The Interaction between Oral Bacteria and 3D Titanium Porous Surfaces Produced by Selective Laser Melting—A Narrative Review
Abstract
:1. Introduction
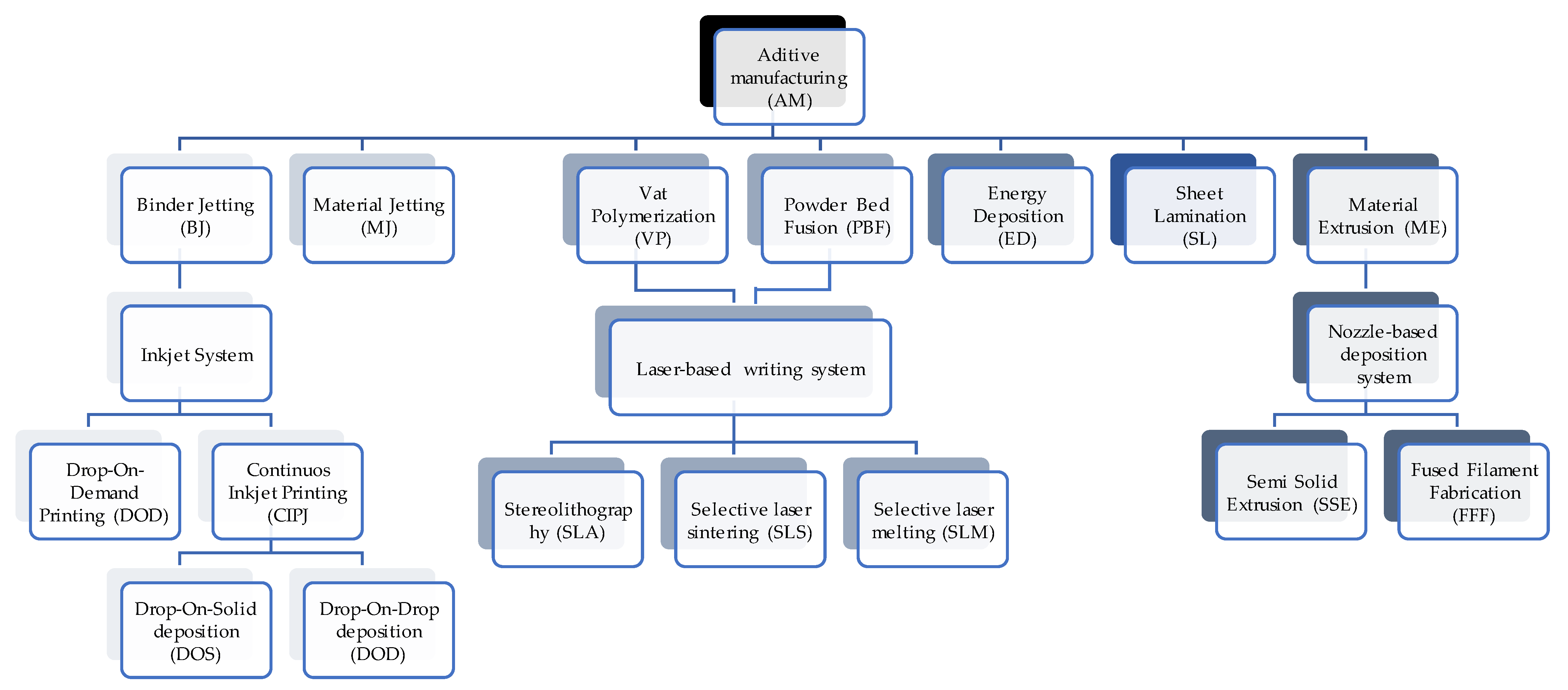
2. Selective Laser Melting (SLM) Technique
3. Factors Influencing Bacterial Adhesion to Titanium
4. Prevention and Treatment Strategies
4.1. Surface Topography and Porous Structure
4.2. Surface Roughness and Biofilm Formation
4.3. Surface Treatment Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Elhadad, A.A.; Rosa-Sainz, A.; Cañete, R.; Peralta, E.; Begines, B.; Balbuena, M.; Alcudia, A.; Torres, Y. Applications and Multidisciplinary Perspective on 3D Printing Techniques: Recent Developments and Future Trends. Mater. Sci. Eng. R Rep. 2023, 156, 156. [Google Scholar] [CrossRef]
- Galati, M.; Minetola, P. Analysis of Density, Roughness, and Accuracy of the Atomic Diffusion Additive Manufacturing (ADAM) Process for Metal Parts. Materials 2019, 12, 4122. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. Mechanical Performance of Additively Manufactured Meta-Biomaterials. Acta Biomater. 2019, 85, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.C.; Palmer, P.; Ji, H.-F.; Ehrlich, G.D.; Król, J.E. Bacterial Biofilm Growth on 3D-Printed Materials. Front. Microbiol. 2021, 12, 646303. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Liaw, C.Y.; Guvendiren, M. Current and Emerging Applications of 3D Printing in Medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Lin, G. 3D Printing Technology and Its Application in Industrial Manufacturing. IOP Conf. Ser. Mater. Sci. Eng. 2020, 782, 022065. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Zhu, S.; Shi, Y. Microstructural, Mechanical and In Vitro Biological Properties of Ti6Al4V-5Cu Alloy Fabricated by Selective Laser Melting. Mater. Charact. 2023, 200, 112858. [Google Scholar] [CrossRef]
- Ni, J.; Ling, H.; Zhang, S.; Wang, Z.; Peng, Z.; Benyshek, C.; Zan, R.; Miri, A.K.; Li, Z.; Zhang, X.; et al. Three-Dimensional Printing of Metals for Biomedical Applications. Mater. Today Bio. 2019, 3, 100024. [Google Scholar] [CrossRef]
- D’Ercole, S.; Mangano, C.; Cellini, L.; Di Lodovico, S.; Ozkaya, C.A.; Iezzi, G.; Piattelli, A.; Petrini, M. A Novel 3D Titanium Surface Produced by Selective Laser Sintering to Counteract Streptococcus Oralis Biofilm Formation. Appl. Sci. 2021, 11, 11915. [Google Scholar] [CrossRef]
- Revilla-León, M.; Sadeghpour, M.; Özcan, M. A Review of the Applications of Additive Manufacturing Technologies Used to Fabricate Metals in Implant Dentistry. J. Prosthodont. 2020, 29, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Gardan, J. Additive Manufacturing Technologies: State of the Art and Trends. Int. J. Prod. Res. 2015, 54, 3118–3132. [Google Scholar] [CrossRef]
- Gallab, M.; Le, P.T.M.; Shintani, S.A.; Takadama, H.; Ito, M.; Kitagaki, H.; Matsushita, T.; Honda, S.; Okuzu, Y.; Fujibayashi, S.; et al. Mechanical, Bioactive, and Long-Lasting Antibacterial Properties of a Ti Scaffold with Gradient Pores Releasing Iodine Ions. Biomater. Adv. 2024, 158, 213781. [Google Scholar] [CrossRef] [PubMed]
- Hindy, A.; Farahmand, F.; Pourdanesh, F.; Torshabi, M.; Al Janabi, A.H.; Rasoulianboroujeni, M.; Tayebi, L.; Tabatabaei, F.S. Synthesis and Characterization of 3D-Printed Functionally Graded Porous Titanium Alloy. J. Mater. Sci. 2020, 55, 9082–9084. [Google Scholar] [CrossRef]
- Abate, K.M.; Nazir, A.; Jeng, J.Y. A Short Review on Cellular Structure Design and Selective Laser Melting Using Bio-Compatible Ti6Al4V Material. Biomed. J. Sci. Tech. Res. 2020, 27, 20519–20523. [Google Scholar] [CrossRef]
- Manea, A.; Bran, S.; Baciut, M.; Armencea, G.; Pop, D.; Berce, P.; Vodnar, D.-C.; Hedesiu, M.; Dinu, C.; Petrutiu, A.; et al. Sterilization Protocol for Porous Dental Implants Made by Selective Laser Melting. Med. Pharm. Rep. 2018, 91, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, R.; Lopes, S.I.; Prashanth, K.G. Selective Laser Melting and Spark Plasma Sintering: A Perspective on Functional Biomaterials. J. Funct. Biomater. 2023, 14, 521. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M.; Traini, T.; Sinjari, B.; Nostro, A.; Caputi, S.; Cellini, L. Porphyromonas Gingivalis Biofilm Formation in Different Titanium Surfaces, an In Vitro Study. Clin. Oral Implant. Res. 2016, 27, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.M.; Rezaienia, A.; Wen, P.; Condoor, S.; Parkar, N.; King, W.; Korakianitis, T. Medical Applications for 3D Printing: Recent Developments. Mo. Med. 2018, 115, 75–81. [Google Scholar]
- Sun, Z. 3D Printing in Medicine: Current Applications and Future Directions. Quant. Imaging Med. Surg. 2018, 8, 1069–1077. [Google Scholar] [CrossRef]
- Kollath, V.O.; Chen, Q.; Mullens, S.; Luyten, J.; Traina, K.; Boccaccini, A.R.; Cloots, R. Electrophoretic Deposition of Hydroxyapatite and Hydroxyapatite–Alginate on Rapid Prototyped 3D Ti6Al4V Scaffolds. J. Mater. Sci. 2016, 51, 2338–2346. [Google Scholar] [CrossRef]
- Nagarajan, B.; Hu, Z.; Song, X.; Zhai, W.; Wei, J. Development of Micro Selective Laser Melting: The State of the Art and Future Perspectives. Engineering 2019, 5, 702–720. [Google Scholar] [CrossRef]
- Kawalkar, R.; Dubey, H.K.; Lokhande, S.P. A review for advancements in standardization for additive manufacturing. Mater Today: Proceedings. 2022, 50, 1983–1990. [Google Scholar] [CrossRef]
- Iezzi, G.; Zavan, B.; Petrini, M.; Ferroni, L.; Pierfelice, T.V.; D’Amora, U.; Ronca, A.; D’Amico, E.; Mangano, C. 3D Printed Dental Implants with a Porous Structure: The In Vitro Response of Osteoblasts, Fibroblasts, Mesenchymal Stem Cells, and Monocytes. J. Dent. 2024, 140, 104778. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Amirthalingam, S.; Kim, S.L.; Lee, S.S.; Rangasamy, J.; Hwang, N.S. Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-Dimensional (3D) Printed Scaffold and Material Selection for Bone Repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Ahsan, M.N.; Paul, C.P.; Kukreja, L.M.; Pinkerton, A.J. Porous Structures Fabrication by Continuous and Pulsed Laser Metal Deposition for Biomedical Applications; Modelling and Experimental Investigation. J. Mater. Process. Technol. 2011, 211, 602–609. [Google Scholar] [CrossRef]
- Chen, M.; Li, H.; Wang, X.; Qin, G.; Zhang, E. Improvement in Antibacterial Properties and Cytocompatibility of Titanium by Fluorine and Oxygen Dual Plasma-Based Surface Modification. Appl. Surf. Sci. 2019, 463, 261–274. [Google Scholar] [CrossRef]
- Barros, R.N.; de Gouvêa, C.V.D. Prophylactic Agents and Bacterial Adherence to Titanium. Clin. Oral Implant. Res. 2011, 22, 1221–1226. [Google Scholar] [CrossRef]
- Ilea, A.; Timuş, D.; Petrescu, N.B.; Soriţău, O.; Boşca, B.A.; Mager, V.; Barbu-Tudoran, L.; Băbţan, A.M.; Câmpian, R.S.; Barabás, R. An In Vitro Study on the Biocompatibility of Titanium Implants Made by Selective Laser Melting. Biotechnol. Bioprocess Eng. 2019, 24, 782–792. [Google Scholar] [CrossRef]
- Zhang, Y.; Jianzhong, L.I.; Che, S. Electropolishing Mechanism of Ti-6Al-4V Alloy Fabricated by Selective Laser Melting. Int. J. Electrochem. Sci. 2018, 13, 4792–4807. [Google Scholar] [CrossRef]
- Tardelli, J.D.C.; da Costa Valente, M.L.; Otani, L.B.; Batalha, R.L.; Alves, F.; Pereira-da-Siva, M.A.; Bagnato, V.S.; Dibb, R.G.P.; Gargarella, P.; Bolfarini, C.; et al. Analysis of Physical, Chemical, Mechanical, and Microbiological Properties of Ti-35Nb-7Zr-5Ta and Ti-6Al-4V Discs Obtained by Machining and Additive Manufacturing. Ceram. Int. 2024, 50, 2845–2854. [Google Scholar] [CrossRef]
- Jiang, Q.L.; Huang, T.Y.; Zhang, Z.Y.; Zhai, D.J.; Wang, H.B.; Feng, K.Q.; Xiang, L. Study of Multifunctional Bioactive Films on 3D Printed Titanium Alloy by Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2024, 478, 130431. [Google Scholar] [CrossRef]
- Dinda, G.P.; Song, L.; Mazumder, J. Fabrication of Ti-6Al-4V Scaffolds by Direct Metal Deposition. Met. Mater. Trans. A 2008, 39, 2914–2922. [Google Scholar] [CrossRef]
- Guo, N.; Leu, M.C. Additive Manufacturing: Technology, Applications and Research Needs. Front. Mech. Eng. 2013, 8, 215–243. [Google Scholar] [CrossRef]
- Duarte, P.M.; Reis, A.F.; de Freitas, P.M.; Ota-Tsuzuki, C. Bacterial Adhesion on Smooth and Rough Titanium Surfaces after Treatment with Different Instruments. J. Periodontol. 2009, 80, 1824–1832. [Google Scholar] [CrossRef]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial Biofilms: Development, Dispersal, and Therapeutic Strategies in the Dawn of the Postantibiotic Era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Taniuchi, A.; May, T.; Kawata, K.; Okabe, S. Increased Antibiotic Resistance of Escherichia coli in Mature Biofilms. Appl. Environ. Microbiol. 2009, 75, 4093–4100. [Google Scholar] [CrossRef]
- Król, J.E.; Wojtowicz, A.J.; Rogers, L.M.; Heuer, H.; Smalla, K.; Krone, S.M.; Top, E.M. Invasion of E. coli Biofilms by Antibiotic Resistance Plasmids. Plasmid 2013, 70, 110–119. [Google Scholar] [CrossRef]
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface Topographical Factors Influencing Bacterial Attachment. Adv. Colloid Interface Sci. 2012, 179–182, 142–149. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q. Role of Surface Roughness in the Wettability, Surface Energy and Flotation Kinetics of Calcite. Powder Technol. 2020, 371, 55–63. [Google Scholar] [CrossRef]
- Bollen, C.M.L.; Lambrechts, P.; Quirynen, M. Comparison of Surface Roughness of Oral Hard Materials to the Threshold Surface Roughness for Bacterial Plaque Retention: A Review of the Literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef] [PubMed]
- McGaffey, M.; zur Linden, A.; Bachynski, N.; Oblak, M.; James, F.; Weese, J.S. Manual Polishing of 3D Printed Metals Produced by Laser Powder Bed Fusion Reduces Biofilm Formation. PLoS ONE 2019, 14, e0212995. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; Pierfelice, T.V.; D’amico, E.; Di Pietro, N.; Pandolfi, A.; D’arcangelo, C.; De Angelis, F.; Mandatori, D.; Schiavone, V.; Piattelli, A.; et al. Influence of Nano, Micro and Macro Topography of Dental Implant Surfaces on Human Gingival Fibroblasts. Int. J. Mol. Sci. 2021, 22, 9871. [Google Scholar] [CrossRef] [PubMed]
- D’Ercole, S.; Di Campli, E.; Pilato, S.; Iezzi, G.; Cellini, L.; Piattelli, A.; Petrini, M. Streptococcus oralis Biofilm Formation on Titanium Surfaces. Int. J. Oral Maxillofac. Implant. 2021, 36, 929–936. [Google Scholar] [CrossRef]
- D’Ercole, S.; Cellini, L.; Pilato, S.; Di Lodovico, S.; Iezzi, G.; Piattelli, A.; Petrini, M. Material Characterization and Streptococcus oralis Adhesion on Polyetheretherketone (PEEK) and Titanium Surfaces Used in Implantology. J. Mater. Sci. Mater. Med. 2020, 31, 84. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, R.; Ullah, I.; Zhang, S.; Sun, Z.; Ren, L.; Yang, K. Rough Surface of Copper-Bearing Titanium Alloy with Multifunctions of Osteogenic Ability and Antibacterial Activity. J. Mater. Sci. Technol. 2020, 48, 130–139. [Google Scholar] [CrossRef]
- Gallorini, M.; Zara, S.; Ricci, A.; Mangano, F.G.; Cataldi, A.; Mangano, C. The Open Cell Form of 3D-Printed Titanium Improves Osteconductive Properties and Adhesion Behavior of Dental Pulp Stem Cells. Materials 2021, 14, 5308. [Google Scholar] [CrossRef]
- Wang, N.; Maskomani, S.; Meenashisundaram, G.K.; Fuh, J.Y.H.; Dheen, S.T.; Anantharajan, S.K. A Study of Titanium and Magnesium Particle-Induced Oxidative Stress and Toxicity to Human Osteoblasts. Mater. Sci. Eng. C 2020, 117, 111285. [Google Scholar] [CrossRef]
- Van Hooreweder, B.; Lietaert, K.; Neirinck, B.; Lippiatt, N.; Wevers, M. CoCr F75 Scaffolds Produced by Additive Manufacturing: Influence of Chemical Etching on Powder Removal and Mechanical Performance. J. Mech. Behav. Biomed. Mater. 2017, 70, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Roncati, L.; Gatti, A.M.; Pusiol, T.; Barbolini, G.; Maiorana, A.; Montanari, S. ESEM Detection of Foreign Metallic Particles inside Ameloblastomatous Cells. Ultrastruct. Pathol. 2015, 39, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, D.G.; Nalli, G.; Verdú, S.; Paparella, M.L.; Cabrini, R.L. Exfoliative Cytology and Titanium Dental Implants: A Pilot Study. J. Periodontol. 2013, 84, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Fretwurst, T.; Buzanich, G.; Nahles, S.; Woelber, J.P.; Riesemeier, H.; Nelson, K. Metal Elements in Tissue with Dental Peri-Implantitis: A Pilot Study. Clin. Oral Implant. Res. 2016, 27, 1178–1186. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, H.; Zhou, J.; Li, F.; Wang, J.; Chen, M.; Liu, Q. Cytotoxicity, DNA Damage, and Apoptosis Induced by Titanium Dioxide Nanoparticles in Human Non-Small Cell Lung Cancer A549 Cells. Environ. Sci. Pollut. Res. 2015, 22, 5519–5530. [Google Scholar] [CrossRef] [PubMed]
- Dumbleton, J.H.; Manley, M.T. Metal-on-Metal Total Hip Replacement: What Does the Literature Say? J. Arthroplast. 2005, 20, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Götz, H.E.; Müller, M.; Emmel, A.; Holzwarth, U.; Erben, R.G.; Stangl, R. Effect of Surface Finish on the Osseointegration of Laser-Treated Titanium Alloy Implants. Biomaterials 2004, 25, 4057–4064. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Krishna, B.V.; Bandyopadhyay, A.; Bose, S. Processing and Biocompatibility Evaluation of Laser Processed Porous Titanium. Acta Biomater. 2007, 3, 1007–1018. [Google Scholar] [CrossRef]
- Pattanayak, D.K.; Fukuda, A.; Matsushita, T.; Takemoto, M.; Fujibayashi, S.; Sasaki, K.; Nishida, N.; Nakamura, T.; Kokubo, T. Bioactive Ti Metal Analogous to Human Cancellous Bone: Fabrication by Selective Laser Melting and Chemical Treatments. Acta Biomater. 2011, 7, 1398–1406. [Google Scholar] [CrossRef]
- Cheng, A.; Humayun, A.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Additively Manufactured 3D Porous Ti-6Al-4V Constructs Mimic Trabecular Bone Structure and Regulate Osteoblast Proliferation, Differentiation and Local Factor Production in a Porosity and Surface Roughness Dependent Manner. Biofabrication 2014, 6, 045007. [Google Scholar] [CrossRef]
- Li, J.P.; Habibovic, P.; van den Doel, M.; Wilson, C.E.; de Wijn, J.R.; van Blitterswijk, C.A.; de Groot, K. Bone Ingrowth in Porous Titanium Implants Produced by 3D Fiber Deposition. Biomaterials 2007, 28, 2810–2820. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of Pore Size on Bone Ingrowth into Porous Titanium Implants Fabricated by Additive Manufacturing: An In Vivo Experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, X.; Yin, S.; Liu, L.; Liu, X.; Zhao, G.; Ma, W.; Qi, W.; Ren, Z.; Liao, H.; et al. Influence of the Pore Size and Porosity of Selective Laser Melted Ti6Al4V ELI Porous Scaffold on Cell Proliferation, Osteogenesis and Bone Ingrowth. Mater. Sci. Eng. C 2020, 106, 110289. [Google Scholar] [CrossRef]
- Hu, X.; Xu, R.; Yu, X.; Chen, J.; Wan, S.; Ouyang, J.; Deng, F. Enhanced Antibacterial Efficacy of Selective Laser Melting Titanium Surface with Nanophase Calcium Phosphate Embedded to TiO2 Nanotubes. Biomed. Mater. 2018, 13, 045015. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Chen, X.-S.; Zhang, C.-Y.; Liu, Y.; Wang, J.; Deng, F.-L. Improved Bioactivity of Selective Laser Melting Titanium: Surface Modification with Micro-/Nano-Textured Hierarchical Topography and Bone Regeneration Performance Evaluation. Mater. Sci. Eng. C 2016, 68, 229–240. [Google Scholar] [CrossRef]
- Tsukanaka, M.; Fujibayashi, S.; Takemoto, M.; Matsushita, T.; Kokubo, T.; Nakamura, T.; Sasaki, K.; Matsuda, S. Bioactive Treatment Promotes Osteoblast Differentiation on Titanium Materials Fabricated by Selective Laser Melting Technology. Dent. Mater. J. 2016, 35, 118–125. [Google Scholar] [CrossRef]
- Longhitano, G.A.; Larosa, M.A.; Munhoz, A.L.J.; de Carvalho Zavaglia, C.A.; Ierardi, M.C.F. Surface Finishes for Ti-6Al-4V Alloy Produced by Direct Metal Laser Sintering. Mater. Res. 2015, 18, 838–842. [Google Scholar] [CrossRef]
- Jiang, G.; Shi, D. Coating of Hydroxyapatite on Highly Porous Al2O3 Substrate for Bone Substitutes. J. Biomed. Mater. Res. 1998, 43, 77–81. [Google Scholar] [CrossRef]
- Braem, A.; Chaudhari, A.; Vivan Cardoso, M.; Schrooten, J.; Duyck, J.; Vleugels, J. Peri- and Intra-Implant Bone Response to Microporous Ti Coatings with Surface Modification. Acta Biomater. 2014, 10, 986–995. [Google Scholar] [CrossRef]
- Yu, P.; Lu, F.; Zhu, W.; Wang, D.; Zhu, X.; Tan, G.; Wang, X.; Zhang, Y.; Li, L.; Ning, C. Bio-Inspired Citrate Functionalized Apatite Coating on Rapid Prototyped Titanium Scaffold. Appl. Surf. Sci. 2014, 313, 947–953. [Google Scholar] [CrossRef]
- Zhang, Q.; Leng, Y.; Xin, R. A Comparative Study of Electrochemical Deposition and Biomimetic Deposition of Calcium Phosphate on Porous Titanium. Biomaterials 2005, 26, 2857–2865. [Google Scholar] [CrossRef]
- Habibovic, P.; Barrè, F.; Van Blitterswijk, C.A.; De Groot, K.; Layrolle, P. Biomimetic Hydroxyapatite Coating on Metal Implants. J. Am. Ceram. Soc. 2002, 85, 517–522. [Google Scholar] [CrossRef]
- Chai, Y.C.; Truscello, S.; Van Bael, S.; Luyten, F.P.; Vleugels, J.; Schrooten, J. Perfusion Electrodeposition of Calcium Phosphate on Additive Manufactured Titanium Scaffolds for Bone Engineering. Acta Biomater. 2011, 7, 2310–2319. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Sohier, J.; Gaillard, C.; Quillard, S.; Dorget, M.; Layrolle, P. Rapid Prototyped Porous Titanium Coated with Calcium Phosphate as a Scaffold for Bone Tissue Engineering. Biomaterials 2008, 29, 2608–2615. [Google Scholar] [CrossRef]
- Seuss, S.; Lehmann, M.; Boccaccini, A.R. Alternating Current Electrophoretic Deposition of Antibacterial Bioactive Glass-Chitosan Composite Coatings. Int. J. Mol. Sci. 2014, 15, 12231–12242. [Google Scholar] [CrossRef]
- Chen, Q.; Cordero-Arias, L.; Roether, J.A.; Cabanas-Polo, S.; Virtanen, S.; Boccaccini, A.R. Alginate/Bioglass® Composite Coatings on Stainless Steel Deposited by Direct Current and Alternating Current Electrophoretic Deposition. Surf. Coat. Technol. 2013, 233, 49–56. [Google Scholar] [CrossRef]
- Petrini, M.; Mangano, C.; Cellini, L.; Di Giulio, M.; Iezzi, G.; Piattelli, A.; D’Ercole, S. Material Characterization and Bacterial Interaction of Titanium Discs Produced by Selective Laser Melting. Mater. Charact. 2022, 189, 111989. [Google Scholar] [CrossRef]
- Liu, R.; Tang, Y.; Liu, H.; Zeng, L.; Ma, Z.; Li, J.; Zhao, Y.; Ren, L.; Yang, K. Effects of Combined Chemical Design (Cu Addition) and Topographical Modification (SLA) of Ti-Cu/SLA for Promoting Osteogenic, Angiogenic and Antibacterial Activities. J. Mater. Sci. Technol. 2020, 47, 202–215. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, H.; Zhang, E.; You, J.; Ma, X.; Bai, X. Antibacterial Activities and Biocompatibilities of Ti-Ag Alloys Prepared by Spark Plasma Sintering and Acid Etching. Mater. Sci. Eng. C 2018, 92, 121–131. [Google Scholar] [CrossRef]
- Kang, M.-K.; Moon, S.-K.; Kwon, J.-S.; Kim, K.-M.; Kim, K.-N. Antibacterial Effect of Sand Blasted, Large-Grit, Acid-Etched Treated Ti-Ag Alloys. Mater. Res. Bull. 2012, 47, 2952–2955. [Google Scholar] [CrossRef]
- van Hengel, I.A.J.; Riool, M.; Fratila-Apachitei, L.E.; Witte-Bouma, J.; Farrell, E.; Zadpoor, A.A.; Zaat, S.A.J.; Apachitei, I. Selective Laser Melting Porous Metallic Implants with Immobilized Silver Nanoparticles Kill and Prevent Biofilm Formation by Methicillin-Resistant Staphylococcus aureus. Biomaterials 2017, 140, 1–15. [Google Scholar] [CrossRef]
- Macpherson, A.; Li, X.; McCormick, P.; Ren, L.; Yang, K.; Sercombe, T.B. Antibacterial Titanium Produced Using Selective Laser Melting. JOM 2017, 69, 2719–2724. [Google Scholar] [CrossRef]
- Ji, H.; Zhao, M.C.; Xie, B.; Zhao, Y.C.; Yin, D.; Gao, C.; Shuai, C.; Atrens, A. Corrosion and Antibacterial Performance of Novel Selective-Laser-Melted (SLMed) Ti-XCu Biomedical Alloys. J. Alloys Compd. 2021, 864, 158415. [Google Scholar] [CrossRef]
- Pokrowiecki, R.; Zaręba, T.; Szaraniec, B.; Pałka, K.; Mielczarek, A.; Menaszek, E.; Tyski, S. In Vitro Studies of Nanosilver-Doped Titanium Implants for Oral and Maxillofacial Surgery. Int. J. Nanomed. 2017, 2017, 4285–4297. [Google Scholar] [CrossRef]
- Yavari, S.A.; Loozen, L.; Paganelli, F.L.; Bakhshandeh, S.; Lietaert, K.; Groot, J.A.; Fluit, A.C.; Boel, C.H.E.; Alblas, J.; Vogely, H.C.; et al. Antibacterial Behavior of Additively Manufactured Porous Titanium with Nanotubular Surfaces Releasing Silver Ions. ACS Appl. Mater. Interfaces 2016, 8, 17080–17089. [Google Scholar] [CrossRef]
- Ercan, B.; Taylor, E.; Alpaslan, E.; Webster, T.J. Diameter of Titanium Nanotubes Influences Anti-Bacterial Efficacy. Nanotechnology 2011, 22, 295102. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A Review of the Biomaterials Technologies for Infection-Resistant Surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Mangano, C.; Piattelli, A.; Scarano, A.; Raspanti, M.; Shibli, J.A.; Mangano, F.G.; Perrotti, V.; Iezzi, G. A Light and Scanning Electron Study of Human Direct Metal Forming Dental Implants. Int. J. Periodontics Restor. Dent. 2014, 34, e9–e17. [Google Scholar] [CrossRef]
- Mangano, C.; Mangano, F.G.; Shibli, J.A.; Roth, L.A.; D’ Addazio, G.; Piattelli, A.; Iezzi, G. Immunohistochemical Evaluation of Peri-Implant Soft Tissues around Machined and Direct Metal Laser Sintered (DMLS) Healing Abutments in Humans. Int. J. Environ. Res. Public Health 2018, 15, 1611. [Google Scholar] [CrossRef]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S. The Influence of Surface Modification on Bacterial Adhesion to Titanium-Based Substrates. ACS Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef]
- Tardelli, J.D.C.; Bagnato, V.S.; dos Reis, A.C. Bacterial Adhesion Strength on Titanium Surfaces Quantified by Atomic Force Microscopy: A Systematic Review. Antibiotics 2023, 12, 994. [Google Scholar] [CrossRef]
- Xue, C.; Shi, X.; Fang, X.; Tao, H.; Zhu, H.; Yu, F.; Ding, X.; Liu, M.; Fang, F.; Yang, F.; et al. The “Pure Marriage” between 3D Printing and Well-Ordered Nanoarrays by Using PEALD Assisted Hydrothermal Surface Engineering. ACS Appl. Mater. Interfaces 2016, 8, 8393–8400. [Google Scholar] [CrossRef]
- Miao, X.; Liao, H.; Deng, Z.; Li, C.; Wu, T.; Zhang, H.; Liu, M.; Cheng, X.; Wang, X. “Dandelion” Inspired Dual-Layered Nanoarrays with Two Model Releasing Features for the Surface Modification of 3D Printing Implants. ACS Biomater. Sci. Eng. 2017, 3, 2259–2266. [Google Scholar] [CrossRef]
- Palka, L.; Mazurek-Popczyk, J.; Arkusz, K.; Baldy-Chudzik, K. Susceptibility to Biofilm Formation on 3D-Printed Titanium Fixation Plates Used in the Mandible: A Preliminary Study. J. Oral Microbiol. 2020, 12, 1838164. [Google Scholar] [CrossRef]
- Sahm, B.D.; da Costa Valente, M.L.; dos Reis, A.C. Use of Surface Nanotechnology in 3D Implants for Antimicrobial Action: A Systematic Review. J. Oral Maxillofac. Surg. Med. Pathol. 2024, 36, 273–277. [Google Scholar] [CrossRef]
- Huang, H.H.; Ho, C.-T.; Lee, T.H.; Lee, T.L.; Liao, K.K.; Chen, F.L. Effect of Surface Roughness of Ground Titanium on Initial Cell Adhesion. Biomol. Eng. 2004, 21, 93–97. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of Material Characteristics and/or Surface Topography on Biofilm Development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Martinez, M.A.F.; Balderrama, Í.d.F.; Karam, P.S.B.H.; de Oliveira, R.C.; de Oliveira, F.A.; Grandini, C.R.; Vicente, F.B.; Stavropoulos, A.; Zangrando, M.S.R.; Sant’Ana, A.C.P. Surface Roughness of Titanium Disks Influences the Adhesion, Proliferation and Differentiation of Osteogenic Properties Derived from Human. Int. J. Implant Dent. 2020, 6, 46. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y.F. Concise Review of Mechanisms of Bacterial Adhesion to Biomaterials and of Techniques Used in Estimating Bacteria-Material Interactions. Eur. Cell Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Bollen, C.M.L.; Papaioannou, W.; Van Eldere, J.; Schepers, E.; Quirynen, M.; Van Steenberghe, D. The Influence of Abutment Surface Roughness on Plaque Accumulation and Peri-Implant Mucositis. Clin. Oral Implant. Res. 1996, 7, 201–211. [Google Scholar] [CrossRef]

| Author | Surface Treatment | Control Group | Pathogenic Species | Pre-Incubation of Samples in Saliva | Results |
|---|---|---|---|---|---|
| van Hengel et al., 2017 [82] | Silver nanoparticles in an oxide surface layer grown using Plasma Electrolytic Oxidation (PEO) in Ca/P-based electrolytes | Ti–6Al–4V | S. aureus | No | A decline in the quantity of CFU within an ex vivo infection model in mouse |
| Macpherson et al., 2017 [83] | Elemental addition of Ag or Cu | Ti–6Al–4V | E. coli | No | The Cu-containing alloy exhibited moderate antibacterial properties, outperforming the Ag-containing alloy |
| Hu et al., 2018 [64] | Sandblasting, anodization and electrochemical deposition of nanophase calcium phosphate (CaP) | TiO2 | S. mutans and S. sanguinis | No | Reduction in the number of both types of bacteria in samples with surface treatment, compared to untreated TiO2 SLM |
| D’Ercole et al., 2021 [10] | Open cell form (interconnected pores) | Ti–6Al–4V | S. oralis | Yes | Three-dimensional discs exhibited considerably lower CFU levels and biofilm biomass than machined surfaces |
| Ji et al., 2021 [84] | Cu in varying concentrations (0, 3, 5, 7 and 10 wt%) | Ti | E. coli | No | The Ti-3Cu alloy showed 99% antibacterial efficacy against E. coli, while alloys with 5%, 7%, and 10% Cu achieved nearly 100% efficacy |
| Petrini et al., 2022 [78] | Electrochemical polishing (EL) and Organic acids-etching (OAE) | Ti–6Al–4V | S. oralis | Yes | OAE exhibited significantly lower CFU counts and biofilm biomass formation compared to EL and machined samples |
| Gallab et al., 2024 [13] | NaOH-CaCl2-heat-ICl3 | Ti | S. aureus | No | Antimicrobial action lasting beyond three months, resulting in the complete elimination of bacteria |
| Jiang et al., 2024 [33] | Plasma Electrolytic Oxidation (PEO) in Zn and Sr-based electrolytes | Ti–6Al–4V | S. aureus | No | The PEO film demonstrated notable antibacterial characteristics |
| Tardelli et al., 2024 [32] | Ti–35Nb–7Zr–5Ta | Ti–6Al–4V | S. aureus | No | There was no difference in the CFU of bacteria among the groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotta, T.C.; D’Ercole, S.; Iezzi, G.; Pedrazzi, V.; Galo, R.; Petrini, M. The Interaction between Oral Bacteria and 3D Titanium Porous Surfaces Produced by Selective Laser Melting—A Narrative Review. Biomimetics 2024, 9, 461. https://doi.org/10.3390/biomimetics9080461
Dotta TC, D’Ercole S, Iezzi G, Pedrazzi V, Galo R, Petrini M. The Interaction between Oral Bacteria and 3D Titanium Porous Surfaces Produced by Selective Laser Melting—A Narrative Review. Biomimetics. 2024; 9(8):461. https://doi.org/10.3390/biomimetics9080461
Chicago/Turabian StyleDotta, Tatiane Cristina, Simonetta D’Ercole, Giovanna Iezzi, Vinicius Pedrazzi, Rodrigo Galo, and Morena Petrini. 2024. "The Interaction between Oral Bacteria and 3D Titanium Porous Surfaces Produced by Selective Laser Melting—A Narrative Review" Biomimetics 9, no. 8: 461. https://doi.org/10.3390/biomimetics9080461







