Effects of Incorporating Ionic Crosslinking on 3D Printing of Biomass–Fungi Composite Materials
Abstract
:1. Introduction
2. Methodology
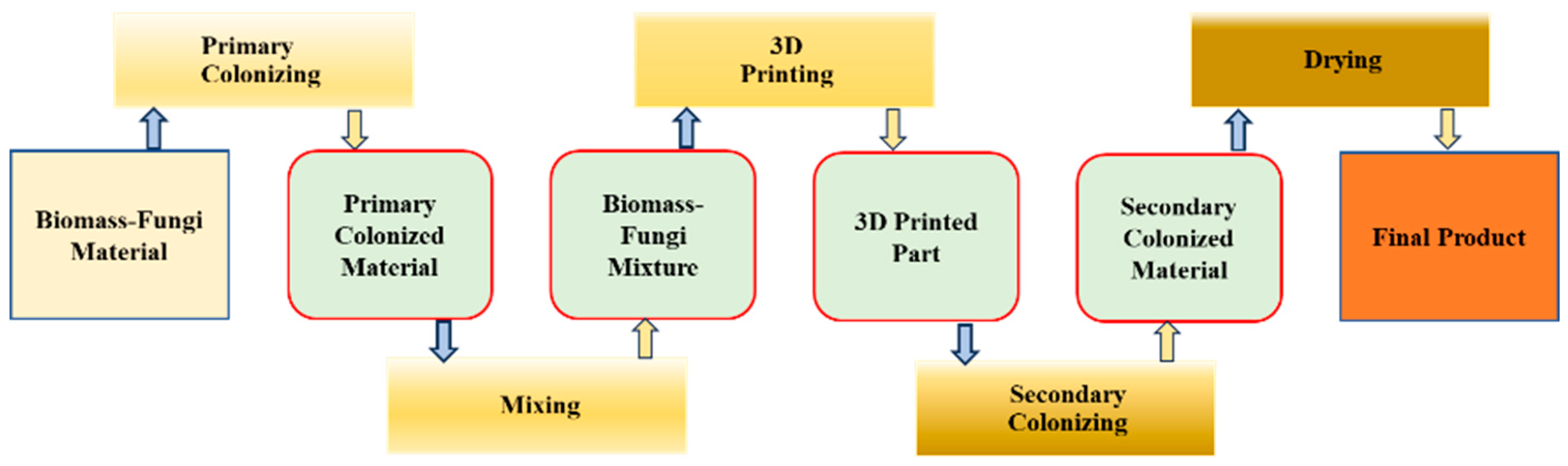
2.1. Procurement of Materials
2.2. Preparation of Biomass–Fungi Mixtures with Different SA Concentrations
2.2.1. Preparation of Primary Colonized Material
2.2.2. Preparation of SA Solutions
2.2.3. Preparation of Biomass–Fungi Mixtures with Different SA Concentrations
2.3. Preparation of CaCl2 Crosslinking Agent
2.4. 3D Printing
2.5. Crosslinking
2.6. Assessment Methods for Quality of 3D Printed Samples
2.6.1. Geometric Accuracy of 3D Printed Samples
2.6.2. Height Shrinkage of the 3D Printed Samples
2.7. Measurements of Rheological Properties of Biomass–Fungi Mixtures
2.8. Measurements of Chemical Properties of 3D Printed Samples
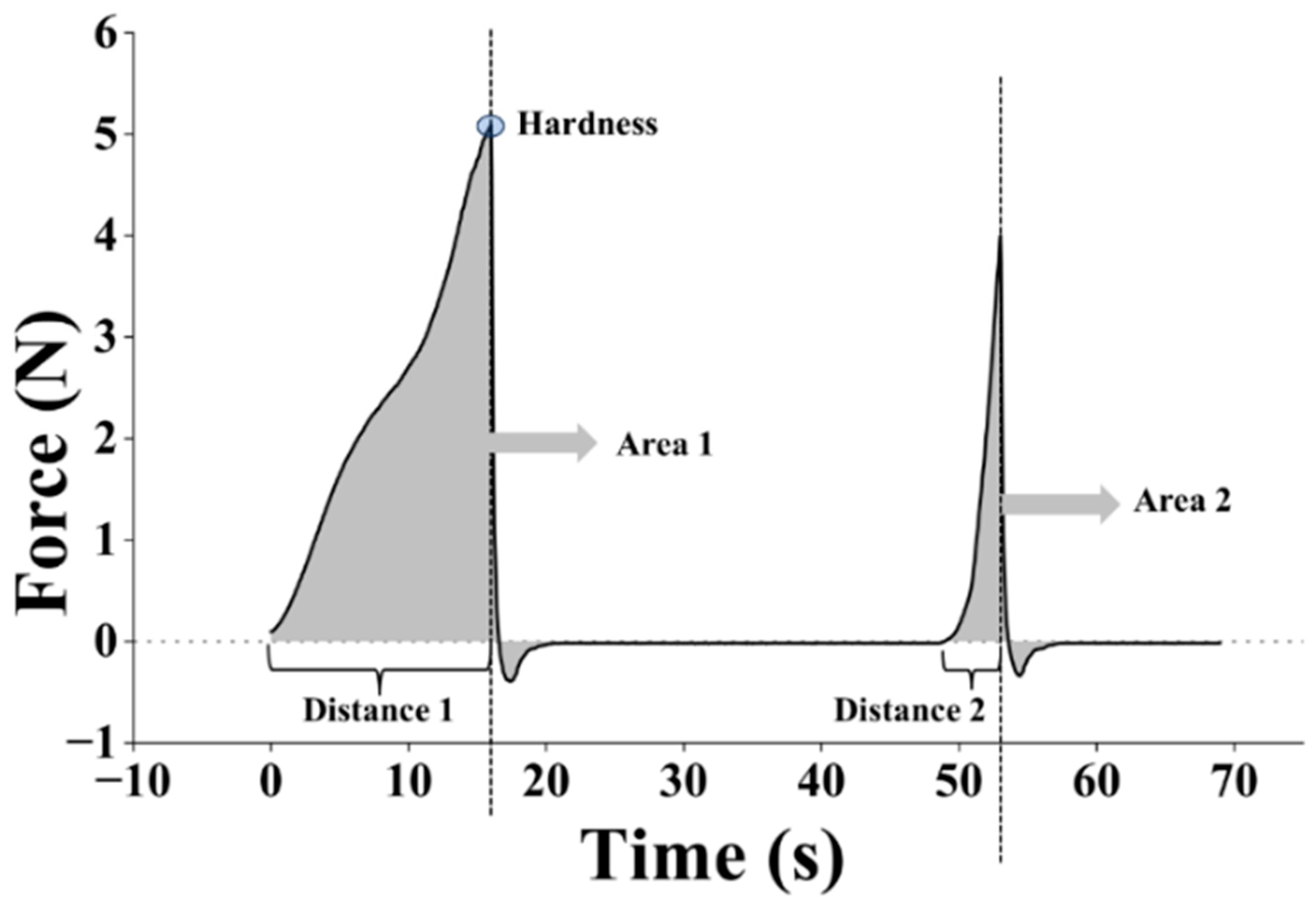
2.9. Measurements of Textural Properties of Biomass–Fungi Mixtures
2.10. Statistical Analysis and Data Processing
3. Results and Discussion
3.1. Effects of SA Concentration and Crosslinking Exposure Time on Quality of 3D Printed Samples
3.1.1. Geometric Accuracy of 3D Printed Samples
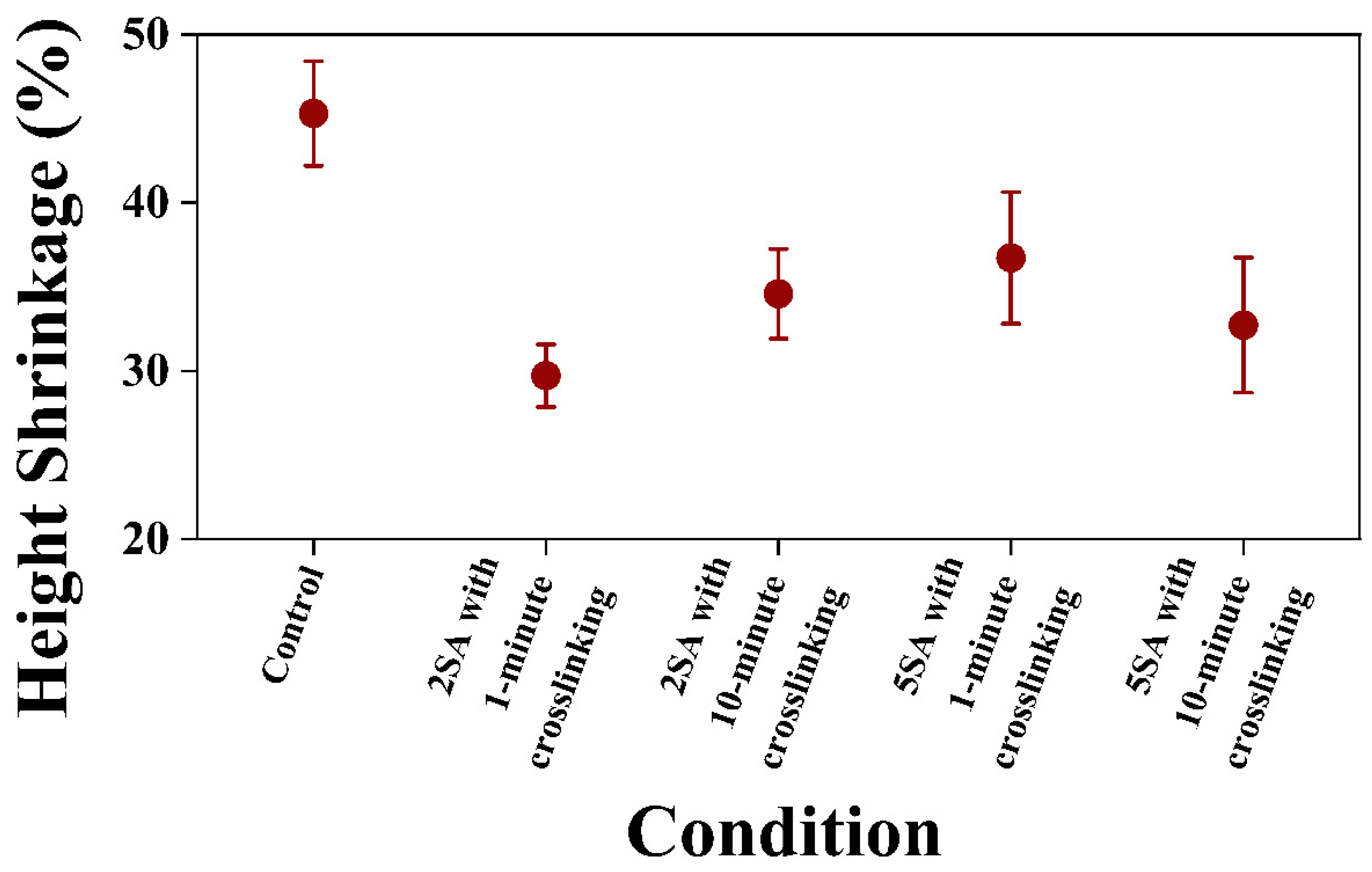
3.1.2. Height Shrinkage of 3D Printed Samples
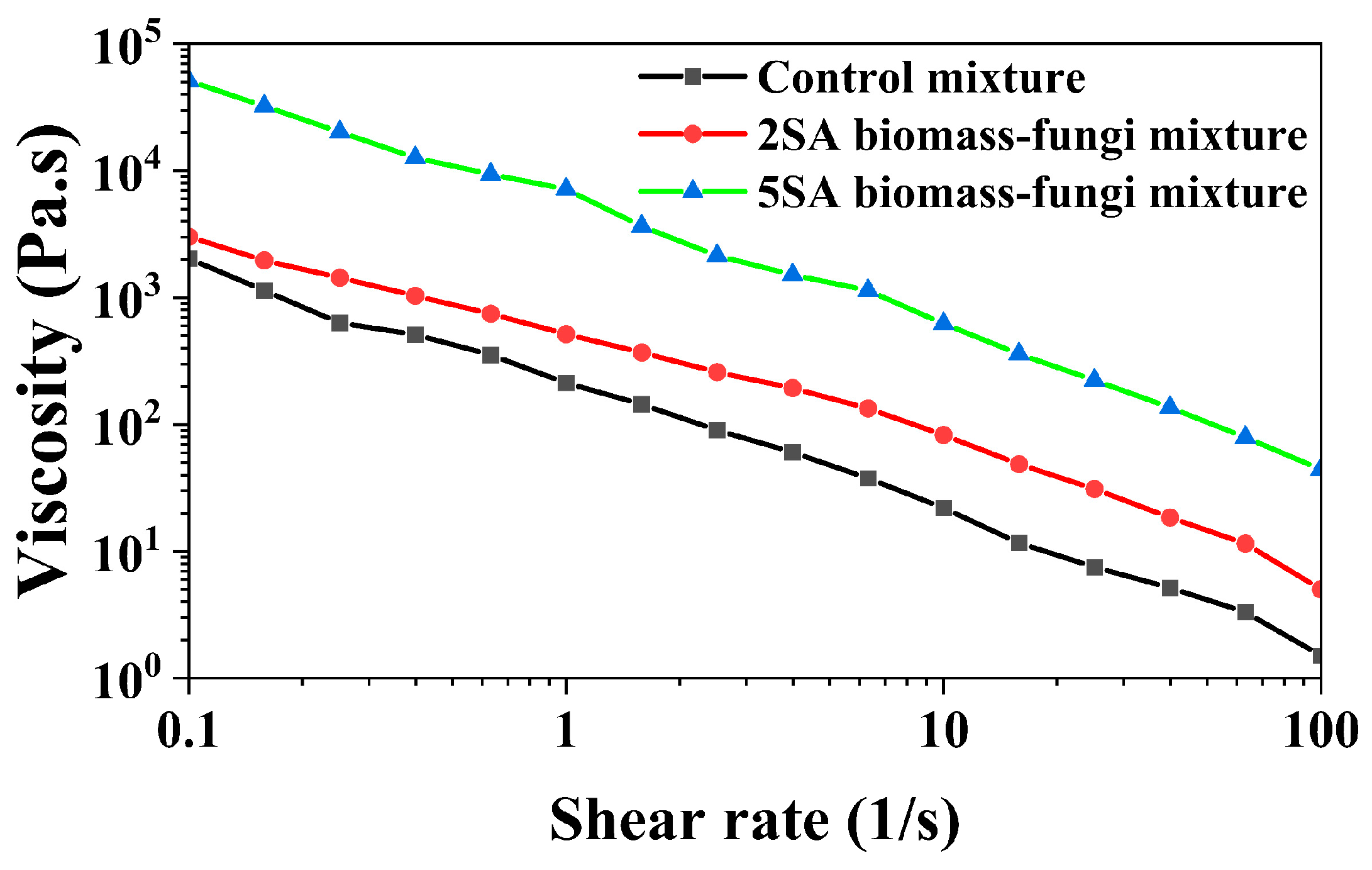
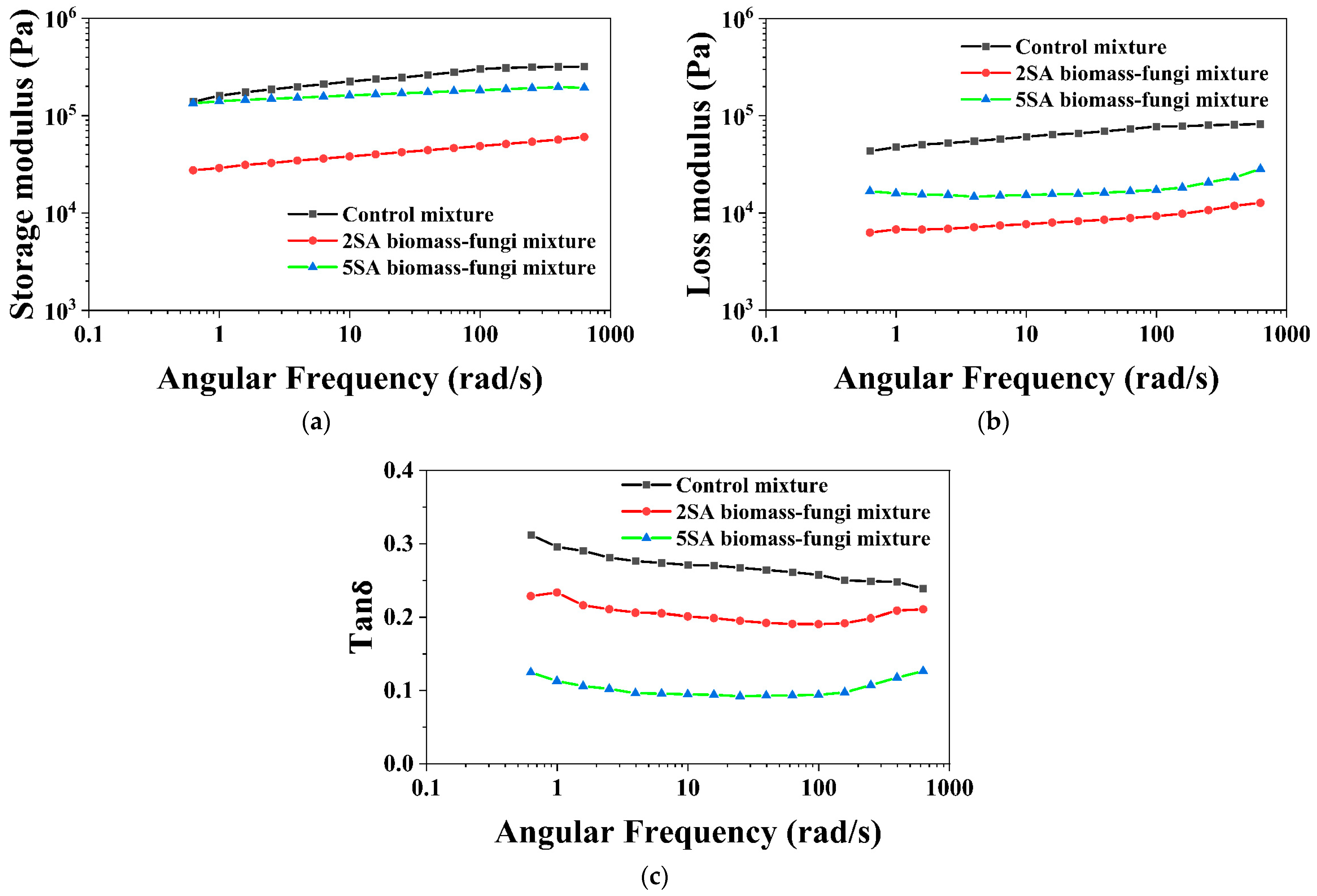
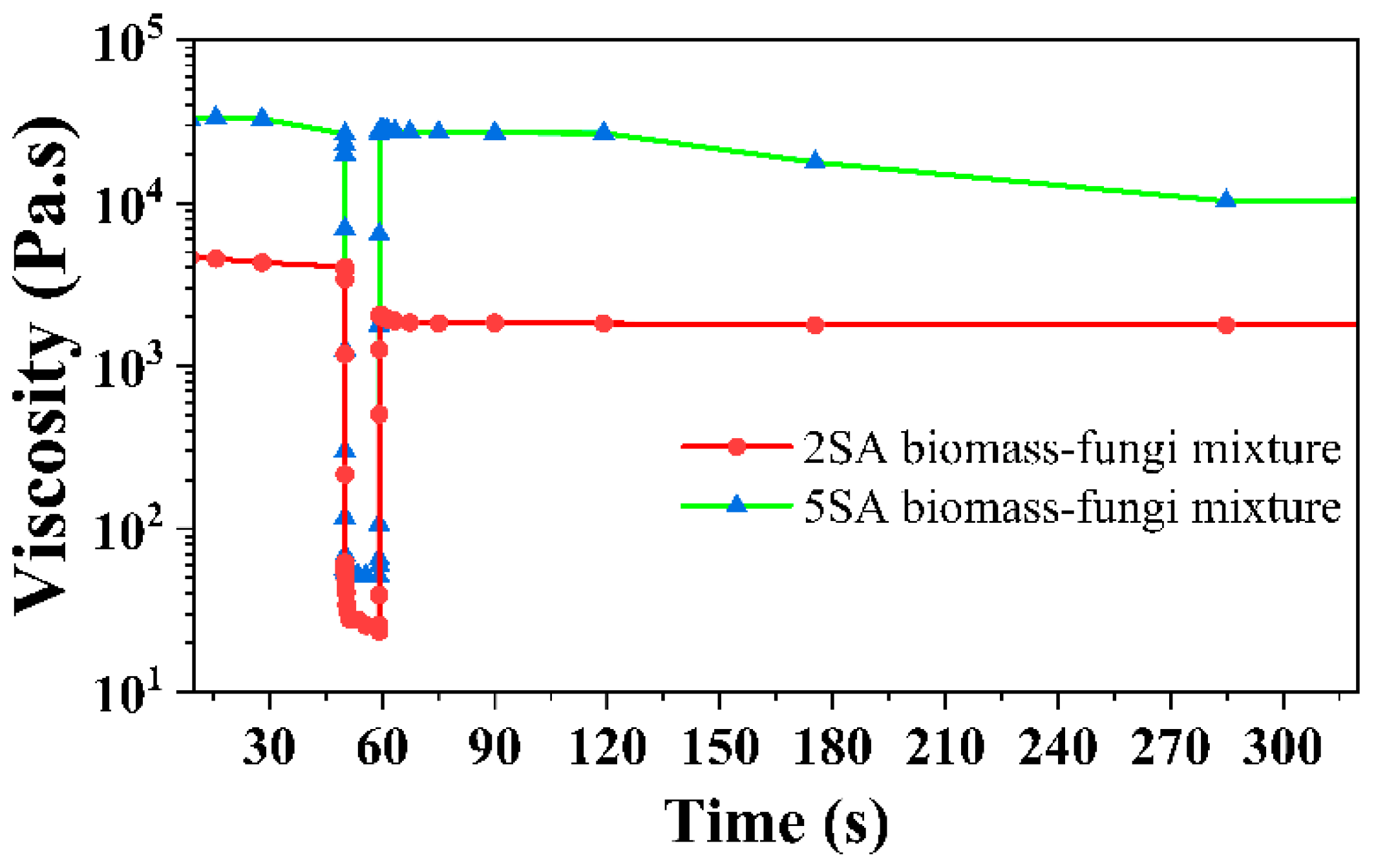
3.2. Effects of SA Concentration on Rheological Properties of Biomass–Fungi Mixtures
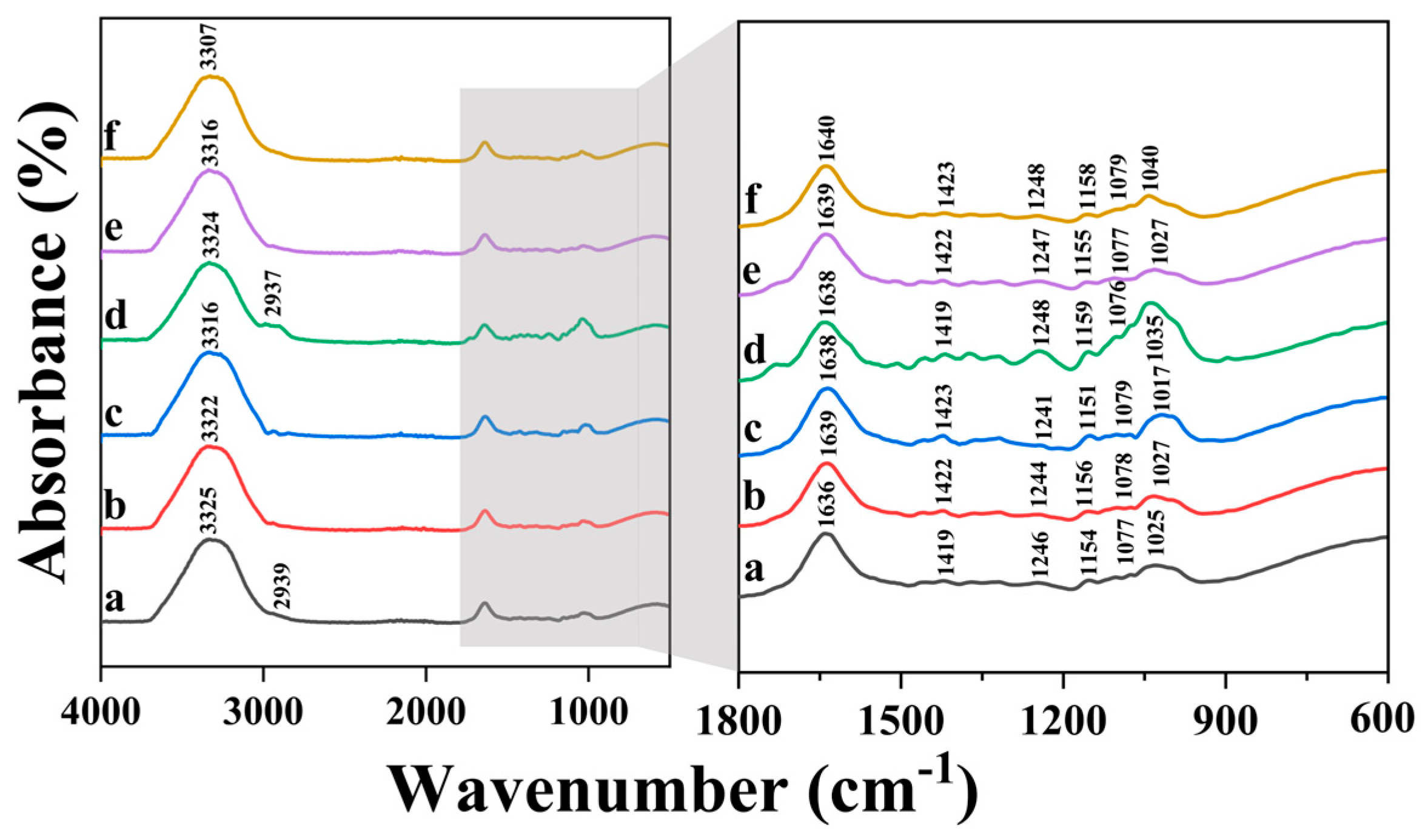
3.3. Effects of SA Concentration and Crosslinking Exposure Time on Chemical Properties of 3D Printed Samples
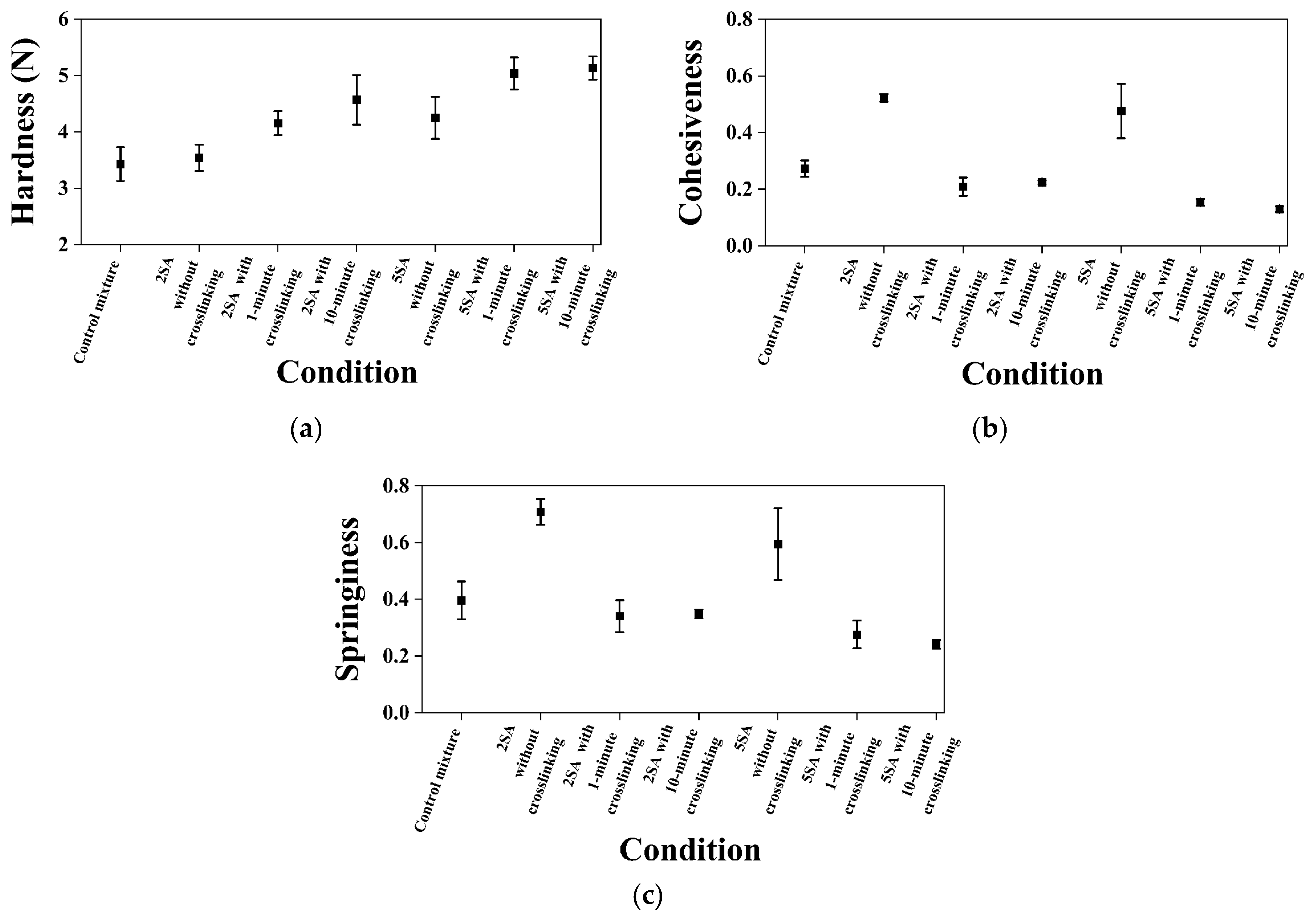
3.4. Effects of SA Concentration and Crosslinking Exposure Time on Textural Properties of Biomass–Fungi Mixtures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soh, E.; Chew, Z.Y.; Saeidi, N.; Javadian, A.; Hebel, D.; Le Ferrand, H. Development of an extrudable paste to build mycelium-bound composites. Mater. Des. 2020, 195, 109058. [Google Scholar] [CrossRef]
- Ecovative Design. Available online: https://grow.bio/pages/grow-it-yourself-education-and-instruction-docs (accessed on 2 July 2023).
- Abhijith, R.; Ashok, A.; Rejeesh, C. Sustainable packaging applications from mycelium to substitute polystyrene: A review. Mater. Today Proc. 2018, 5, 2139–2145. [Google Scholar] [CrossRef]
- Holt, G.A.; Mcintyre, G.; Flagg, D.; Bayer, E.; Wanjura, J.; Pelletier, M. Fungal mycelium and cotton plant materials in the manufacture of biodegradable molded packaging material: Evaluation study of select blends of cotton byproducts. J. Biobased Mater. Bioenergy 2012, 6, 431–439. [Google Scholar] [CrossRef]
- Rahman, A.M.; Rahman, T.T.; Pei, Z.; Ufodike, C.O.; Lee, J.; Elwany, A. Additive Manufacturing Using Agriculturally Derived Biowastes: A Systematic Literature Review. Bioengineering 2023, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Elsacker, E.; Vandelook, S.; Van Wylick, A.; Ruytinx, J.; De Laet, L.; Peeters, E. A comprehensive framework for the production of mycelium-based lignocellulosic composites. Sci. Total Environ. 2020, 725, 138431. [Google Scholar] [CrossRef] [PubMed]
- Ecovative at MoMA PS1. Available online: https://www.bfi.org/2014/07/28/ecovative-at-moma-ps1/ (accessed on 28 July 2014).
- Gandia, A.; van den Brandhof, J.G.; Appels, F.V.; Jones, M.P. Flexible fungal materials: Shaping the future. Trends Biotechnol. 2021, 39, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Pelletier, M.; Holt, G.; Wanjura, J.; Lara, A.; Tapia-Carillo, A.; McIntyre, G.; Bayer, E. An evaluation study of pressure-compressed acoustic absorbers grown on agricultural by-products. Ind. Crops Prod. 2017, 95, 342–347. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Rahman, A.M.; Wei, X.; Pei, Z.; Truong, D.; Lucht, M.; Zou, N. 3D Printing of Biomass–Fungi Composite Material: Effects of Mixture Composition on Print Quality. J. Manuf. Mater. Process. 2021, 5, 112. [Google Scholar] [CrossRef]
- Elsacker, E.; Peeters, E.; De Laet, L. Large-scale robotic extrusion-based additive manufacturing with living mycelium materials. Sustain. Futures 2022, 4, 100085. [Google Scholar] [CrossRef]
- Ghazvinian, A. A Sustainable Alternative to Architectural Materials: Mycelium-Based Bio-Composites. Proc. Divergence Archit. Res. Atlanta GA USA 2021, 15, 159–167. [Google Scholar]
- Ghazvinian, A.; Gursoy, B. Basics of Building with Mycelium-Based Bio-Composites: A Review of Built Projects and Related Material Research. J. Green Build. 2022, 17, 37–69. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced materials from fungal mycelium: Fabrication and tuning of physical properties. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hasan, K.F.; Horváth, P.G.; Zsolt, K.; Alpár, T. Design and Fabrication Technology in Biocomposite Manufacturing, in Value-Added Biocomposites; CRC Press: Boca Raton, FL, USA, 2021; pp. 157–188. [Google Scholar]
- Bhardwaj, A.; Vasselli, J.; Lucht, M.; Pei, Z.; Shaw, B.; Grasley, Z.; Wei, X.; Zou, N. 3D Printing of Biomass-Fungi Composite Material: A Preliminary Study. Manuf. Lett. 2020, 24, 96–99. [Google Scholar] [CrossRef]
- Rahman, A.M.; Bhardwaj, A.; Pei, Z.; Ufodike, C.; Castell-Perez, E. The 3D Printing of Biomass-Fungi Composites: Effects of Waiting Time after Mixture Preparation on Mechanical Properties, Rheological Properties, Minimum Extrusion Pressure, and Print Quality of the Prepared Mixture. J. Compos. Sci. 2022, 6, 237. [Google Scholar] [CrossRef]
- Mohseni, A.; Vieira, F.R.; Pecchia, J.A.; Gürsoy, B. Three-Dimensional Printing of Living Mycelium-Based Composites: Material Compositions, Workflows, and Ways to Mitigate Contamination. Biomimetics 2023, 8, 257. [Google Scholar] [CrossRef]
- Soh, E.; Teoh, J.H.; Leong, B.; Xing, T.; Le Ferrand, H. 3D printing of mycelium engineered living materials using a waste-based ink and non-sterile conditions. Mater. Des. 2023, 236, 112481. [Google Scholar] [CrossRef]
- Ghazvinian, A.; Gürsoy, B. Mycelium-based composite graded materials: Assessing the effects of time and substrate mixture on mechanical properties. Biomimetics 2022, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.M.; Bhardwaj, A.; Vasselli, J.G.; Pei, Z.; Shaw, B.D. Three-Dimensional Printing of Biomass–Fungi Biocomposite Materials: The Effects of Mixing and Printing Parameters on Fungal Growth. J. Manuf. Mater. Process. 2023, 8, 2. [Google Scholar] [CrossRef]
- Panchal, N.; Patel, D.; Shah, N. Synthesis of hydrogels. In Proceedings of the 4th International Conference on Multidisciplinary Research & Practice (4ICMRP-2017), Ahmedabad, India, 22 December 2017. [Google Scholar]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Chen, H.; Abdullayev, A.; Bekheet, M.F.; Schmidt, B.; Regler, I.; Pohl, C.; Vakifahmetoglu, C.; Czasny, M.; Kamm, P.H.; Meyer, V.; et al. Extrusion-based additive manufacturing of fungal-based composite materials using the tinder fungus Fomes fomentarius. Fungal Biol. Biotechnol. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Liu, S.; Bastola, A.K.; Li, L. A 3D Printable and Mechanically Robust Hydrogel Based on Alginate and Graphene Oxide. ACS Appl. Mater. Interfaces 2017, 9, 41473–41481. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.; Singh, A.; Sudha; Sahay, N.S.; Sharma, J.; Roy, A.; Kumari, M.M.; Rana, D.; Thakran, S.; Deka, D.; et al. Piriformospora indica: An Axenically Culturable Mycorrhiza-Like Endosymbiotic Fungus. In Fungal Associations; Hock, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 125–150. [Google Scholar]
- Rahman, A.M.; Bedsole, C.O.; Akib, Y.M.; Hamilton, J.; Rahman, T.T.; Shaw, B.D.; Pei, Z. Effects of Sodium Alginate and Calcium Chloride on Fungal Growth and Viability in Biomass-Fungi Composite Materials Used for 3D Printing. Biomimetics 2024, 9, 251. [Google Scholar] [CrossRef]
- Shaw, B.D.; Hoch, H.C. Ions as Regulators of Growth and Development, in Biology of the Fungal Cell; Springer: Berlin/Heidelberg, Germany, 2001; pp. 73–89. [Google Scholar]
- Kokova, V.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Delattre, C.; Molinié, R.; Petit, E.; Elboutachfaiti, R.; Murdjeva, M.; Apostolova, E. Extraction, Structural Characterization, and In Vivo Anti-Inflammatory Effect of Alginate from Cystoseira crinita (Desf.) Borry Harvested in the Bulgarian Black Sea. Mar. Drugs 2023, 21, 245. [Google Scholar] [CrossRef]
- Thakare, K.; Jerpseth, L.; Pei, Z.; Qin, H. Applying Layer-by-layer Photo-crosslinking in Green Bioprinting: Shape Fidelity and Cell Viability of Printed Hydrogel Constructs Containing Algae Cells. J. Manuf. Sci. Eng. 2022, 144, 094502. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Liu, C.; Regenstein, J.M.; Liu, X.; Zhou, P. Rheological and mechanical behavior of milk protein composite gel for extrusion-based 3D food printing. LWT 2019, 102, 338–346. [Google Scholar] [CrossRef]
- Richard, E.; Chang, T.-h.; Sutherland, J.W. Statistical Quality Design and Control; Prentice Hall: Englewood Cliffs, NJ, USA, 1992. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S. Experimental Designs Using ANOVA; Thomson/Brooks/Cole: Belmont, CA, USA, 2007; Volume 724. [Google Scholar]
- Godoi, F.C.; Prakash, S.; Bhandari, B.R. 3D printing technologies applied for food design: Status and prospects. J. Food Eng. 2016, 179, 44–54. [Google Scholar] [CrossRef]
- Lille, M.; Nurmela, A.; Nordlund, E.; Metsä-Kortelainen, S.; Sozer, N. Applicability of protein and fiber-rich food materials in extrusion-based 3D printing. J. Food Eng. 2018, 220, 20–27. [Google Scholar] [CrossRef]
- Huan, S.; Mattos, B.D.; Ajdary, R.; Xiang, W.; Bai, L.; Rojas, O.J. Two-phase emulgels for direct ink writing of skin-bearing architectures. Adv. Funct. Mater. 2019, 29, 1902990. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, T.; Duan, S.; Qin, Z.; Li, C.; Zhang, Z.; Liu, A.; Wu, D.; Chen, H.; Han, G.; et al. Effects of sodium alginate and rice variety on the physicochemical characteristics and 3D printing feasibility of rice paste. LWT 2020, 127, 109360. [Google Scholar] [CrossRef]
- Chen, H.; Xie, F.; Chen, L.; Zheng, B. Effect of rheological properties of potato, rice and corn starches on their hot-extrusion 3D printing behaviors. J. Food Eng. 2019, 244, 150–158. [Google Scholar] [CrossRef]
- Studart, A.R. Additive manufacturing of biologically-inspired materials. Chem. Soc. Rev. 2016, 45, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Hecht, F.M.; Rheinlaender, J.; Schierbaum, N.; Goldmann, W.H.; Fabry, B.; Schäffer, T.E. Imaging viscoelastic properties of live cells by AFM: Power-law rheology on the nanoscale. Soft Matter 2015, 11, 4584–4591. [Google Scholar] [CrossRef] [PubMed]
- Bahcegul, E.G.; Bahcegul, E.; Ozkan, N. 3D printing of crude lignocellulosic biomass extracts containing hemicellulose and lignin. Ind. Crops Prod. 2022, 186, 115234. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, M.; Devahastin, S. Improvement of 3D printability of buckwheat starch-pectin system via synergistic Ca2+-microwave pretreatment. Food Hydrocoll. 2021, 113, 106483. [Google Scholar] [CrossRef]
- Walayat, N.; Xiong, Z.; Xiong, H.; Moreno, H.M.; Li, Q.; Nawaz, A.; Zhang, Z.; Wang, P.; Niaz, N. The effectiveness of egg white protein and β-cyclodextrin during frozen storage: Functional, rheological and structural changes in the myofibrillar proteins of Culter alburnus. Food Hydrocoll. 2020, 105, 105842. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Z.; Liu, Y.; Zhao, Y.; Yuan, X.; Kang, Z.; Zhu, M.; Ma, H. High-pressure processing influences the conformation, water distribution, and gel properties of pork myofibrillar proteins containing Artemisia sphaerocephala Krasch gum. Food Chem. X 2022, 14, 100320. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Joshua, A.; Ding, L.; Wang, B.; Feng, X.; Mao, Z.; Xu, H.; Sui, X. Rheology of regenerated cellulose suspension and influence of sodium alginate. Int. J. Biol. Macromol. 2020, 148, 811–816. [Google Scholar] [CrossRef]
- Sang, S.; Ou, C.; Fu, Y.; Su, X.; Jin, Y.; Xu, X. Complexation of fish skin gelatin with glutentin and its effect on the properties of wheat dough and bread. Food Chem. X 2022, 14, 100319. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, M.; Devahastin, S. Color/aroma changes of 3D-Printed buckwheat dough with yellow flesh peach as triggered by microwave heating of gelatin-gum Arabic complex coacervates. Food Hydrocoll. 2021, 112, 106358. [Google Scholar] [CrossRef]
- Sritham, E.; Gunasekaran, S. FTIR spectroscopic evaluation of sucrose-maltodextrin-sodium citrate bioglass. Food Hydrocoll. 2017, 70, 371–382. [Google Scholar] [CrossRef]
- Xu, Y.; Zhan, C.; Fan, L.; Wang, L.; Zheng, H. Preparation of dual crosslinked alginate–chitosan blend gel beads and in vitro controlled release in oral site-specific drug delivery system. Int. J. Pharm. 2007, 336, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Badita, C.; Aranghel, D.; Burducea, C.; Mereuta, P. Characterization of sodium alginate based films. Rom. J. Phys 2020, 65, 1–8. [Google Scholar]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.; Mendes, A.; Bártolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Breene, W.M. Application of texture profile analysis to instrumental food texture evaluation. J. Texture Stud. 1975, 6, 53–82. [Google Scholar] [CrossRef]
- Friedman, H.H.; Whitney, J.E.; Szczesniak, A.S. The texturometer—A new instrument for objective texture measurement. J. Food Sci. 1963, 28, 390–396. [Google Scholar] [CrossRef]
- Henry, W.F.; Katz, M.H.; Pilgrim, F.J.; May, A.T. Texture of semi-solid foods: Sensory and physical correlates. J. Food Sci. 1971, 36, 155–161. [Google Scholar] [CrossRef]
- Szczesniak, A.S. Textural characterization of temperature sensitive foods. J. Texture Stud. 1975, 6, 139–156. [Google Scholar] [CrossRef]
- Steffe, J.F. Rheological Methods in Food Process Engineering; Freeman Press: New York, NY, USA, 1996. [Google Scholar]
- Fermin, B.C.; Hahm, T.; Radinsky, J.A.; Kratochvil, R.J.; Hall, J.E.; Lo, Y.M. Effect of proline and glutamine on the functional properties of wheat dough in winter wheat varieties. J. Food Sci. 2005, 70, E273–E278. [Google Scholar] [CrossRef]
- Andrade, L.; Andrade, C.; Dias, M.; Nascimento, C.; Mendes, M. Chlorella and spirulina microalgae as sources of functional foods. Nutraceuticals Food Suppl. 2018, 6, 45–58. [Google Scholar]
- Pavlath, A.; Gossett, C.; Camirand, W.; Robertson, G. Ionomeric films of alginic acid. J. Food Sci. 1999, 64, 61–63. [Google Scholar] [CrossRef]
- Overview of TPA. Available online: https://texturetechnologies.com/resources/texture-profile-analysis (accessed on 12 May 2024).
- Lau, M.; Tang, J.; Paulson, A. Texture profile and turbidity of gellan/gelatin mixed gels. Food Res. Int. 2000, 33, 665–671. [Google Scholar] [CrossRef]


| Same Day after Printing | 5 Days after Printing | ||||||
|---|---|---|---|---|---|---|---|
| Condition | Photograph | Height Deviation (%) | Length Deviation (%) | Photograph | Height Deviation (%) | Length Deviation (%) | Observation |
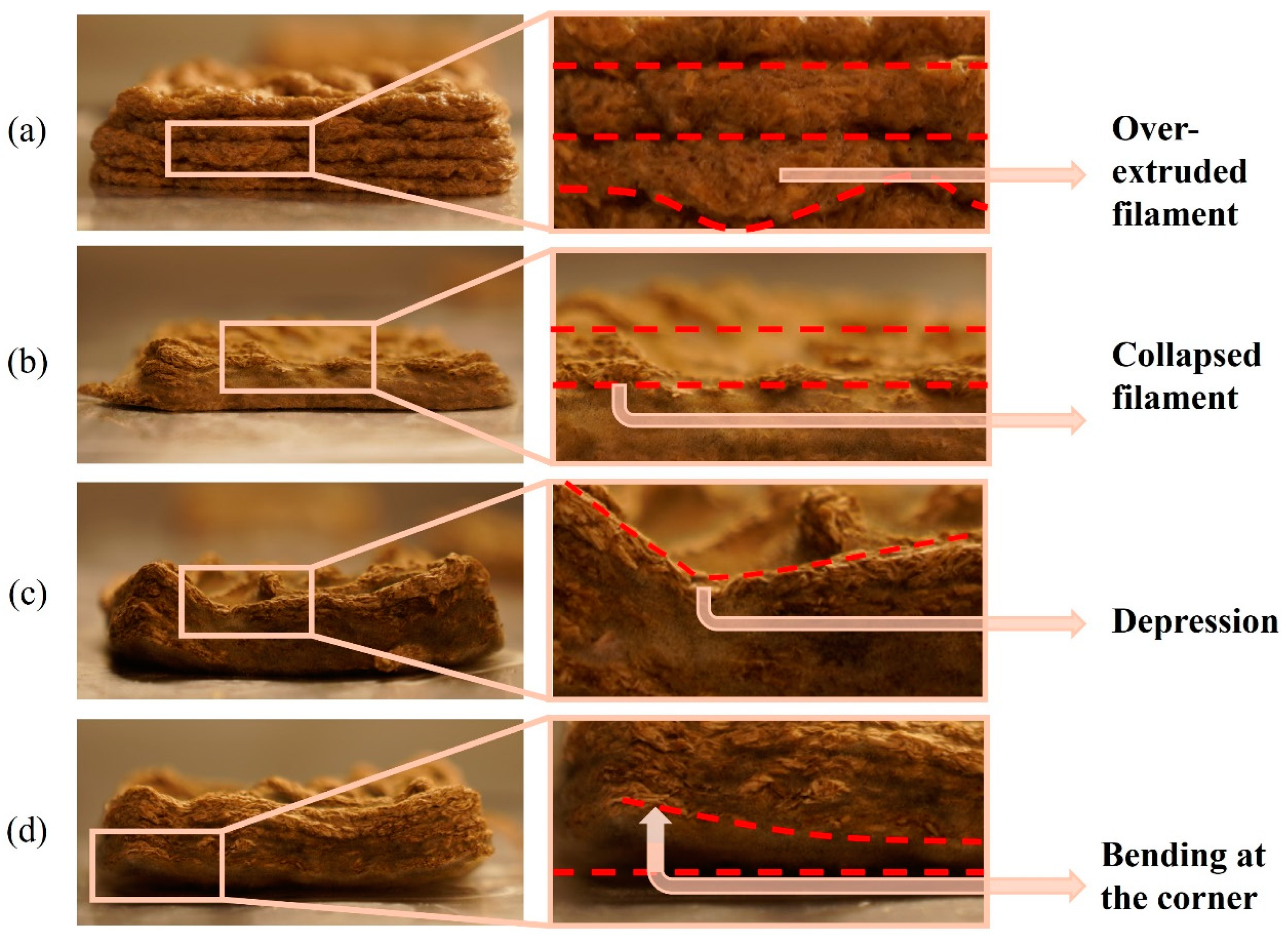
| 0SA biomass–fungi mixture (control mixture) without crosslinking |  | 15.43 ± 5.48 | 2.72 ± 0.94 |  | 53.73 ± 17.04 | 7.44 ± 2.12 | The bottom layers became wider as the subsequent layers were printed. After 5 days, the structure collapsed at some corners. |
| 2SA biomass–fungi mixture with 1-min crosslinking |  | 17.82 ± 1.96 | 3.12 ± 2.42 |  | 42.26 ± 11.28 | 16.2 ± 2.81 | Good geometric accuracy, and no depressions observed. |
| 2SA biomass–fungi mixture with 10-min crosslinking |  | 15.28 ± 1.17 | 5.45 ± 3.39 |  | 44.58 ± 10.77 | 18.32 ± 2.30 | Good geometric accuracy initially, but after 5 days, depressions were observed in a few areas. |
| 5SA biomass–fungi mixture with 1-min crosslinking |  | 26.14 ± 6.92 | 5.02 ± 1.45 |  | 53.42 ± 12.24 | 9.44 ± 3.09 | Good geometric accuracy initially, but after 5 days, the printed samples bent in some corners, and a few depressions were observed. |
| 5SA biomass–fungi mixture with 10-min crosslinking |  | 21.22 ± 2.42 | 10.41 ± 2.03 |  | 46.97 ± 13.68 | 15.18 ± 3.51 | Good geometric accuracy initially, but after 5 days, significant depressions were observed, and printed samples bent in all the corners. |
| Mixture | K (Pa·s) | n | R2 | η (Pa·s) (at 10 s−1) |
|---|---|---|---|---|
| Control mixture | 169 | −0.066 | 0.9572 | 21.986 |
| 2SA Biomass–fungi mixture | 2503 | −0.172 | 0.9971 | 133.894 |
| 5SA Biomass–fungi mixture | 5566 | 0.039 | 0.9985 | 623.684 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, A.M.; Akib, Y.M.; Bedsole, C.O.; Pei, Z.; Shaw, B.D.; Ufodike, C.O.; Castell-Perez, E. Effects of Incorporating Ionic Crosslinking on 3D Printing of Biomass–Fungi Composite Materials. Biomimetics 2024, 9, 411. https://doi.org/10.3390/biomimetics9070411
Rahman AM, Akib YM, Bedsole CO, Pei Z, Shaw BD, Ufodike CO, Castell-Perez E. Effects of Incorporating Ionic Crosslinking on 3D Printing of Biomass–Fungi Composite Materials. Biomimetics. 2024; 9(7):411. https://doi.org/10.3390/biomimetics9070411
Chicago/Turabian StyleRahman, Al Mazedur, Yeasir Mohammad Akib, Caleb Oliver Bedsole, Zhijian Pei, Brian D. Shaw, Chukwuzubelu Okenwa Ufodike, and Elena Castell-Perez. 2024. "Effects of Incorporating Ionic Crosslinking on 3D Printing of Biomass–Fungi Composite Materials" Biomimetics 9, no. 7: 411. https://doi.org/10.3390/biomimetics9070411
APA StyleRahman, A. M., Akib, Y. M., Bedsole, C. O., Pei, Z., Shaw, B. D., Ufodike, C. O., & Castell-Perez, E. (2024). Effects of Incorporating Ionic Crosslinking on 3D Printing of Biomass–Fungi Composite Materials. Biomimetics, 9(7), 411. https://doi.org/10.3390/biomimetics9070411









