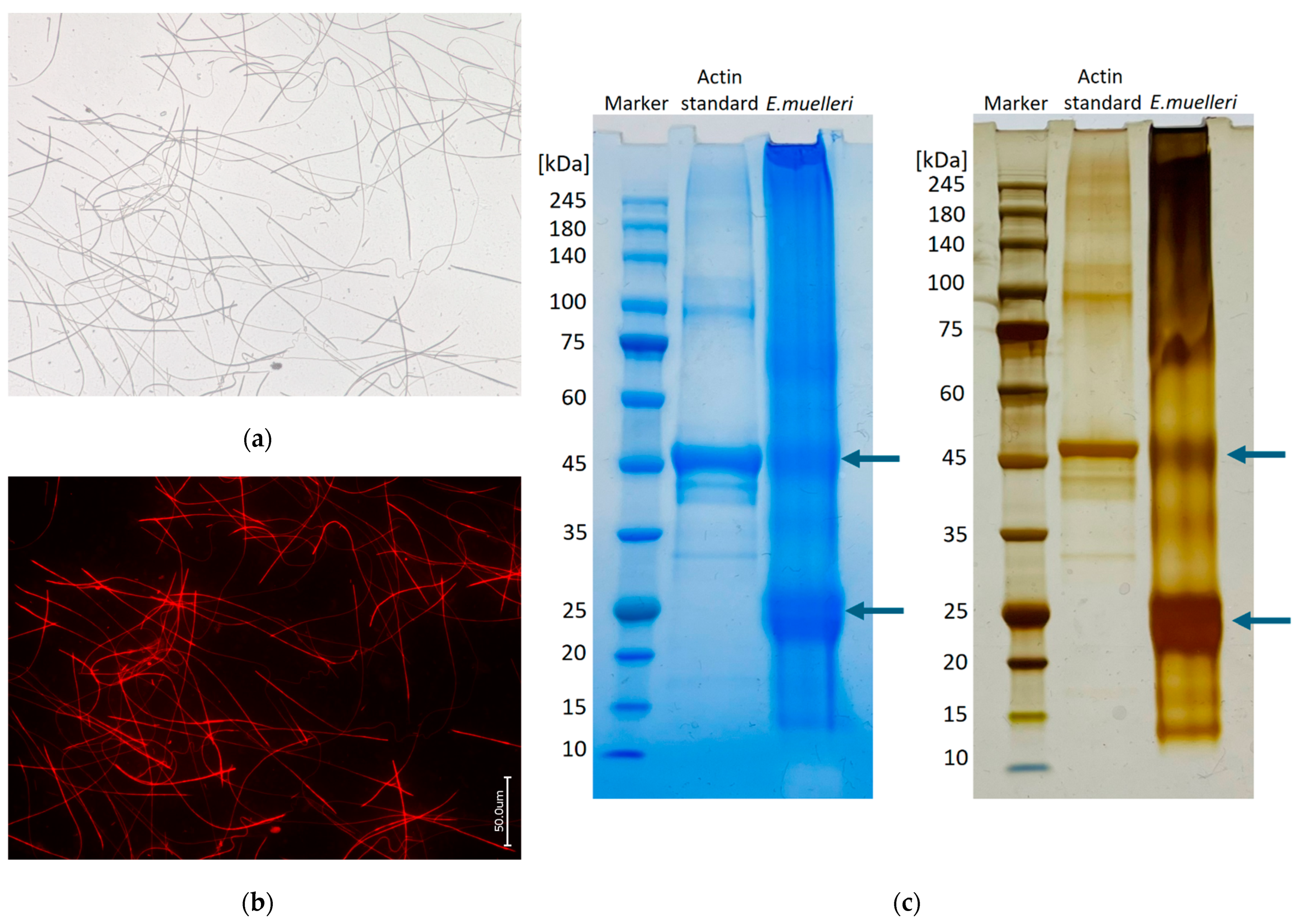
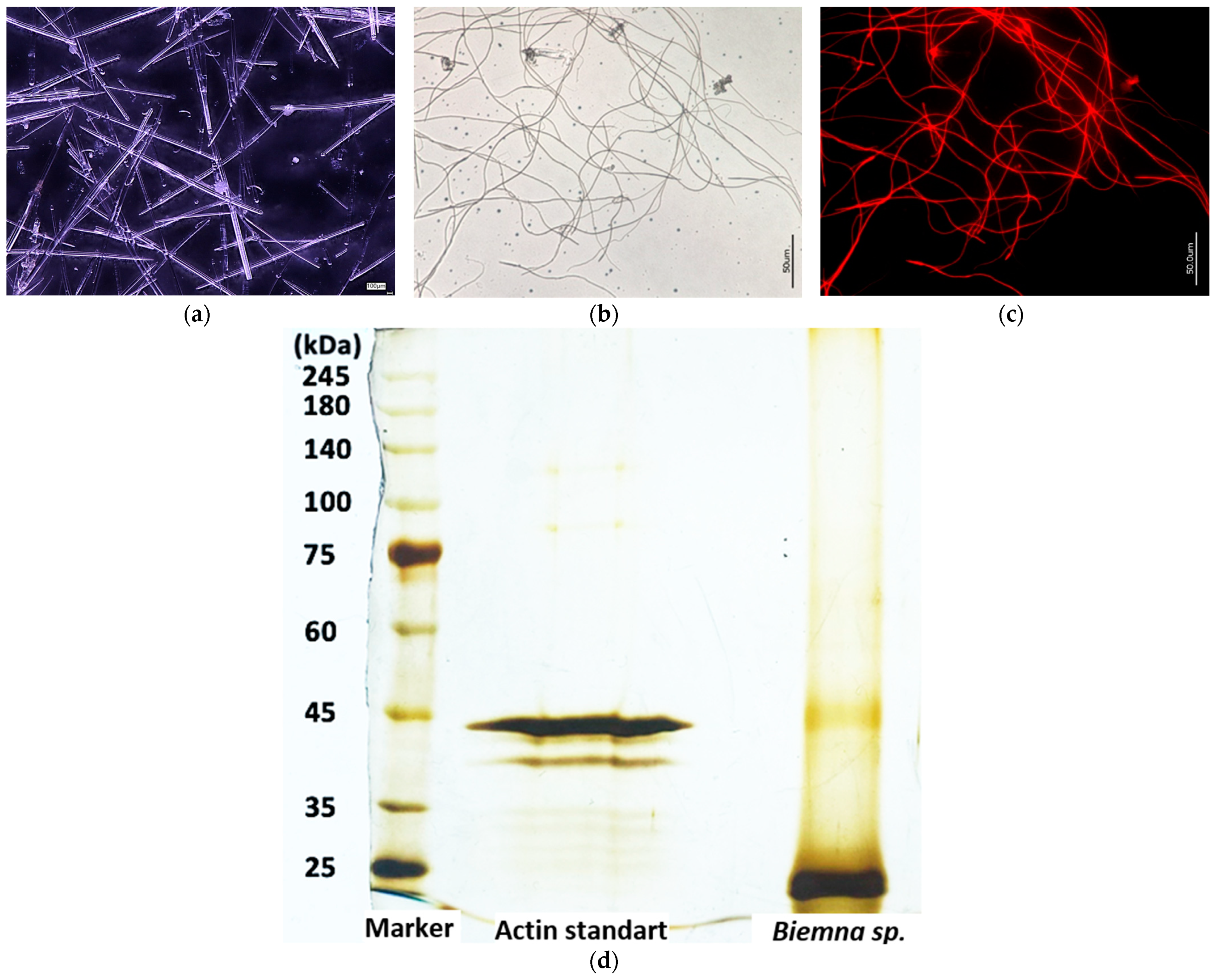
2.6. SDS-PAGE
3. Results
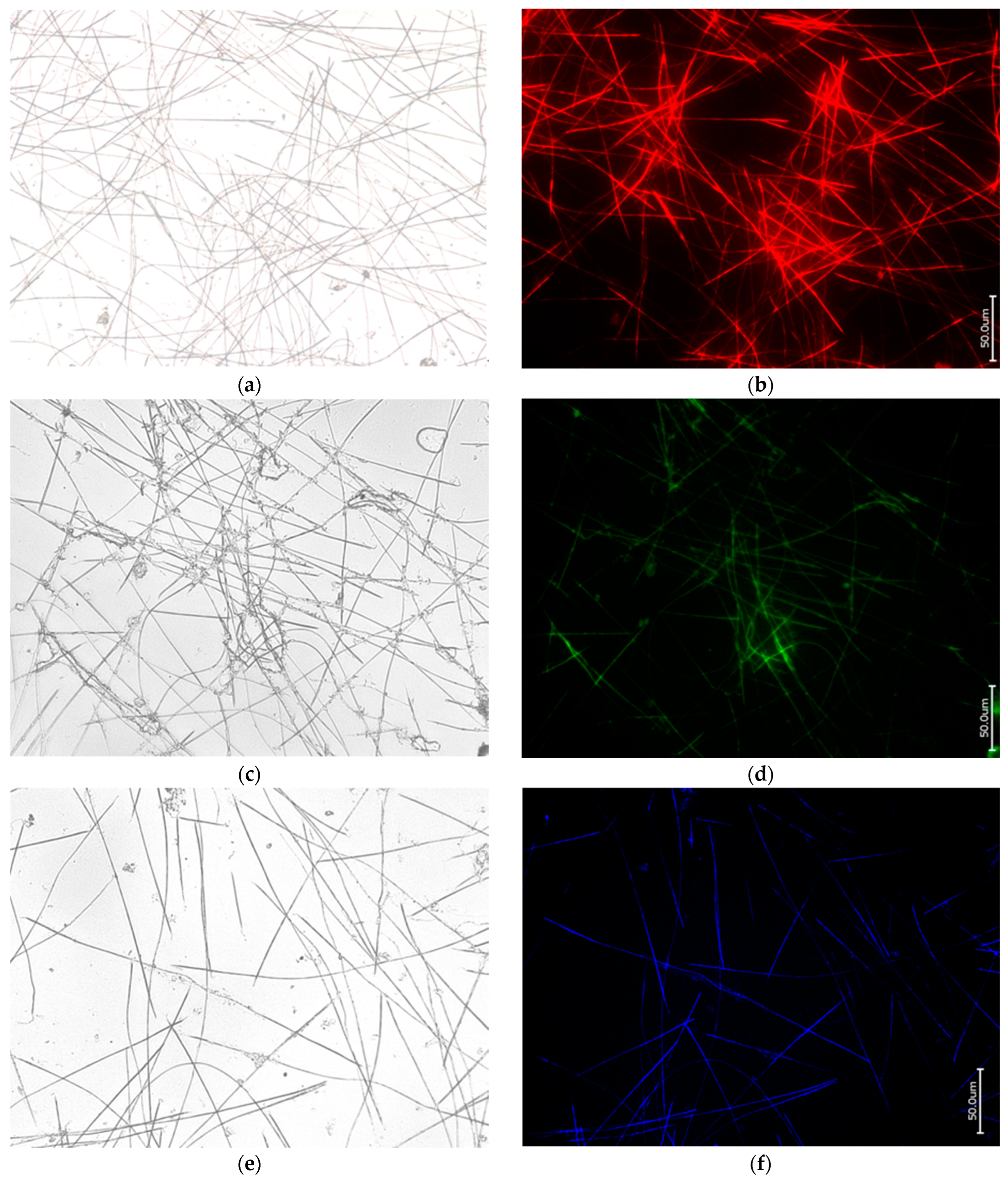
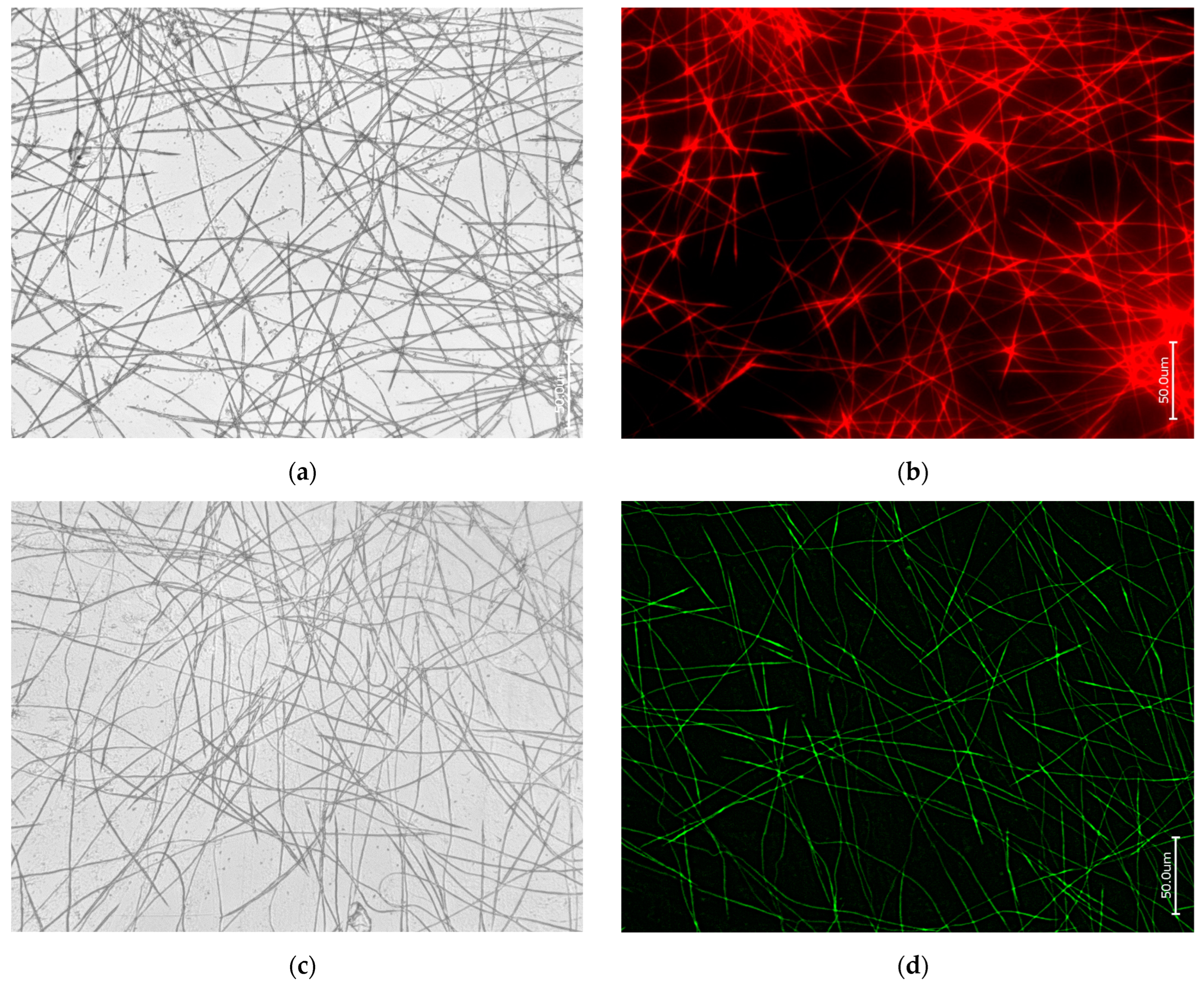
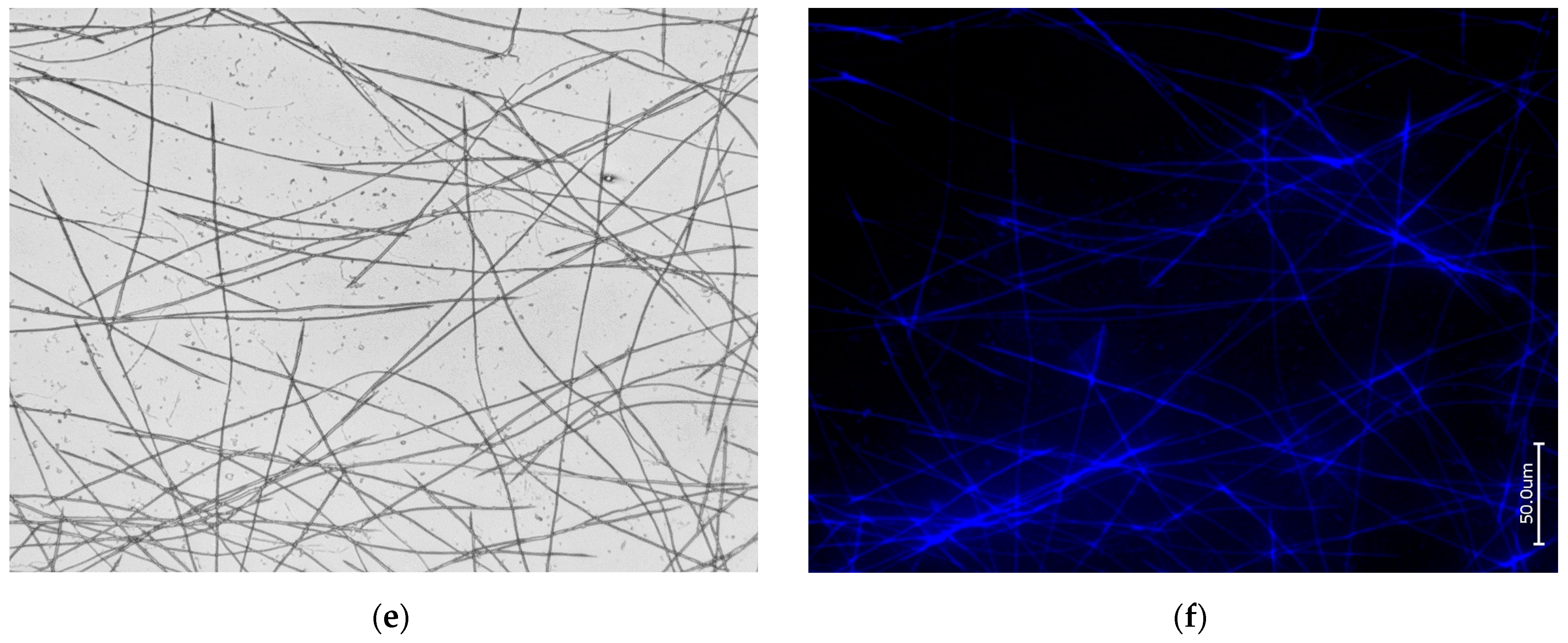
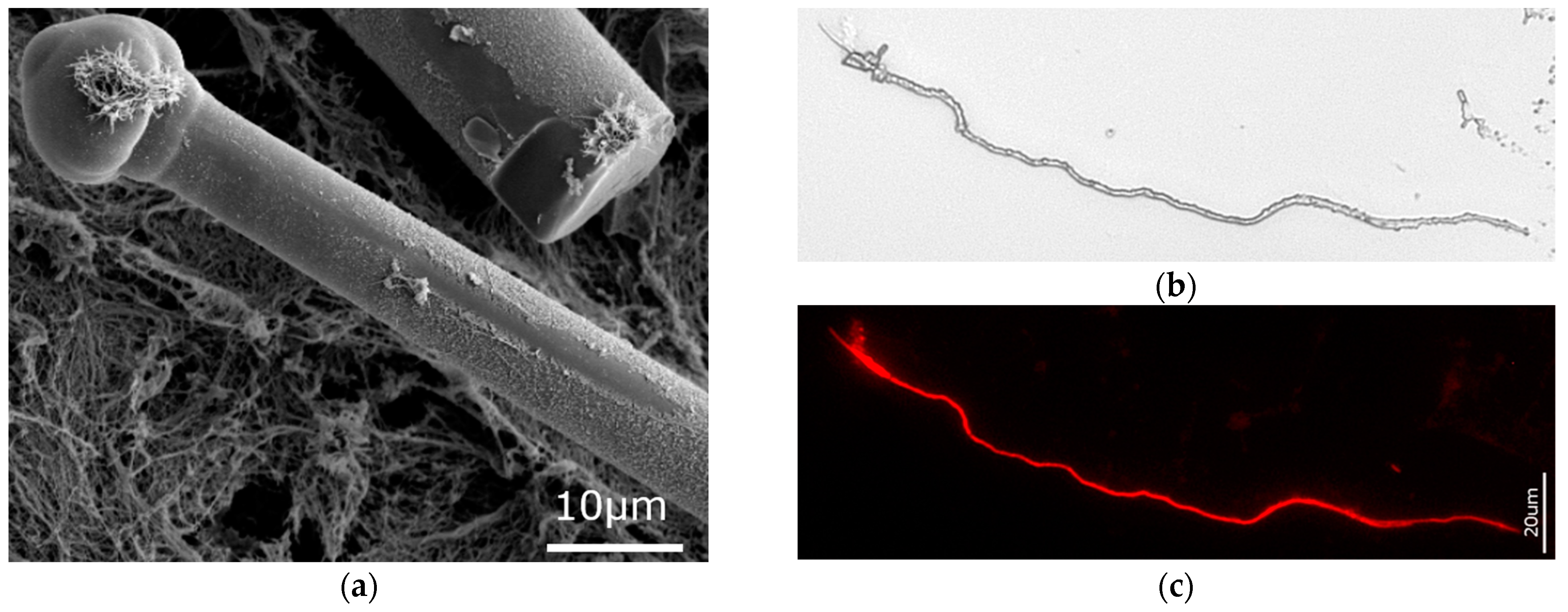
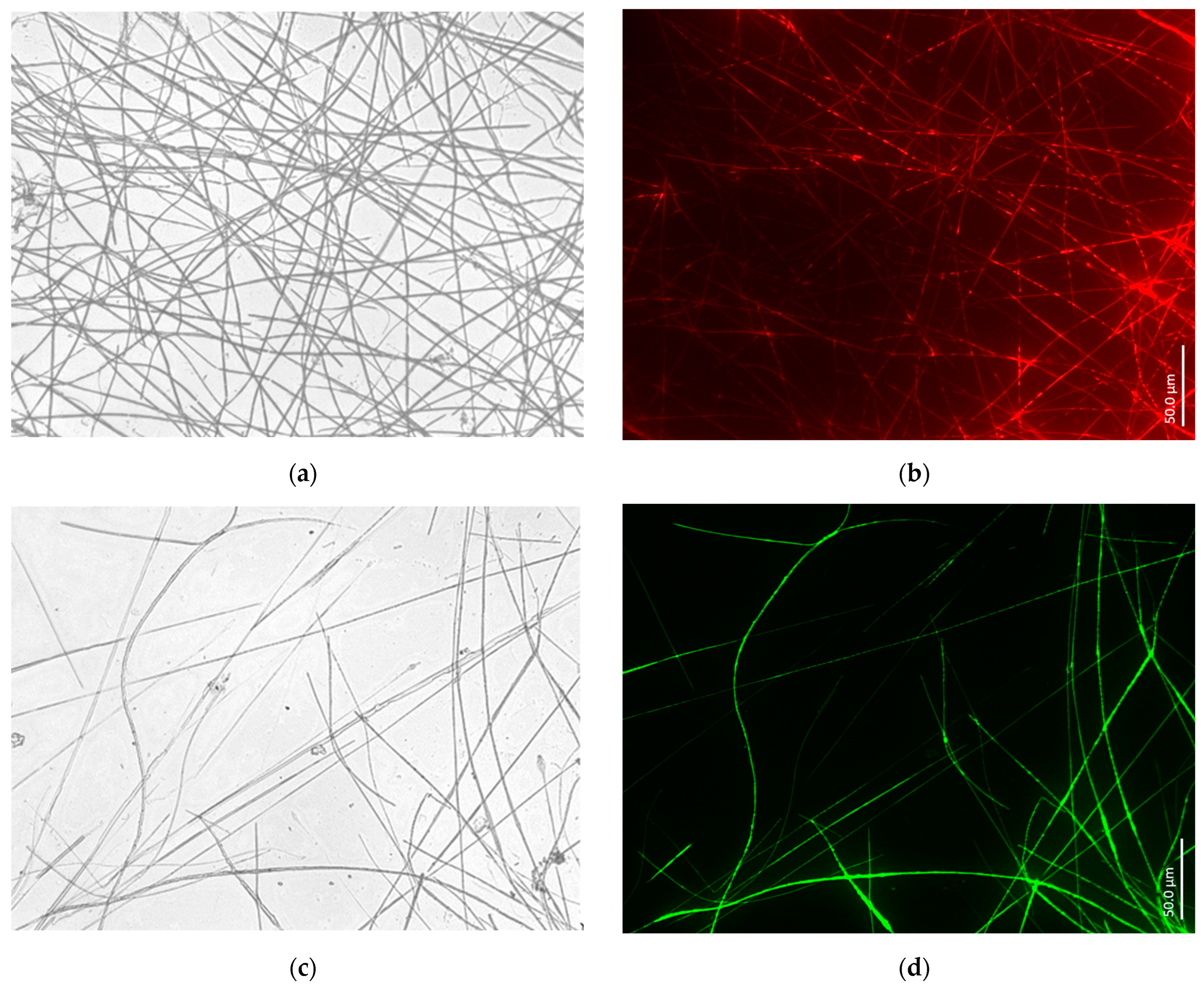
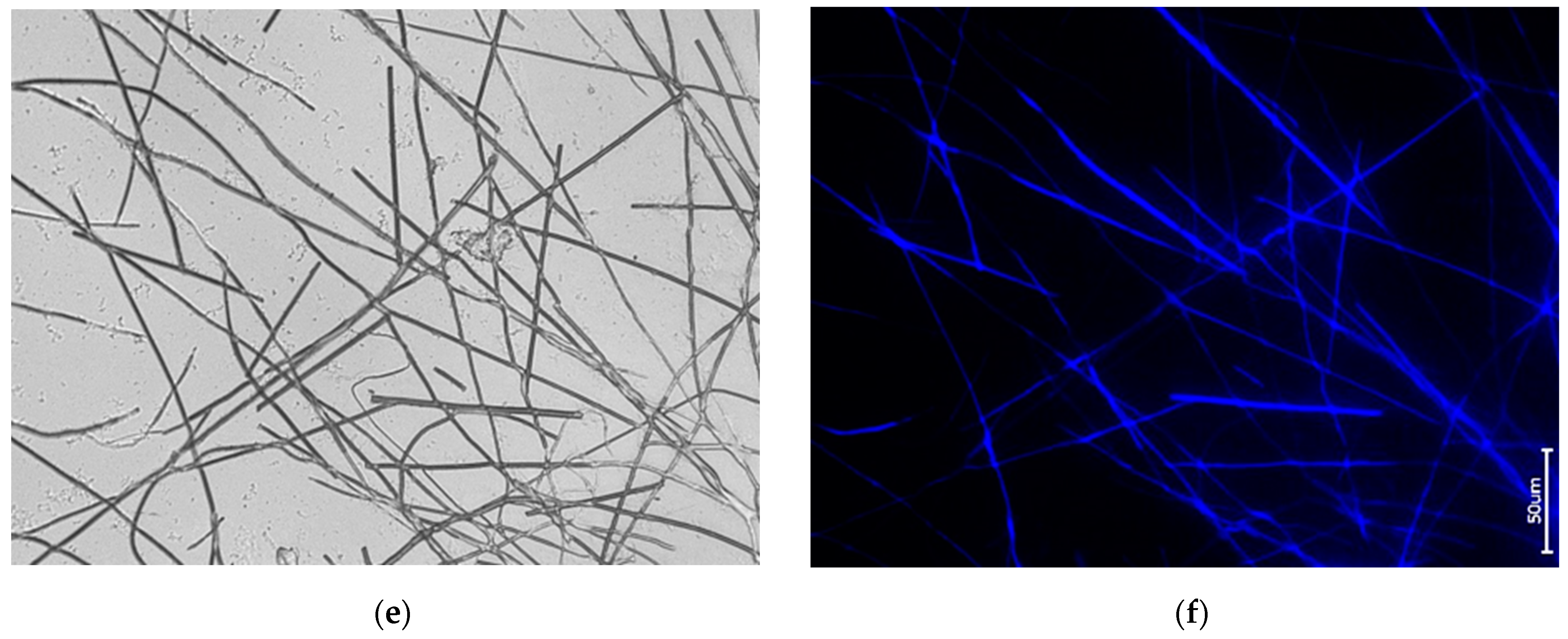
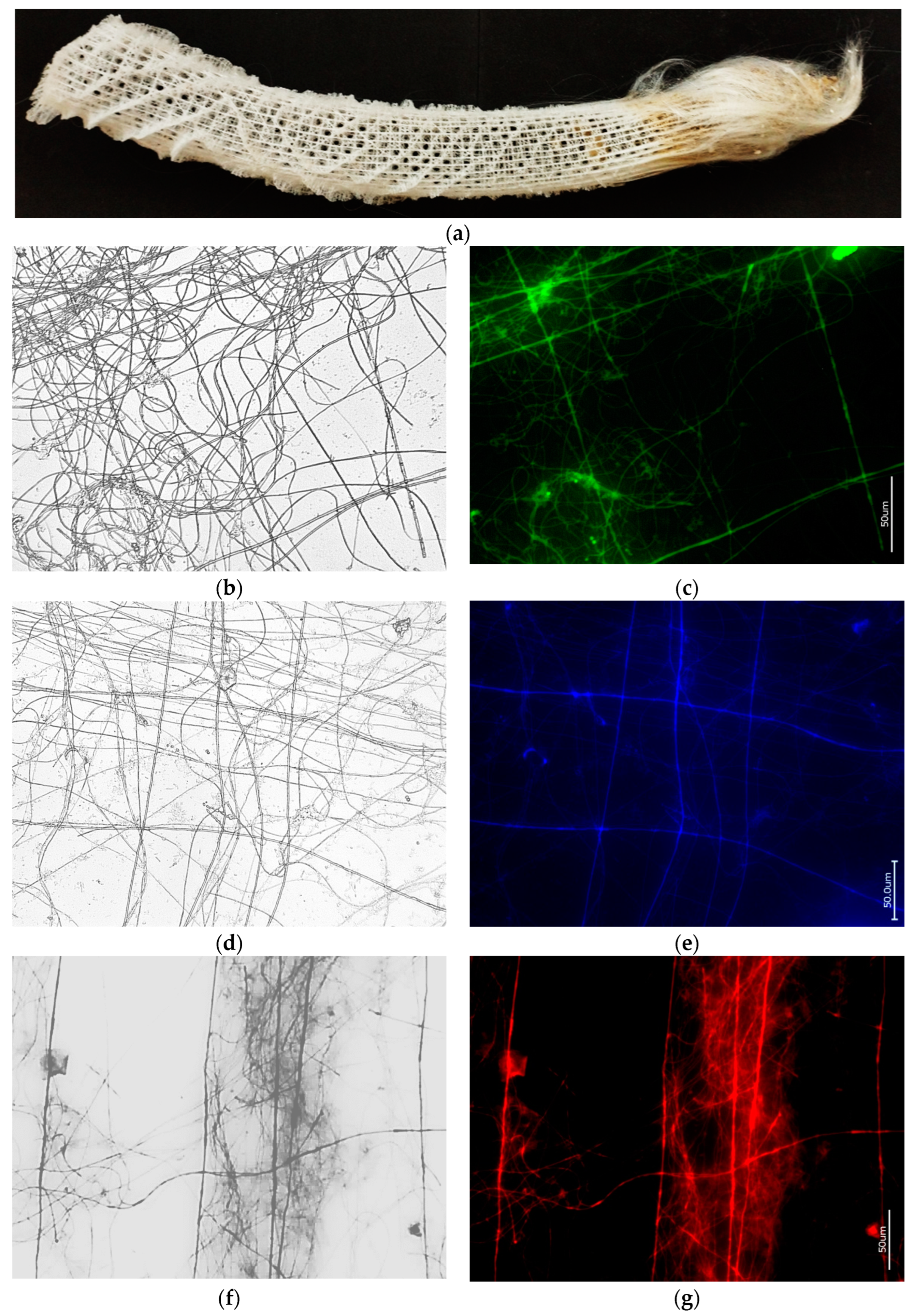
3.1. Actin within Spicules of Freshwater Demosponges
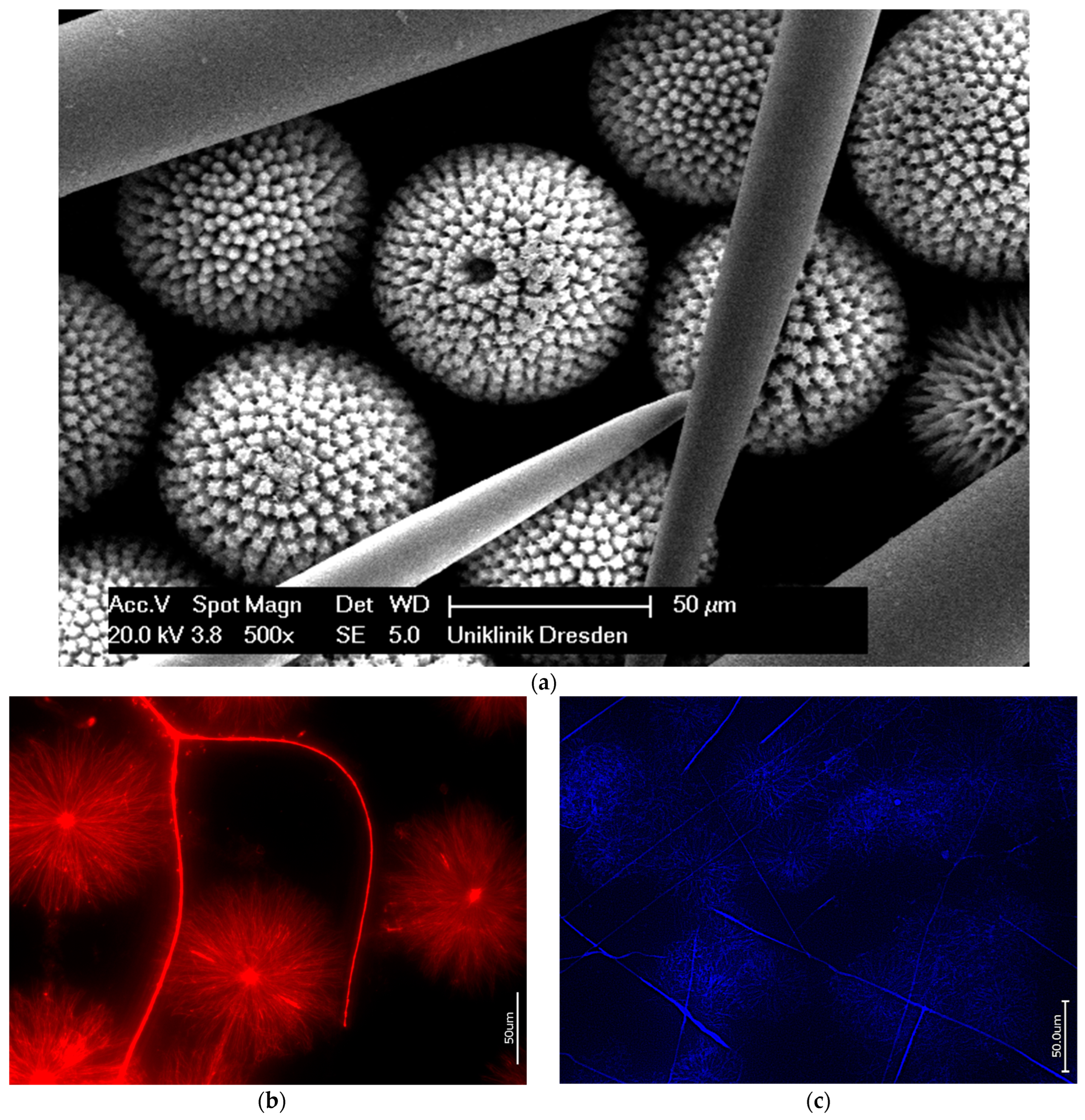
3.2. Actin within Spicules of Marine Demosponges
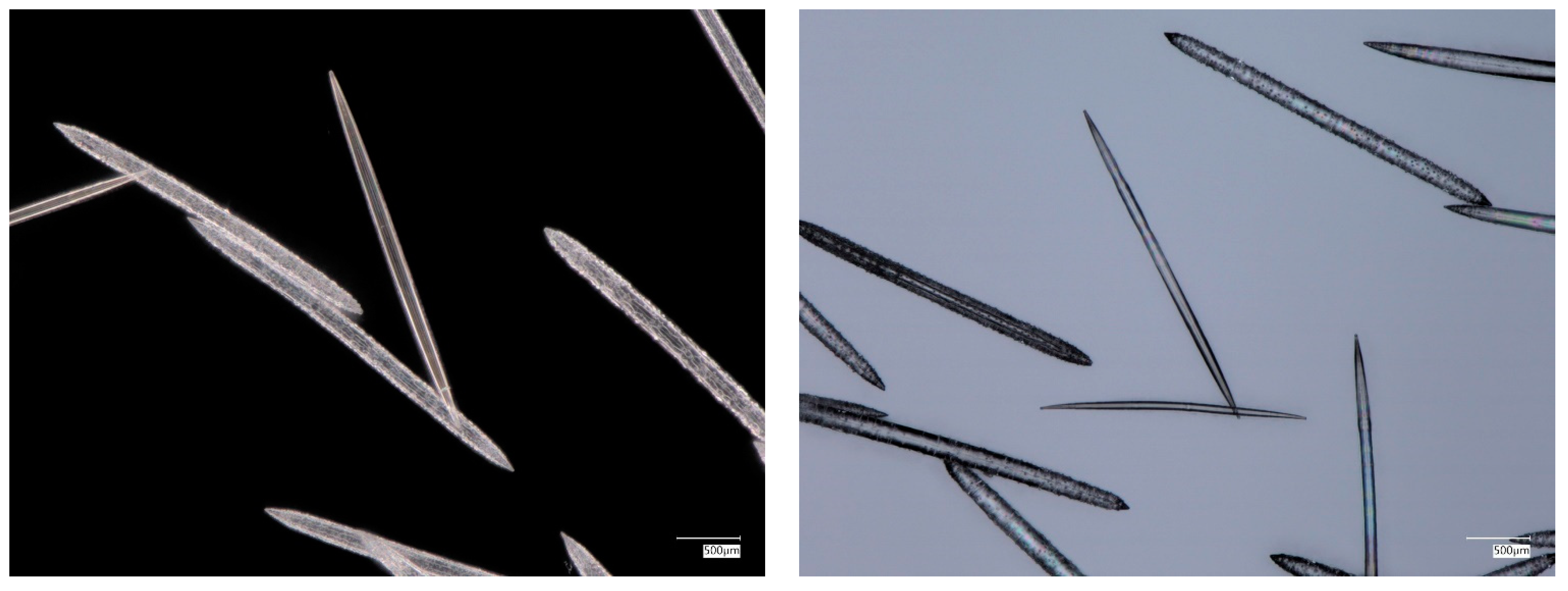
3.3. Actin in the Skeleton of Glass Sponges
4. Discussion
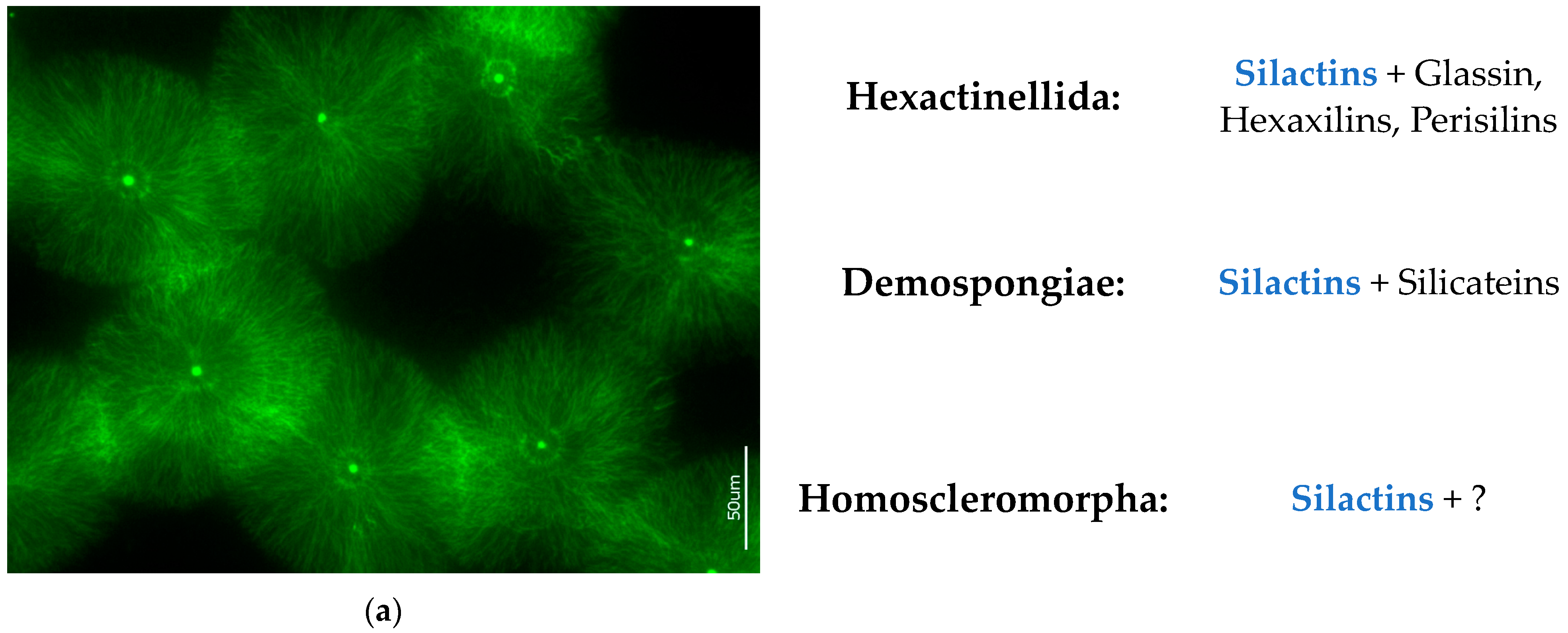
- (a)
- Genomic data. There is no evidence of silicatein genes, but those for glassin, as well as collagens and actins, have been reported in the genome of the reef-building psychrophilic glass sponge Aphrocallistes vastus (order Sceptrulophora) [51]. Also, in the genome of the Mediterranean Oopsacas minuta (order Lyssacinosida) glass sponge, there is no evidence of silicatein, silintaphin, or galectin genes, but actin and glassin genes have been recently reported [52].
- (b)
- Data on inhibition of actin polymerization. It is well recognized that latrunculin B binds to actin monomers and inhibits F-actin polymerization [53]. In recent experiments involving the cultivation of the Spongilla lacustris freshwater demosponge from its gemmules, it was shown with strong evidence that actin inhibition by latrunculin B prevents spicule formation [24]. In the samples of hatched gemmules, in the presence of latrunculin B, siliceous spicules never appeared; however, the young sponges grew. To our best knowledge, there are no data on the inhibitory effects of latrunculins against the biosynthesis or self-assembly of silicateins. Consequently, the occurrence of silicateins within sclerocytes of demosponges did not lead to the formation of spicules, but the absence of actin had a decisive impact on spiculogenesis. Put simply, the implication is no actin, no spicules!
- (c)
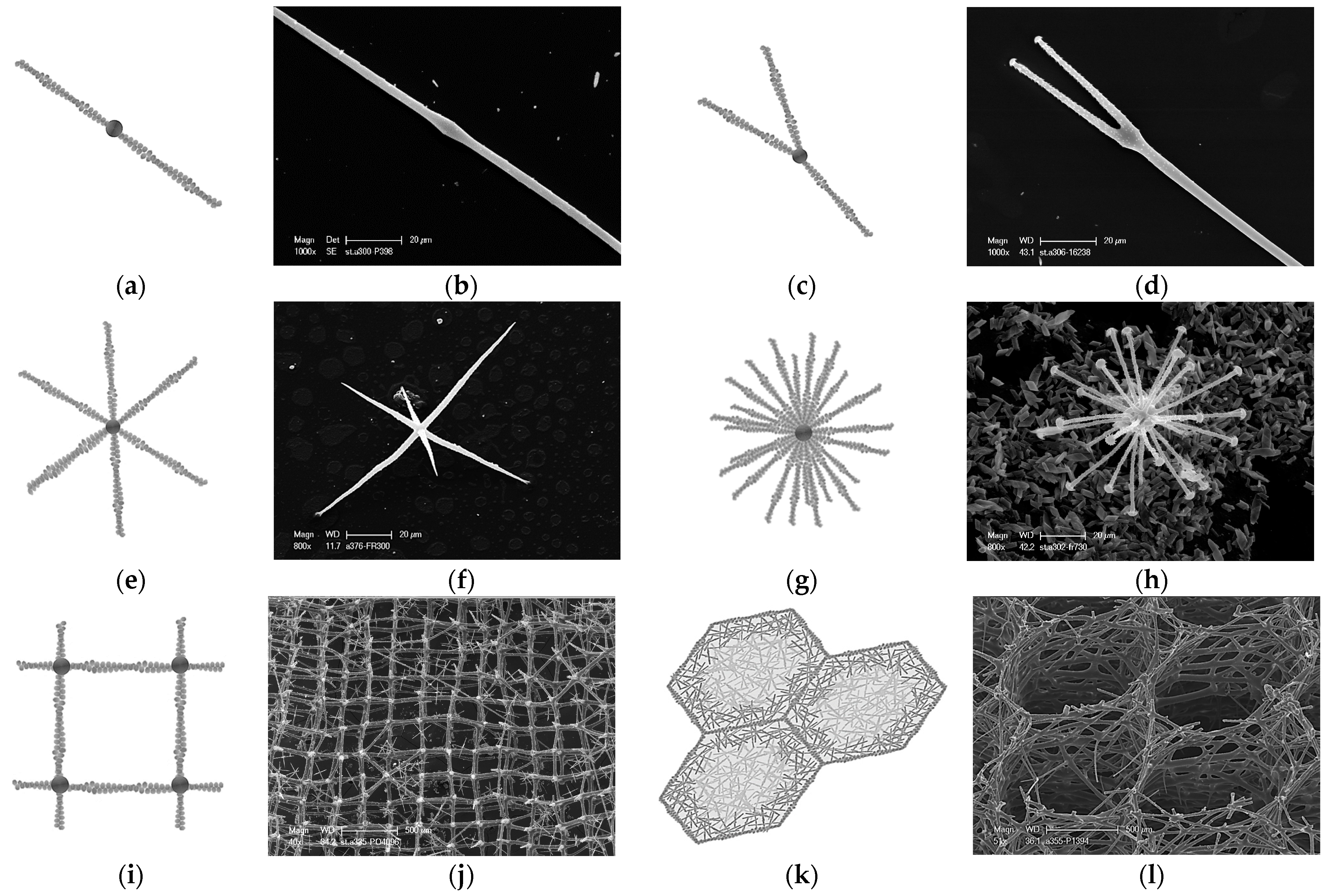
- Data on structural features characteristic only of actin. Such phenomena known from structural biology as bifurcation, dichotomic growth, and branching represent characteristic features only of actin [48,54]. They are also responsible for the formation of a broad range of higher-order 3D suprafilamentous structures of F-actin: bundles, aggregates, branched, cross-linked, and dendritic filamentous constructs [55,56,57] (see Figure 10). It should be noted that the micrometer size and the quantity of actin filaments that have been isolated from the skeletal formations of diverse demosponges (see Figure 9 as an example) and hexactinellids (see the corresponding images in [22,24]) are not surprising. For example, up to 500 actin filaments have been found in the actin bundles in bristle sprouts of Drosophila fruit flies [58]. Also, in the same organism, the bristle cell extension is supported by up to 400 µm-long F-actin bundles assembled together [59,60]. The unique surface ornamentation and sophisticated microarchitecture of some star-like microscleres in demosponges (i.e., sterrasters of Geodia sponges) [11,21,24] may seem somewhat extraordinary, and the possible participation of radially oriented actin in this kind of spiculogenesis seems doubtful in principle. However, such a radial structural orientation has already been reported for intracellular actins in Drosophila S2 and in Xenopus XTC cells [61], as well as in filopodia [62] or lamellipodia of motile cells [63], in flagella [64], and in diverse neurons [65,66,67]. Regarding biosilica-producing organisms, the occurrence of radially oriented actin filaments has been reported within the frustules of such diatoms as Coscinodiscus granii and Cyclotella cryptica [26,27]. A fundamental remark should be made here: none of the above-mentioned proteins involved in biosilicification in sponges (i.e., silicateins, glassins, etc.) or in diatoms (i.e., silaffins, silacidins, etc.) possess structural features similar to those of actin.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Schröder, H.C.; Wiens, M.; Schloßmacher, U.; Müller, W.E. Biosilica: Molecular Biology, Biochemistry and Function in Demosponges as well as its Applied Aspects for Tissue Engineering. Adv. Mar. Biol. 2012, 62, 231–271. [Google Scholar] [PubMed]
- Sahebi, M.; Hanafi, M.M.; Siti Nor Akmar, A.; Rafii, M.Y.; Azizi, P.; Tengoua, F.F.; Nurul Mayzaitul Azwa, J.; Shabanimofrad, M. Importance of silicon and mechanisms of biosilica formation in plants. BioMed Res. Int. 2015, 2015, 396010. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Jesionowski, T.; Ehrlich, H. Biosilica as a source for inspiration in biological materials science. Am. Mineral. 2018, 103, 665–691. [Google Scholar] [CrossRef]
- Ikeda, T. Bacterial biosilicification: A new insight into the global silicon cycle. Biosci. Biotechnol. Biochem. 2021, 85, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Kim, D.H.; Youn, S.; Pack, S.P. Biomimetic Diatom Biosilica and Its Potential for Biomedical Applications and Prospects: A Review. Int. J. Mol. Sci. 2024, 25, 2023. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wan, B.; Yuan, X.; Muscente, A.D.; Xiao, S. Spiculogenesis and biomineralization in early sponge animals. Nat. Commun. 2019, 10, 3348. [Google Scholar] [CrossRef] [PubMed]
- Turner, E.C. Possible poriferan body fossils in early Neoproterozoic microbial reefs. Nature 2021, 596, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Pisera, A.; Łukowiak, M.; Masse, S.; Tabachnick, K.; Fromont, J.; Ehrlich, H.; Bertolino, M. Insights into the structure and morphogenesis of the giant basal spicule of the glass sponge Monorhaphis chuni. Front. Zool. 2021, 18, 58. [Google Scholar]
- Dendy, A. The tetraxonid sponge-spicule: A study in evolution. Acta Zool. 1921, 2, 95–152. [Google Scholar] [CrossRef]
- Uriz, M.J.; Turon, X.; Becerro, M.A.; Agell, G. Siliceous spicules and skeleton frameworks in sponges: Origin, diversity, ultrastructural patterns, and biological functions. Microsc. Res. Tech. 2003, 62, 279–299. [Google Scholar] [CrossRef]
- Cárdenas, P. Surface Microornamentation of Demosponge Sterraster Spicules, Phylogenetic and Paleontological Implications. Front. Mar. Sci. 2020, 7, 613610. [Google Scholar] [CrossRef]
- Łukowiak, M.; Van Soest, R.; Klautau, M.; Pérez, T.; Pisera, A.; Tabachnick, K. The terminology of sponge spicules. J. Morphol. 2022, 283, 1517–1548. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, S.S.; Kiørboe, T.; Larsen, P.S.; Leys, S.P.; Yahel, G.; Walther, J.H. Hydrodynamics of sponge pumps and evolution of the sponge body plan. eLife 2020, 9, e61012. [Google Scholar] [CrossRef]
- Tabachnick, K.R. Adaptation of the Hexactinellid Sponges to Deep-Sea Life. In Fossil and Recent Sponges; Reitner, J., Keupp, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 378–386. [Google Scholar]
- Aizenberg, J.; Weaver, J.C.; Thanawala, M.S.; Sundar, V.C.; Morse, D.E.; Fratzl, P. Materials science: Skeleton of Euplectella sp.: Structural hierarchy from the nanoscale to the macroscale. Science 2005, 309, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Deutzmann, R.; Brunner, E.; Cappellini, E.; Koon, H.; Solazzo, C.; Yang, Y.; Ashford, D.; Thomas-Oates, J.; Lubeck, M.; et al. Mineralization of the metre-long biosilica structures of glass sponges is templated on hydroxylated collagen. Nat. Chem. 2010, 2, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Silica biomineralization, Sponges. In Encyclopedia of Geobiology. Encyclopedia of Earth Sciences Series; Springer: Dordrecht, The Netherlands, 2011; pp. 796–808. [Google Scholar]
- Ehrlich, H.; Brunner, E.; Simon, P.; Bazhenov, V.V.; Botting, J.P.; Tabachnick, K.R.; Springer, A.; Kummer, K.; Vyalikh, D.V.; Molodtsov, S.L.; et al. Calcite reinforced silica-silica joints in the biocomposite skeleton of deep-sea glass sponges. Adv. Funct. Mater. 2011, 21, 3473–3481. [Google Scholar] [CrossRef]
- Dendy, A. Studies on the Comparative Anatomy of Sponges; Kessinger Publishing: Whitefish, MT, USA, 1888; p. 274. [Google Scholar]
- Bidder, G. The origin of sponge spicules. Nature 1925, 115, 298–299. [Google Scholar] [CrossRef]
- Schoeppler, V.; Reich, E.; Vacelet, J.; Rosenthal, M.; Pacureanu, A.; Rack, A.; Zaslansky, P.; Zolotoyabko, E.; Zlotnikov, I. Shaping highly regular glass architectures: A lesson from nature. Sci. Adv. 2017, 3, aao2047. [Google Scholar] [CrossRef]
- Voronkina, A.; Romanczuk-Ruszuk, E.; Przekop, R.E.; Lipowicz, P.; Gabriel, E.; Heimler, K.; Rogoll, A.; Vogt, C.; Frydrych, M.; Wienclaw, P.; et al. Honeycomb Biosilica in Sponges: From Under-standing Principles of Unique Hierarchical Organization to Assessing Biomimetic Potential. Biomimetics 2023, 8, 234. [Google Scholar] [CrossRef]
- Shimizu, K.; Nishi, M.; Sakate, Y.; Kawanami, H.; Bito, T.; Arima, J.; Leria, L.; Maldonado, M. Silica-associated proteins from hexactinellid sponges support an alternative evolutionary scenario for biomineralization in Porifera. Nat. Commun. 2024, 15, 181. [Google Scholar] [CrossRef]
- Ehrlich, H.; Luczak, M.; Ziganshin, R.; Mikšík, I.; Wysokowski, M.; Simon, P.; Baranowska-Bosiacka, I.; Kupnicka, P.; Ereskovsky, A.; Galli, R.; et al. Arrested in Glass: Actin within Sophisticated Architectures of Biosilica in Sponges. Adv. Sci. 2022, 9, 2105059. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Actin and the realization of unique biosilica-based architectures in sponges. In 11th World Sponge Conference. Book of Abstracts; Naturalis Biodiversity Center: Leiden, The Netherlands, 10 October 2022. [Google Scholar]
- Tesson, B.; Hildebrand, M. Extensive and intimate association of the cytoskeleton with forming silica in diatoms: Control over patterning on the meso- and micro-scale. PLoS ONE 2010, 5, 14300. [Google Scholar] [CrossRef] [PubMed]
- Tesson, B.; Hildebrand, M. Dynamics of silica cell wall morphogenesis in the diatom Cyclotella cryptica: Substructure formation and the role of microfilaments. J. Struct. Biol. 2010, 169, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Povarova, N.V.; Barinov, N.A.; Baranov, M.S.; Markina, N.M.; Varizhuk, A.M.; Pozmogova, G.E.; Klinov, D.V.; Kozhemyako, V.B.; Lukyanov, K.A. Efficient silica synthesis from tetra(glycerol)orthosilicate with cathepsin- and silicatein-like proteins. Sci. Rep. 2018, 8, 16759. [Google Scholar] [CrossRef] [PubMed]
- Talevski, T.; Talevska Leshoska, A.; Pejoski, E.; Pejin, B.; Machałowski, T.; Wysokowski, M.; Tsurkan, M.V.; Petrova, O.; Sivkov, V.; Martinovic, R.; et al. Identification and first insights into the structure of chitin from the endemic freshwater demosponge Ochridaspongia rotunda (Arndt, 1937). Int. J. Biol. Macromol. 2020, 162, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Pospich, S.; Merino, F.; Raunser, S. Structural Effects and Functional Implications of Phalloidin and Jasplakinolide Binding to Actin Filaments. Structure 2020, 28, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Melak, M.; Plessner, M.; Grosse, R. Actin visualization at a glance. J. Cell Sci. 2017, 130, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.L.; Cassimeris, L.; Fechheimer, M.; Zigmond, S.H. Mechanisms responsible for F-actin stabilization after lysis of polymorphonuclear leukocytes. J. Cell Biol. 1992, 116, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, K.; Black, B.; Mohanty, S. Introduction of impermeable actin-staining molecules to mammalian cells by optoporation. Sci. Rep. 2014, 4, 6553. [Google Scholar] [CrossRef]
- Romani, M.; Auwerx, J. Phalloidin staining of actin filaments for visualization of muscle fibers in Caenorhabditis elegans. Bio-Protocol 2021, 11, e4183. [Google Scholar]
- Frigeri, L.G.; Radabaugh, T.R.; Haynes, P.A.; Hildebrand, M. Identification of proteins from a cell wall fraction of the diatom Thalassiosira pseudonana: Insights into silica structure formation. Mol. Cell Proteom. 2006, 5, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tu, S.; Gong, W.; Tao, W.; Tang, M.; Wei, Y.; Kuang, C.; Liu, X.; Zhang, Y.H.; Hao, X. Three-dimensional multi-color optical nanoscopy at sub-10-nm resolution based on small-molecule organic probes. Cell Rep. Methods 2023, 3, 100556. [Google Scholar] [CrossRef]
- Manconi, R.; Pronzato, R. Suborder Spongillina subord. nov.: Freshwater Sponges. In Systema Porifera; Hooper, J.N.A., Van Soest, R.W.M., Willenz, P., Eds.; Springer: Boston, MA, USA, 2002. [Google Scholar]
- Morrow, C.; Cárdenas, P. Proposal for a revised classification of the Demospongiae (Porifera). Front. Zool. 2015, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Krasko, A.; Le Pennec, G.; Steffen, R.; Wiens, M.; Ammar, M.S.A.; Müller, I.M.; Schröder, H.C. Molecular mechanism of spicule formation in the demosponge Suberites domuncula: Silicatein-collagen-myotrophin. Regul. Altern. Splic. 2003, 33, 195–221. [Google Scholar]
- Müller, W.E.; Boreiko, A.; Wang, X.; Belikov, S.I.; Wiens, M.; Grebenjuk, V.A.; Schlossmacher, U.; Schröder, H.C. Silicateins, the major biosilica forming enzymes present in demosponges: Protein analysis and phylogenetic relationship. Gene 2007, 395, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Rouiller, I.; Xu, X.P.; Amann, K.J.; Egile, C.; Nickell, S.; Nicastro, D.; Li, R.; Pollard, T.D.; Volkmann, N.; Hanein, D. The structural basis of actin filament branching by the Arp2/3 complex. J. Cell Biol. 2008, 180, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, P.; Rapp, H.T.; Klitgaard, A.B.; Best, M.; Thollesson, M.; Tendal, O.S. Taxonomy, biogeography and DNA barcodes of Geodia species (Porifera, Demospongiae, Tetractinellida) in the Atlantic boreo-arctic region. Zool. J. Linn. Soc. 2013, 169, 251–311. [Google Scholar] [CrossRef]
- Tabachnick, K.; Janussen, D.; Menshenina, L. Cold Biosilicification in Metazoan: Psychrophilic Glass Sponges. In Extreme Biomimetics; Ehrlich, H., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 53–80. [Google Scholar]
- Dohrmann, M.; Kelley, C.; Kelly, M.; Pisera, A.; Hooper, J.N.A.; Reiswig, H.M. An integrative systematic framework helps to reconstruct skeletal evolution of glass sponges (Porifera, Hexactinellida). Front. Zool. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C.; Milliron, G.W.; Allen, P.; Miserez, A.; Rawal, A.; Garay, J.; Morse, D.E. Unifying Design Strategies in Demosponge and Hexactinellid Skeletal Systems. J. Adhes. 2010, 86, 72–95. [Google Scholar] [CrossRef]
- Fraccaroli, A.; Franco, C.A.; Rognoni, E.; Neto, F.; Rehberg, M.; Aszodi, A.; Wedlich-Söldner, R.; Pohl, U.; Gerhardt, H.; Montanez, E. Visualization of endothelial actin cytoskeleton in the mouse retina. PLoS ONE 2012, 7, e47488. [Google Scholar] [CrossRef]
- Kalil, K.; Li, L.; Hutchins, B.I. Signaling mechanisms in cortical axon growth, guidance, and branching. Front. Neuroanat. 2011, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Svitkina, T.M.; Borisy, G.G. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 1999, 145, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Kalman, D.; Gomperts, S.N.; Hardy, S.; Kitamura, M.; Bishop, J.M. Ras family GTPases control growth of astrocyte processes. Mol. Biol. Cell 1999, 10, 1665–1683. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Parreno, J.; Nowak, R.B.; Biswas, S.K.; Wang, K.; Hoshino, M.; Uesugi, K.; Yagi, N.; Moncaster, J.A.; Lo, W.K.; et al. Age-related changes in eye lens biomechanics, morphology, refractive index and transparency. Aging 2019, 11, 12497–12531. [Google Scholar] [CrossRef] [PubMed]
- Francis, W.R.; Eitel, M.; Vargas, S.; Garcia-Escudero, C.A.; Conci, N.; Deister, F.; Mah, J.L.; Guiglielmoni, N.; Krebs, S.; Blum, H.; et al. The genome of the reef-building glass sponge Aphrocallistes vastus provides insights into silica biomineralization. R. Soc. Open Sci. 2023, 10, 230423. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.; Schenkelaars, Q.; Jourda, C.; Duchesne, M.; Belahbib, H.; Rocher, C.; Selva, M.; Riesgo, A.; Vervoort, M.; Leys, S.P.; et al. The compact genome of the sponge Oopsacas minuta (Hexactinellida) is lacking key metazoan core genes. BMC Biol. 2023, 21, 139. [Google Scholar] [CrossRef]
- Moscatelli, A.; Idilli, A.I.; Rodighiero, S.; Caccianiga, M. Inhibition of actin polymerisation by low concentration Latrunculin B affects endocytosis and alters exocytosis in shank and tip of tobacco pollen tubes. Plant Biol. 2012, 14, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Blanchoin, L.; Mullins, R.D. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 545–576. [Google Scholar] [CrossRef] [PubMed]
- Martiel, J.L.; Michelot, A.; Boujemaa-Paterski, R.; Blanchoin, L.; Berro, J. Force Production by a Bundle of Growing Actin Filaments Is Limited by Its Mechanical Properties. Biophys. J. 2020, 118, 39. [Google Scholar] [CrossRef]
- Castaneda, N.; Park, J.; Kang, E.H. Regulation of Actin Bundle Mechanics and Structure by Intracellular Environmental Factors. Front. Phys. 2021, 9, 675885. [Google Scholar] [CrossRef]
- Chen, X.; Roeters, S.J.; Cavanna, F.; Alvarado, J.; Baiz, C.R. Crowding alters F-actin secondary structure and hydration. Commun. Biol. 2023, 6, 900. [Google Scholar] [CrossRef] [PubMed]
- Tilney, L.G.; Tilney, M.S.; Guild, G.M. F actin bundles in Drosophila bristles I. Two filament cross-links are involved in bundling. J. Cell Biol. 1995, 130, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Tilney, L.G.; Connelly, P.S.; Vranich, K.A.; Shaw, M.K.; Guild, G.M. Regulation of actin filament cross-linking and bundle shape in Drosophila bristles. J. Cell Biol. 2000, 148, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Guild, G.M.; Connelly, P.S.; Ruggiero, L.; Vranich, K.A.; Tilney, L.G. Actin filament bundles in Drosophila wing hairs: Hairs and bristles use different strategies for assembly. Mol. Biol. Cell 2005, 16, 3620–3631. [Google Scholar] [CrossRef] [PubMed]
- Belin, B.J.; Goins, L.M.; Mullins, R.D. Comparative analysis of tools for live cell imaging of actin network architecture. Bioarchitecture 2014, 4, 189–202. [Google Scholar] [CrossRef]
- Schaefer, A.W.; Kabir, N.; Forscher, P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J. Cell Biol. 2002, 158, 139–152. [Google Scholar] [CrossRef]
- Danuser, G.; Oldenbourg, R. Probing f-actin flow by tracking shape fluctuations of radial bundles in lamellipodia of motile cells. Biophys. J. 2000, 79, 191–201. [Google Scholar] [CrossRef]
- Pochitaloff, M.; Miranda, M.; Richard, M.; Chaiyasitdhi, A.; Takagi, Y.; Cao, W.; De La Cruz, E.M.; Sellers, J.R.; Joanny, J.F.; Jülicher, F.; et al. Flagella-like beating of actin bundles driven by self-organized myosin waves. Nat. Phys. 2022, 18, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Hammar, K.; Smith, P.J.S.; Oldenbourg, R. Arrangement of radial actin bundles in the growth cone of Aplysia bag cell neurons shows the immediate past history of filopodial behavior. Proc. Natl. Acad. Sci. USA 1999, 96, 7928–7931. [Google Scholar] [CrossRef]
- Katoh, K.; Hammar, K.; Smith, P.J.S.; Oldenbourg, R. Birefringence imaging directly reveals architectural dynamics of filamentous actin in living growth cones. Mol. Biol. Cell 1999, 10, 197–210. [Google Scholar] [CrossRef]
- Oldenbourg, R.; Katoh, K.; Danuser, G. Mechanism of lateral movement of filopodia and radial actin bundles across neuronal growth cones. Biophys. J. 2000, 78, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Andjus, S.; Tubić, B.; Vasiljević, B.; Nikolić, V.; Paunović, M. Anomalies of Sponge Spicules: Exploring Links to Environmental Pollution. Water 2024, 16, 332. [Google Scholar] [CrossRef]
- de Voogd, N.J.; Alvarez, B.; Boury-Esnault, N.; Cárdenas, P.; Díaz, M.-C.; Dohrmann, M.; Downey, R.; Goodwin, C.; Hajdu, E.; Hooper, J.N.A.; et al. World Porifera Database. Homoscleromorpha. 2024. Available online: http://www.marinespecies.org/porifera/index.php (accessed on 23 May 2024).
- Gazave, E.; Lapébie, P.; Renard, E.; Vacelet, J.; Rocher, C.; Ereskovsky, A.V.; Cárdenas, P.; Borchiellini, C. No longer Demospongiae: Homoscleromorpha formal nomination as a fourth class of Porifera. Hydrobiologia 2012, 687, 3–10. [Google Scholar] [CrossRef]
- Ruiz, C.; Ereskovsky, A.; Pérez, T. New Skeleton-Less Homoscleromorphs (Porifera, Homoscleromorpha) from the Caribbean Sea: Exceptions to Rules Are Definitely Common in Sponge Taxonomy. Zootaxa 2022, 5200, 128–148. [Google Scholar] [CrossRef]
- Stillitani, D.; Ereskovsky, A.V.; Pérez, T.; Ruiz, C.; Laport, M.S.; Puccinelli, G.; Hardoim, C.C.P.; Willenz, P.; Muricy, G. Solving a taxonomic puzzle: Integrative taxonomy reveals new cryptic and polymorphic species of Oscarella (Homoscleromorpha: Oscarellidae). Invertebr. Syst. 2022, 36, 714–750. [Google Scholar] [CrossRef]
- Van de Meene, A.M.L.; Pickett-Heaps, J.D. Valve morphogenesis in the centric diatom Rhizosolenia setigera (Bacillariophyceae, Centrales) and its taxonomic implications. Eur. J. Phycol. 2004, 39, 93–104. [Google Scholar] [CrossRef]
- Durak, G.M.; Brownlee, C.; Wheeler, G.L. The role of the cytoskeleton in biomineralisation in haptophyte algae. Sci. Rep. 2017, 7, 15409. [Google Scholar] [CrossRef]
- Manning, L. Mimicking Biosintering: The Identification of Highly Condensed Surfaces in Bioinspired Silica Materials. Langmuir 2021, 37, 561–568. [Google Scholar] [CrossRef]
- He, M.; Li, Y.; Yin, J.; Sun, Q.; Xiong, W.; Li, S.; Yang, L. Compressive performance and fracture mechanism of bio-inspired heterogeneous glass sponge lattice structures manufactured by selective laser melting. Mater. Des. 2022, 214, 110396. [Google Scholar] [CrossRef]
- Chen, H.; Jia, Z.; Li, L. Lightweight lattice-based skeleton of the sponge Euplectella aspergillum: On the multifunctional design. J. Mech. Behav. Biomed. Mater. 2022, 135, 105448. [Google Scholar] [CrossRef]
- Chen, H. Multiscale Structures and Mechanics of Biomineralized Lattices in Hexactinellid sponges and Echinoderms. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2023. [Google Scholar]
- Fernandes, M.C.; Aizenberg, J.; Weaver, J.C.; Bertoldi, K. Mechanically robust lattices inspired by deep-sea glass sponges. Nat. Mater. 2021, 20, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh, S.; Mahdavinejad, M.; Habib, F. Bio-inspiration from sponge for high-performance building. Naqshejahan 2023, 13, 86–101. [Google Scholar]
- Sarikaya, M.; Fong, H.; Sunderland, N.; Flinn, B.D.; Mayer, G.; Mescher, A.; Gaino, E. Biomimetic model of a sponge-spicular optical fiber—Mechanical properties and structure. J. Mater. Res. 2001, 16, 1420–1428. [Google Scholar] [CrossRef]
- Aizenberg, J.; Sundar, V.C.; Yablon, A.D.; Weaver, J.C.; Chen, G. Biological glass fibers: Correlation between optical and structural properties. Proc. Natl. Acad. Sci. USA 2004, 101, 3358–3363. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Maldonado, M.; Parker, A.R.; Kulchin, Y.N.; Schilling, J.; Köhler, B.; Skrzypczak, U.; Simon, P.; Reiswig, H.M.; Tsurkan, M.V.; et al. Supercontinuum Generation in Naturally Occurring Glass Sponges Spicules. Adv. Opt. Mater. 2016, 4, 1608–1613. [Google Scholar] [CrossRef]
- Zhang, X.; Luan, Y.; Li, Y.; Wang, Z.; Li, Z.; Xu, F.; Guo, Z. Bioinspired design of lightweight laminated structural materials and the intralayer/interlayer strengthening and toughening mechanisms induced by the helical structure. Compos. Struct. 2021, 276, 114575. [Google Scholar] [CrossRef]
- Zhang, X.; Luan, Y.; Li, Y.; Wang, Z.; Li, Z.; Xu, F.; Guo, Z.Z. Unveiling the mechanics of deep-sea sponge-inspired tubular metamaterials: Exploring bending, radial, and axial mechanical behavior. Thin-Walled Struct. 2024, 196, 111476. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Zhang, X.; Xu, F.; Guo, Z.Z. Crashworthiness design of a sponge-inspired multicell tube under axial crushing. Int. J. Mech. Sci. 2023, 244, 108070. [Google Scholar] [CrossRef]
- Morankar, S.; Sundar Sundaram Singaravelu, A.; Niverty, S.; Mistry, Y.; Penick, C.A.; Bhate, D.; Chawla, N. Tensile and fracture behavior of silica fibers from the Venus flower basket (Euplectella aspergillum). Int. J. Solids Struct. 2022, 253, 111622. [Google Scholar] [CrossRef]
- Morankar, S.K.; Mistry, Y.; Bhate, D.; Penick, C.A.; Chawla, N. In situ investigations of failure mechanisms of silica fibers from the venus flower basket (Euplectella Aspergillum). Acta Biomater. 2023, 162, 304–311. [Google Scholar] [CrossRef]
- Tavangarian, F.; Sadeghzade, S.; Davami, K. A novel biomimetic design inspired by nested cylindrical structures of spicules. J. Alloys Compd. 2021, 864, 158197. [Google Scholar] [CrossRef]
- Xiao, H. Nested structure role in the mechanical response of spicule inspired fibers. Bioinspir. Biomim. 2024, 19, 046008. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, Y.; Li, J.; Chen, P.; Liu, Y. Artificial intelligence-enhanced bioinspiration: Design of optimized mechanical lattices beyond deep-sea sponges. Extreme Mech. Lett. 2023, 62, 102033. [Google Scholar] [CrossRef]
- Sharma, D.; Hiremath, S.S. In-plane elastic properties of the Euplectella aspergillum inspired lattice structures: Analytic modelling, finite element modelling and experimental validation. Structures 2023, 48, 962–975. [Google Scholar] [CrossRef]
- Yang, Z.; Xue, J.; Li, T.; Zhai, D.; Yu, X.; Huan, Z.; Wu, C. 3D printing of sponge spicules-inspired flexible bioceramic-based scaffolds. Biofabrication 2022, 14, 035009. [Google Scholar] [CrossRef] [PubMed]
- Robson Brown, K.; Bacheva, D.; Trask, R.S. The structural efficiency of the sea sponge Euplectella aspergillum skeleton: Bio-inspiration for 3D printed architectures. J. R. Soc. Interface 2019, 16, 965. [Google Scholar] [CrossRef] [PubMed]
- Tavangarian, F.; Sadeghzade, S.; Fani, N.; Khezrimotlagh, D.; Davami, K. 3D-printed bioinspired spicules: Strengthening and toughening via stereolithography. J. Mech. Behav. Biomed. Mater. 2024, 136, 105485. [Google Scholar] [CrossRef]
- Martins, E.; Rapp, H.T.; Xavier, J.R.; Diogo, G.S.; Reis, R.L.; Silva, T.H. Macro and Microstructural Characteristics of North Atlantic Deep-Sea Sponges as Bioinspired Models for Tissue Engineering Scaffolding. Front. Mar. Sci. 2021, 7, 613647. [Google Scholar] [CrossRef]

| Biomimetic Directions | References |
|---|---|
| Mimicking biosintering | [75] |
| Bioinspired selective laser melting | [76] |
| Bio-based generative honeycomb design | [22] |
| Multifunctional design | [77,78] |
| Bioinspired architecture: next generation of high-performance buildings, skyscrapers, and bridges | [79,80] |
| Biomimetics of lightweight structural biomaterials | [15,81,82,83,84] |
| Deep-sea sponge-inspired tubular metamaterials | [85] |
| Crashworthiness design of a sponge-inspired multicell tube | [86] |
| Architectured ceramic fibers and damage evolution | [87,88] |
| Computational modeling of spicule-inspired nested structures | [89,90] |
| Artificial intelligence-enhanced bioinspiration | [91] |
| Analytic modeling, finite element modeling, and experimental validation | [92] |
| 3D printing of sponge spicule-inspired flexible bioceramic-based scaffolds | [93,94] |
| Bioinspired stereolithography | [95] |
| Bioinspired models for tissue engineering scaffolding | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehrlich, H.; Voronkina, A.; Tabachniсk, K.; Kubiak, A.; Ereskovsky, A.; Jesionowski, T. Silactins and Structural Diversity of Biosilica in Sponges. Biomimetics 2024, 9, 393. https://doi.org/10.3390/biomimetics9070393
Ehrlich H, Voronkina A, Tabachniсk K, Kubiak A, Ereskovsky A, Jesionowski T. Silactins and Structural Diversity of Biosilica in Sponges. Biomimetics. 2024; 9(7):393. https://doi.org/10.3390/biomimetics9070393
Chicago/Turabian StyleEhrlich, Hermann, Alona Voronkina, Konstantin Tabachniсk, Anita Kubiak, Alexander Ereskovsky, and Teofil Jesionowski. 2024. "Silactins and Structural Diversity of Biosilica in Sponges" Biomimetics 9, no. 7: 393. https://doi.org/10.3390/biomimetics9070393











