Three-Dimensional Finite Element Analysis of Stress Distribution in Dental Implant Prosthesis and Surrounding Bone Using PEEK Abutments
Abstract
1. Introduction
2. Materials and Methods
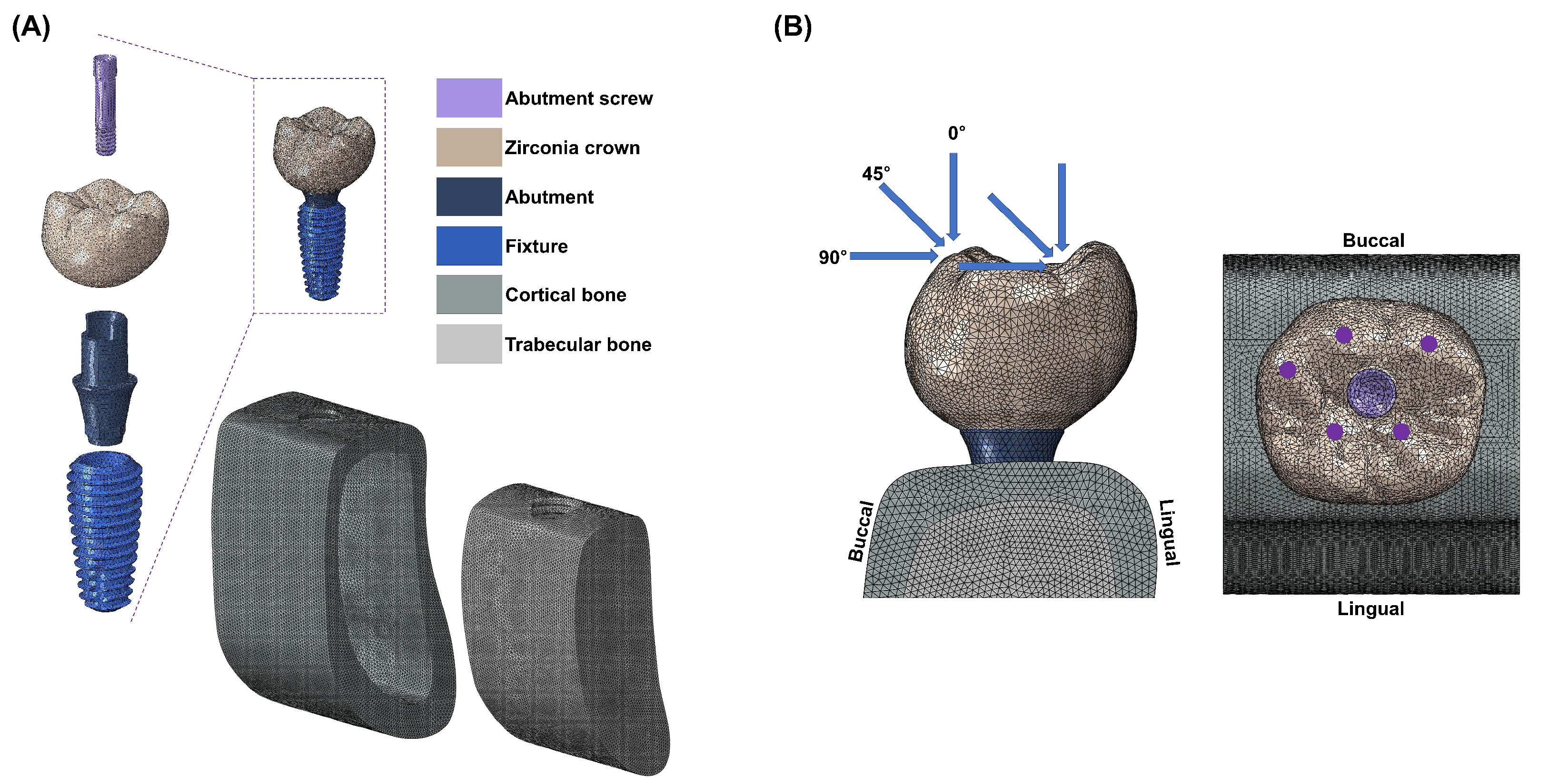
2.1. Finite Element Model
2.2. Boundary and Loading Conditions
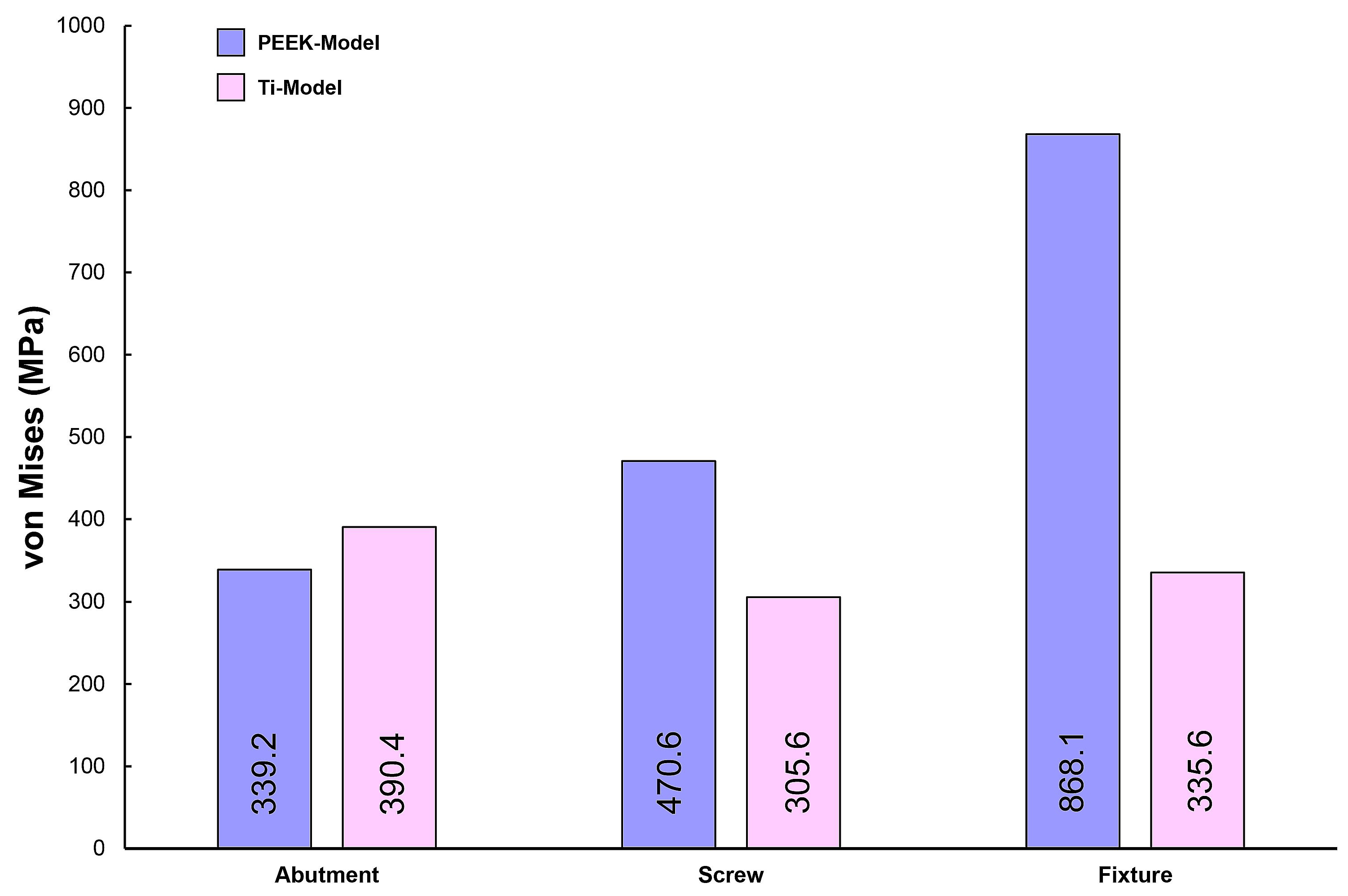
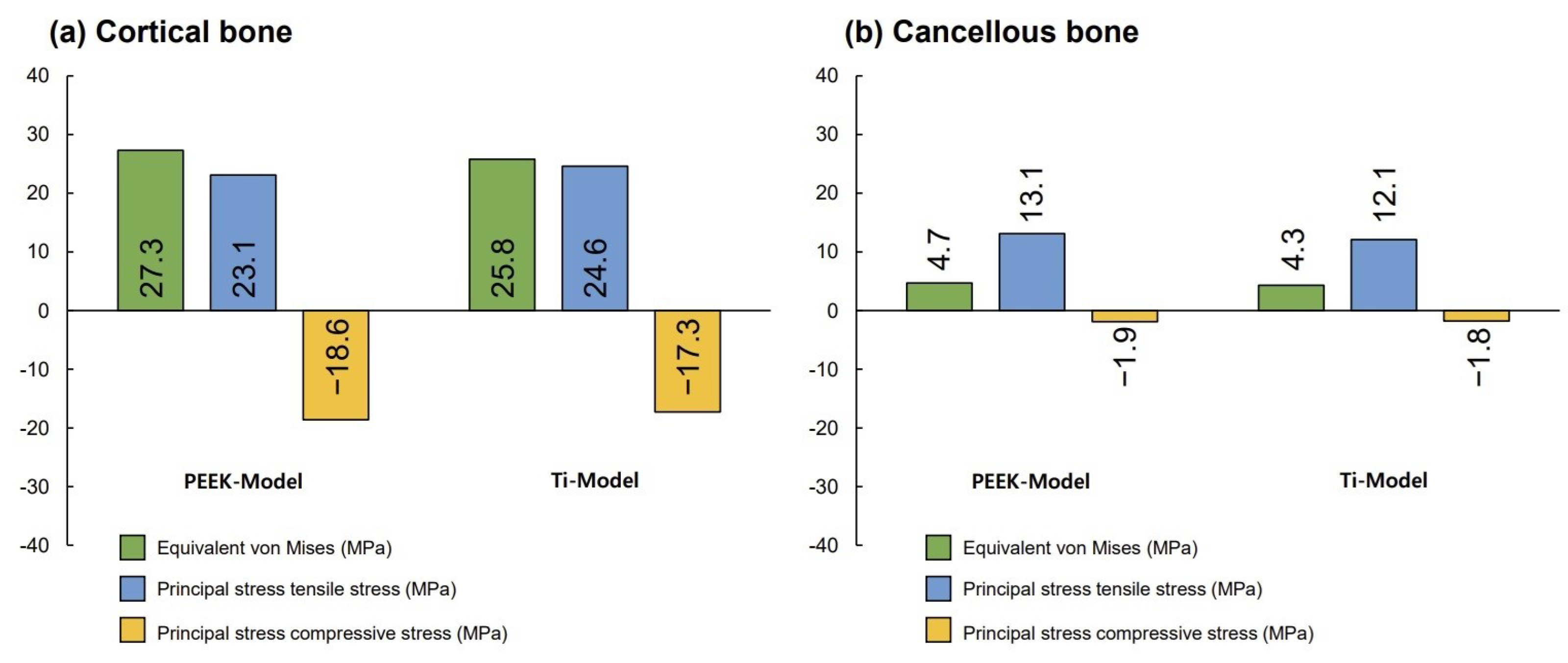
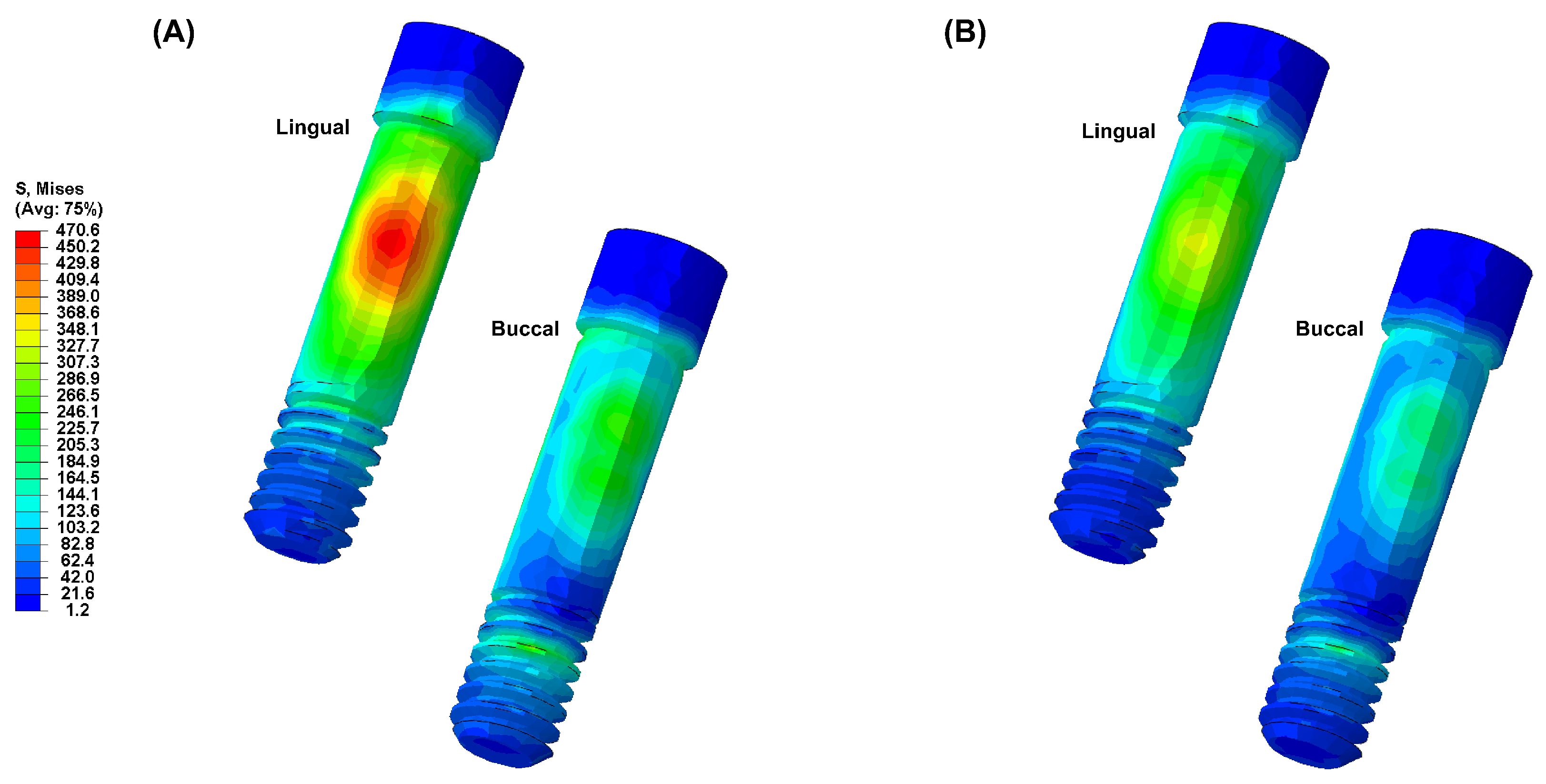
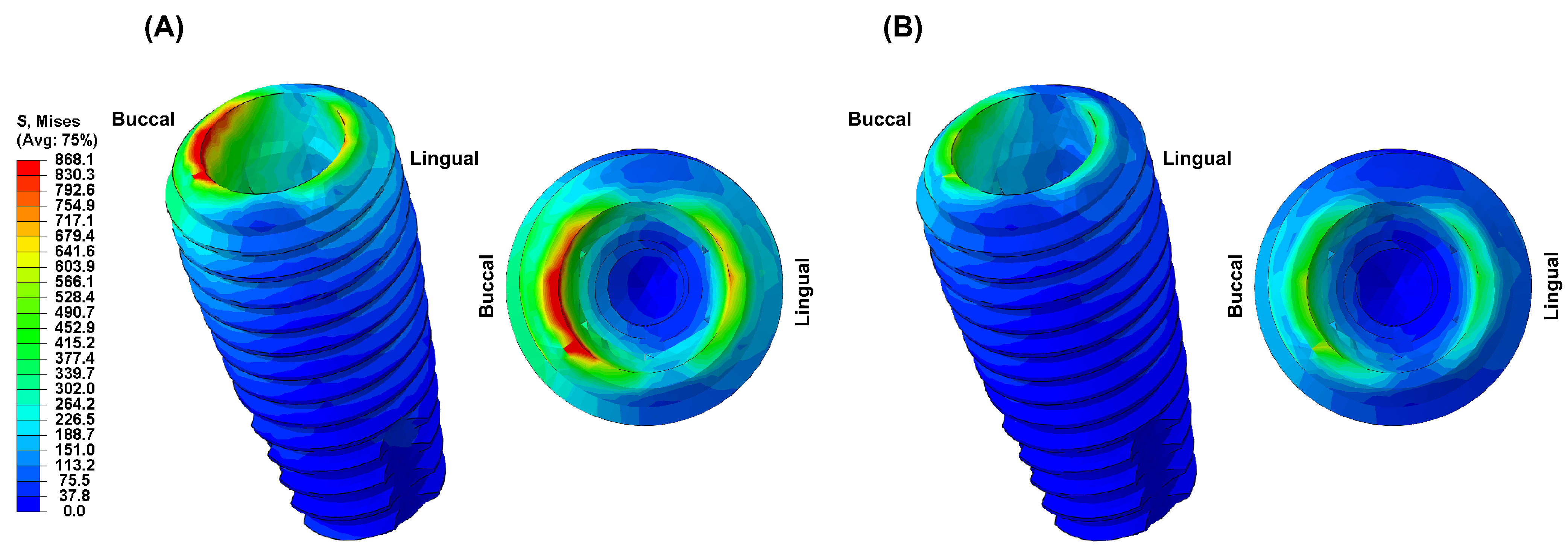
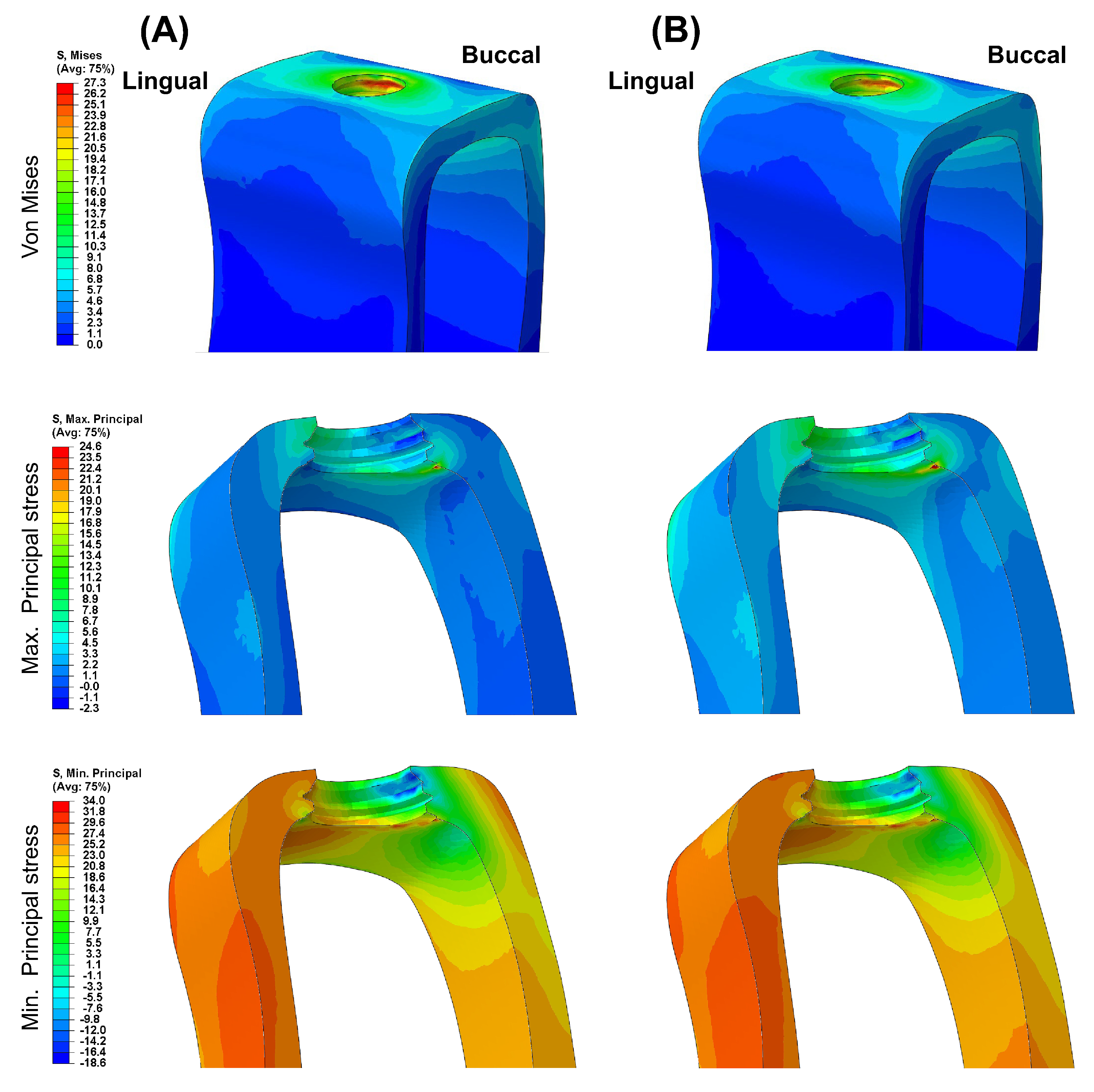
3. Results
4. Discussion
5. Conclusions
- Due to the low elastic modulus of the PEEK abutment, high VMS values were observed in the implant fixture.
- Based on the results of this study, a PEEK abutment requires improved mechanical and physical properties for clinical application, and its clinical use is limited.
- Different abutment materials did not significantly affect the stress distribution and magnitude in the bone around the implant.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velasco-Ortega, E.; Del Rocío Jiménez-Martin, I.; Moreno-Muñoz, J.; Núñez-Márquez, E.; Luis Rondón-Romero, J.; Cabanillas-Balsera, D.; Jiménez-Guerra, Á.; Ortiz-García, I.; López-López, J.; Monsalve-Guil, L. Long-term treatment outcomes of implant prostheses in partially and totally edentulous patients. Materials 2022, 15, 4910. [Google Scholar] [CrossRef] [PubMed]
- Raikar, S.; Talukdar, P.; Kumari, S.; Panda, S.K.; Oommen, V.M.; Prasad, A. Factors affecting the survival rate of dental implants: A retrospective study. J. Int. Soc. Prev. Community Dent. 2017, 7, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Z.; Monje, A.; Huang, W.; Wu, Y.; Wang, G. Intermediate long-term clinical performance of dental implants placed in sites with a previous early implant failure: A retrospective analysis. Clin. Oral. Implant. Res. 2015, 26, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Shyu, Y.T.; Wu, Y.L.; Tsai, M.H.; Chen, H.S.; Wu, A.Y. Effects of marginal bone loss progression on stress distribution in different implant-abutment connections and abutment materials: A 3D finite element analysis study. Materials 2022, 15, 5866. [Google Scholar] [CrossRef] [PubMed]
- Merin, R.L. Repair of peri-implant bone loss after occlusal adjustment: A case report. J. Am. Dent. Assoc. 2014, 145, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- Isidor, F. Influence of forces on peri-implant bone. Clin. Oral. Implant. Res. 2006, 17, 8–18. [Google Scholar] [CrossRef]
- Graves, C.V.; Harrel, S.K.; Rossmann, J.A.; Kerns, D.; Gonzalez, J.A.; Kontogiorgos, E.D.; Al-Hashimi, I.; Abraham, C. The role of occlusion in the dental implant and peri-implant condition: A review. Open Dent. J. 2016, 10, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.M.; Del Bel Cury, A.A.; Pizzoloto, L.; Acapa, I.R.H.; Shibli, J.A.; Bordin, D. Does traumatic occlusal forces lead to peri-implant bone loss? A systematic review. Braz. Oral Res. 2019, 33, e069. [Google Scholar] [CrossRef]
- Di Fiore, A.; Montagner, M.; Sivolella, S.; Stellini, E.; Yilmaz, B.; Brunello, G. Peri-implant bone loss and overload: A systematic review focusing on occlusal analysis through digital and analogic methods. J. Clin. Med. 2022, 11, 4812. [Google Scholar] [CrossRef]
- Brunski, J.B.; Puleo, D.A.; Nanci, A. Biomaterials and biomechanics of oral and maxillofacial implants: Current status and future developments. Int. J. Oral Maxillofac. Implant. 2000, 15, 15–46. [Google Scholar]
- Hsu, Y.T.; Fu, J.H.; Al-Hezaimi, K.; Wang, H.L. Biomechanical implant treatment complications: A systematic review of clinical studies of implants with at least 1 year of functional loading. Int. J. Oral Maxillofac. Implant. 2012, 27, 894–904. [Google Scholar]
- Chang, C.L.; Chen, C.S.; Yeung, T.C.; Hsu, M.L. Biomechanical effect of a zirconia dental implant-crown system: A three-dimensional finite element analysis. Int. J. Oral Maxillofac. Implant. 2012, 27, e49–e57. [Google Scholar]
- Graf, T.; Güth, J.F.; Schweiger, J.; Erdelt, K.J.; Edelhoff, D.; Stimmelmayr, M. Biomechanical behavior of implants with different diameters in relation to simulated bone loss—An in vitro study. Clin. Oral Investig. 2023, 27, 5887–5894. [Google Scholar] [CrossRef] [PubMed]
- Khaohoen, A.; Sornsuwan, T.; Chaijareenont, P.; Poovarodom, P.; Rungsiyakull, C.; Rungsiyakull, P. Biomaterials and clinical application of dental implants in relation to bone density—A narrative review. J. Clin. Med. 2023, 12, 6924. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.H. Comparison of stress distribution in bone and implant-supported dental prosthesis with zirconia and titanium implants: A 3-dimensional finite element analysis. J. Tech. Dent. 2020, 42, 348–354. [Google Scholar] [CrossRef]
- Karthik, K.; Sivakumar Sivaraj Thangaswamy, V. Evaluation of implant success: A review of past and present concepts. J. Pharm. Bioallied. Sci. 2013, 5, S117–S119. [Google Scholar] [CrossRef] [PubMed]
- Baqain, Z.H.; Moqbel, W.Y.; Sawair, F.A. Early dental implant failure: Risk factors. Br. J. Oral Maxillofac. Surg. 2012, 50, 239–243. [Google Scholar] [CrossRef]
- Sarot, J.R.; Contar, C.M.M.; Cruz, A.C.C.D.; de Souza Magini, R. Evaluation of the stress distribution in CFR-PEEK dental implants by the three-dimensional finite element method. J. Mater. Sci. Mater. Med. 2010, 21, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Schwitalla, A.D.; Abou-Emara, M.; Spintig, T.; Lackmann, J.; Müller, W.D. Finite element analysis of the biomechanical effects of PEEK dental implants on the peri-implant bone. J. Biomech. 2015, 48, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Belser, U.C.; Grütter, L.; Vailati, F.; Bornstein, M.M.; Weber, H.P.; Buser, D. Outcome evaluation of early placed maxillary anterior single-tooth implants using objective esthetic criteria: A cross-sectional, retrospective study in 45 patients with a 2-to 4-year follow-up using pink and white esthetic scores. J. Periodontol. 2009, 80, 140–151. [Google Scholar] [CrossRef]
- Houshmand, B.; Ardakani, M.T.; Moscowchi, A.; Oskoui, I.Z. Effect of implant design on stress distribution: A finite element study. J. Long Term Eff. Med. Implant. 2022, 32, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Hong, M.H. Influence of zirconia and titanium fixture materials on stress distribution in abutment screws: A three-dimensional finite element analysis. J. Tech. Dent. 2021, 43, 42–47. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Zarauz, C.; Strasding, M.; Sailer, I.; Zwahlen, M.; Zembic, A. A systematic review of the influence of the implant-abutment connection on the clinical outcomes of ceramic and metal implant abutments supporting fixed implant reconstructions. Clin. Oral Implant. Res. 2018, 29, 160–183. [Google Scholar] [CrossRef]
- Choi, H.; Hong, M.H. Finite element analysis of the effect of tightening torque on the connection stability of a two-piece zirconia implant system. Adv. Mater. Sci. Eng. 2022, 2022, 1456475. [Google Scholar] [CrossRef]
- Pérez-Pevida, E.; Brizuela-Velasco, A.; Chávarri-Prado, D.; Jiménez-Garrudo, A.; Sánchez-Lasheras, F.; Solaberrieta-Méndez, E.; Diéguez-Pereira, M.; Fernández-González, F.J.; Dehesa-Ibarra, B.; Monticelli, F. Biomechanical consequences of the elastic properties of dental implant alloys on the supporting bone: Finite element analysis. Biomed. Res. Int. 2016, 2016, 1850401. [Google Scholar] [CrossRef]
- Jörn, D.; Kohorst, P.; Besdo, S.; Rücker, M.; Stiesch, M.; Borchers, L. Influence of lubricant on screw preload and stresses in a finite element model for a dental implant. J. Prosthet. Dent. 2014, 112, 340–348. [Google Scholar] [CrossRef]
- Ha, S.R.; Kim, S.H.; Lee, J.B.; Han, J.S.; Yeo, I.S.; Yoo, S.H.; Kim, H.K. Biomechanical three-dimensional finite element analysis of monolithic zirconia crown with different cement type. J. Adv. Prosthodont. 2015, 7, 475–483. [Google Scholar] [CrossRef]
- Çağlar, A.; Turhan Bal, B.; Karakoca, S.; Aydın, C.; Yılmaz, H.; Sarısoy, Ş. Three-dimensional finite element analysis of titanium and yttrium-stabilized zirconium dioxide abutments and implants. Int. J. Oral Maxillofac. Implant. 2011, 26, 961–969. [Google Scholar]
- Caglar, A.; Bal, B.T.; Aydin, C.; Yilmaz, H.; Ozkan, S. Evaluation of stresses occurring on three different zirconia dental implants: Three-dimensional finite element analysis. Int. J. Oral Maxillofac. Implant. 2010, 25, 95–103. [Google Scholar]
- Mohamed, M.; Pisavadia, H.; Westover, L. A finite element model for evaluating the effectiveness of the Advanced System for Implant Stability Testing (ASIST). J. Biomech. 2021, 124, 110570. [Google Scholar] [CrossRef]
- Verri, F.R.; Santiago Júnior, J.F.; Almeida, D.A.D.F.; Verri, A.C.G.; de Souza Batista, V.E.; Lemos, C.A.A. Three-dimensional finite element analysis of anterior single implant-supported prostheses with different bone anchorages. Sci. World J. 2015, 2015, 321528. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Choi, H.; Lee, D.H.; Hong, M.H. Comparative finite element analysis of mandibular posterior single zirconia and titanium implants: A 3-dimensional finite element analysis. J. Adv. Prosthodont. 2021, 13, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Licausi, M.P.; Muñoz, A.I.; Borrás, V.A.; Espallargas, N. Tribocorrosion mechanisms of Ti6Al4V in artificial saliva by zero-resistance ammetry (ZRA) technique. J. Bio Tribo Corros. 2015, 1, 8. [Google Scholar] [CrossRef]


| Material | Young’s Modulus (MPa) | Poisson’s Ratio | Reference |
|---|---|---|---|
| Titanium implant | 110,000 | 0.35 | [24] |
| PEEK abutment | 4100 | 0.40 | [19] |
| Titanium abutment | 110,000 | 0.50 | [24] |
| Abutment screw | 110,000 | 0.35 | [24] |
| Zirconia crown | 210,000 | 0.26 | [21] |
| Cortical bone | 13,400 | 0.30 | [24] |
| Cancellous bone | 1370 | 0.31 | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.-H.; Choi, H. Three-Dimensional Finite Element Analysis of Stress Distribution in Dental Implant Prosthesis and Surrounding Bone Using PEEK Abutments. Biomimetics 2024, 9, 472. https://doi.org/10.3390/biomimetics9080472
Hong M-H, Choi H. Three-Dimensional Finite Element Analysis of Stress Distribution in Dental Implant Prosthesis and Surrounding Bone Using PEEK Abutments. Biomimetics. 2024; 9(8):472. https://doi.org/10.3390/biomimetics9080472
Chicago/Turabian StyleHong, Min-Ho, and Hyunsuk Choi. 2024. "Three-Dimensional Finite Element Analysis of Stress Distribution in Dental Implant Prosthesis and Surrounding Bone Using PEEK Abutments" Biomimetics 9, no. 8: 472. https://doi.org/10.3390/biomimetics9080472
APA StyleHong, M.-H., & Choi, H. (2024). Three-Dimensional Finite Element Analysis of Stress Distribution in Dental Implant Prosthesis and Surrounding Bone Using PEEK Abutments. Biomimetics, 9(8), 472. https://doi.org/10.3390/biomimetics9080472






