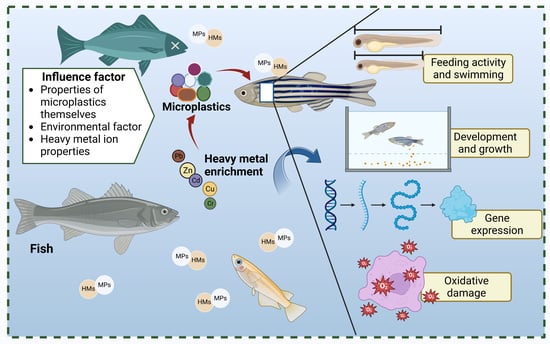
Factors Affecting the Adsorption of Heavy Metals by Microplastics and Their Toxic Effects on Fish
Abstract
:1. Introduction
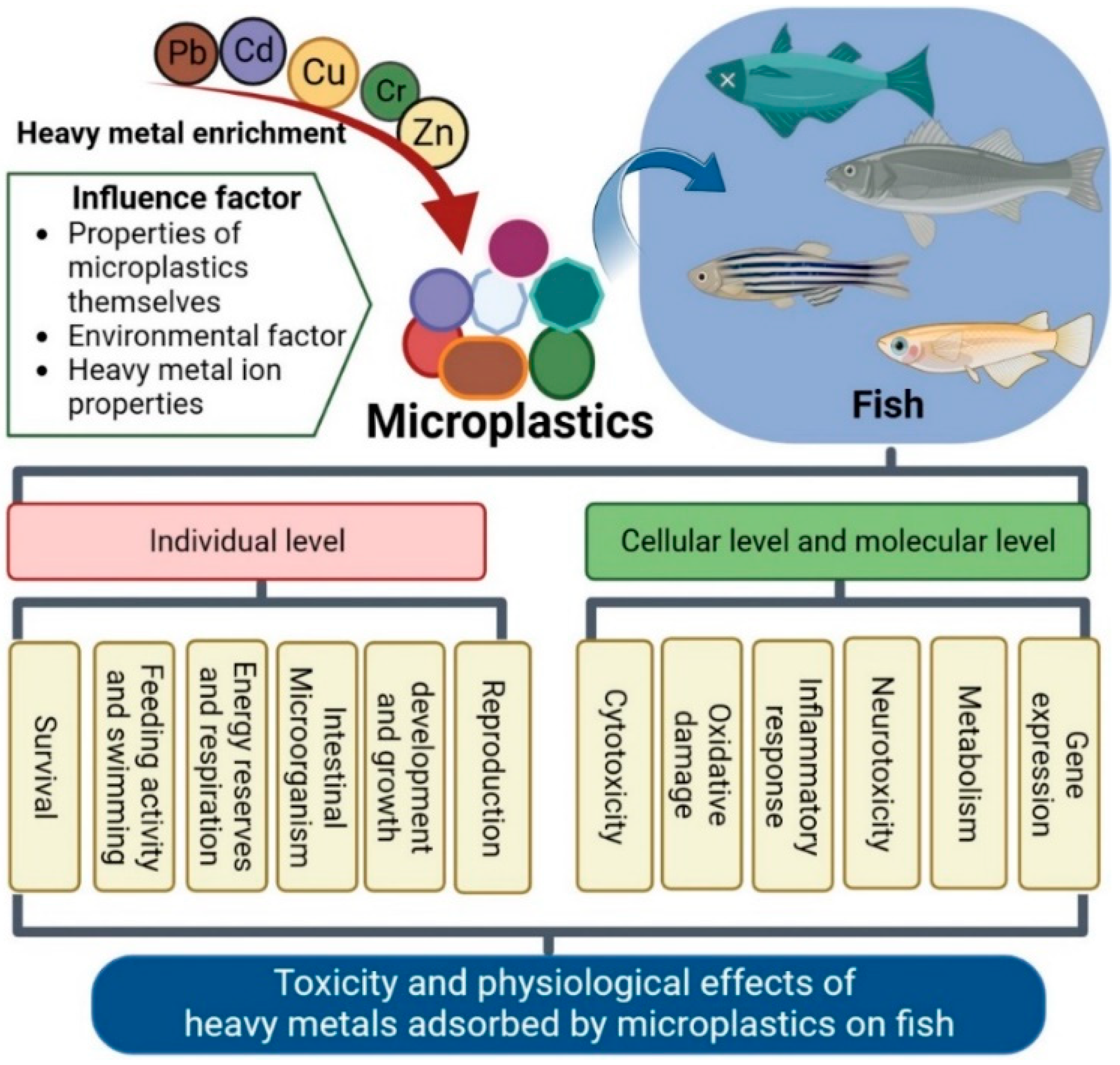
2. Factors Influencing Heavy Metal Enrichment on MPs and Removal Strategies
2.1. Influence of the Properties of MPs
2.2. Effects of Environmental Factors and Properties of HMs
3. Toxic Physiological Effects of MPs with Adsorbed HMs on Fish
3.1. Individual Level
3.1.1. Survival
3.1.2. Feeding Activity and Swimming
3.1.3. Energy Reserves and Respiration
3.1.4. Intestinal Microorganisms
3.1.5. Development and Growth
3.1.6. Reproduction
3.2. Cellular Level
3.2.1. Cytotoxicity
3.2.2. Oxidative Damage
3.2.3. Inflammatory Response
3.2.4. Neurotoxicity
3.2.5. Metabolism
3.3. Molecular Level
Gene Expression
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to Microplastics: An Overview on Possible Human Health Effects. Sci. Total. Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental Occurrences, Fate, and Impacts of Microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef] [PubMed]
- So, M.W.K.; Vorsatz, L.D.; Cannicci, S.; Not, C. Fate of Plastic in the Environment: From Macro to Nano by Macrofauna. Environ. Pollut. 2022, 300, 118920. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J. Plastic Pollution and Potential Solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and Intestinal Effects of Nano- and Microplastics: A Review of the Literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, P.D.; Kim, S.; Sarkar, B.; Oleszczuk, P.; Sang, M.K.; Haque, M.N.; Ahn, J.H.; Bank, M.S.; Ok, Y.S. Effects of Microplastics on the Terrestrial Environment: A Critical Review. Environ. Res. 2022, 209, 112734. [Google Scholar] [CrossRef]
- Priya, A.K.; Jalil, A.A.; Dutta, K.; Rajendran, S.; Vasseghian, Y.; Qin, J.; Soto-Moscoso, M. Microplastics in the Environment: Recent Developments in Characteristic, Occurrence, Identification and Ecological Risk. Chemosphere 2022, 298, 134161. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Labrenz, M. Marine Microbial Assemblages on Microplastics: Diversity, Adaptation, and Role in Degradation. Ann. Rev. Mar. Sci. 2020, 12, 209–232. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of Exposure of Heavy Metals and Their Impact on Health Consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Valente, T.; Ventura, D.; Matiddi, M.; Sbrana, A.; Silvestri, C.; Piermarini, R.; Jacomini, C.; Costantini, M.L. Image Processing Tools in the Study of Environmental Contamination by Microplastics: Reliability and Perspectives. Environ. Sci. Pollut. Res. Int. 2023, 30, 298–309. [Google Scholar] [CrossRef]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.-J. Microplastics in Aquatic Environments: Occurrence, Accumulation, and Biological Effects. Sci. Total. Environ. 2020, 703, 134699. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Karim, M.R.; Zheng, X.; Li, X. Heavy Metal and Metalloid Pollution of Soil, Water and Foods in Bangladesh: A Critical Review. Int. J. Environ. Res. Public Health 2018, 15, 2825. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Q.; Li, Y.; Bi, L.; Jin, L.; Peng, R. Toxic Effects of Cadmium on Fish. Toxics 2022, 10, 622. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, B.; Chen, H.; Yuan, R.; Wang, F. Distribution, Biological Effects and Biofilms of Microplastics in Freshwater Systems—A Review. Chemosphere 2022, 299, 134370. [Google Scholar] [CrossRef] [PubMed]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Al-Emran, M.; Alam, S.I.; Faggio, C. Effects of Heavy Metals on Fish Physiology—A Review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef] [PubMed]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The Potential of Microplastics as Carriers of Metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and Effects of Microplastics with Heavy Metals in Aquatic and Terrestrial Environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [CrossRef]
- Li, B.; Liang, W.; Liu, Q.-X.; Fu, S.; Ma, C.; Chen, Q.; Su, L.; Craig, N.J.; Shi, H. Fish Ingest Microplastics Unintentionally. Environ. Sci. Technol. 2021, 55, 10471–10479. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Xiang, L.; Mi, X.; Duan, M.; Wu, C. Fish Personality Affects Their Exposure to Microplastics. Ecotoxicol. Environ. Saf. 2022, 233, 113301. [Google Scholar] [CrossRef]
- Sequeira, I.F.; Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Worldwide Contamination of Fish with Microplastics: A Brief Global Overview. Mar. Pollut. Bull. 2020, 160, 111681. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Zhu, X.; Sun, C.; Teng, J.; Chen, L.; Shan, E.; Zhao, J. Microplastics in Fish Meals: An Exposure Route for Aquaculture Animals. Sci. Total. Environ. 2022, 807, 151049. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ge, J.; Yu, X. Bioavailability and Toxicity of Microplastics to Fish Species: A Review. Ecotoxicol. Environ. Saf. 2020, 189, 109913. [Google Scholar] [CrossRef] [PubMed]
- Emenike, E.C.; Iwuozor, K.O.; Anidiobi, S.U. Heavy Metal Pollution in Aquaculture: Sources, Impacts and Mitigation Techniques. Biol. Trace Elem. Res. 2022, 200, 4476–4492. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.; Niu, J.; Li, W.; Huang, Y.; Yu, H.; Lai, Z.; Liu, S.; Xu, E.G. How Do Microplastics Adsorb Metals? A Preliminary Study under Simulated Wetland Conditions. Chemosphere 2022, 309, 136547. [Google Scholar] [CrossRef]
- Marchant, D.J.; Iwan Jones, J.; Zemelka, G.; Eyice, O.; Kratina, P. Do Microplastics Mediate the Effects of Chemicals on Aquatic Organisms? Aquat. Toxicol. 2022, 242, 106037. [Google Scholar] [CrossRef]
- Peñalver, R.; Arroyo-Manzanares, N.; López-García, I.; Hernández-Córdoba, M. An Overview of Microplastics Characterization by Thermal Analysis. Chemosphere 2020, 242, 125170. [Google Scholar] [CrossRef]
- Brennecke, D.; Ferreira, E.C.; Costa, T.M.M.; Appel, D.; da Gama, B.A.P.; Lenz, M. Ingested Microplastics (>100 Μm) Are Translocated to Organs of the Tropical Fiddler Crab Uca Rapax. Mar. Pollut. Bull. 2015, 96, 491–495. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, T.; Li, Z.; Ge, W.; Wu, J.; Song, N.; Chai, C. Phthalate Esters in Surface Sediments from Fishing Ports in Circum-Bohai-Sea Region, China. Mar. Pollut. Bull. 2021, 171, 112782. [Google Scholar] [CrossRef]
- Gao, F.; Li, J.; Sun, C.; Zhang, L.; Jiang, F.; Cao, W.; Zheng, L. Study on the Capability and Characteristics of Heavy Metals Enriched on Microplastics in Marine Environment. Mar. Pollut. Bull. 2019, 144, 61–67. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption Behavior of Organic Pollutants on Microplastics. Ecotoxicol. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef]
- Fu, Q.; Tan, X.; Ye, S.; Ma, L.; Gu, Y.; Zhang, P.; Chen, Q.; Yang, Y.; Tang, Y. Mechanism Analysis of Heavy Metal Lead Captured by Natural-Aged Microplastics. Chemosphere 2021, 270, 128624. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Hu, H.; Li, J.; Li, J.; Wang, L.; Liu, L.; Tang, Y. Microplastics Existence Affected Heavy Metal Affinity to Ferrihydrite as a Representative Sediment Mineral. Sci. Total. Environ. 2023, 859, 160227. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, J.; Zhang, W.; Shi, L.; Yi, K.; Yu, H.; Zhang, C.; Li, S.; Li, J. Microplastics as a Vehicle of Heavy Metals in Aquatic Environments: A Review of Adsorption Factors, Mechanisms, and Biological Effects. J. Environ. Manage 2022, 302, 113995. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, S.; Zhang, C.; Pan, Z.; Sun, D.; Zhou, A.; Xu, G.; Zou, J. Interactions Effects of Nano-Microplastics and Heavy Metals in Hybrid Snakehead (Channa Maculata ♀ × Channa Argus ♂). Fish. Shellfish. Immunol. 2022, 124, 74–81. [Google Scholar] [CrossRef]
- Aghilinasrollahabadi, K.; Salehi, M.; Fujiwara, T. Investigate the Influence of Microplastics Weathering on Their Heavy Metals Uptake in Stormwater. J. Hazard. Mater. 2021, 408, 124439. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Martínez, I.E.; Shruti, V.C. Overview of Microplastics Pollution with Heavy Metals: Analytical Methods, Occurrence, Transfer Risks and Call for Standardization. J. Hazard. Mater. 2021, 415, 125755. [Google Scholar] [CrossRef]
- Yu, X.; Lang, M.; Huang, D.; Yang, C.; Ouyang, Z.; Guo, X. Photo-Transformation of Microplastics and Its Toxicity to Caco-2 Cells. Sci. Total. Environ. 2022, 806, 150954. [Google Scholar] [CrossRef]
- Stock, V.; Laurisch, C.; Franke, J.; Dönmez, M.H.; Voss, L.; Böhmert, L.; Braeuning, A.; Sieg, H. Uptake and Cellular Effects of PE, PP, PET and PVC Microplastic Particles. Toxicol. Vitro 2021, 70, 105021. [Google Scholar] [CrossRef]
- Xie, M.; Huang, J.-L.; Lin, Z.; Chen, R.; Tan, Q.-G. Field to Laboratory Comparison of Metal Accumulation on Aged Microplastics in Coastal Waters. Sci. Total. Environ. 2021, 797, 149108. [Google Scholar] [CrossRef]
- Bhagat, J.; Zang, L.; Nishimura, N.; Shimada, Y. Zebrafish: An Emerging Model to Study Microplastic and Nanoplastic Toxicity. Sci. Total. Environ. 2020, 728, 138707. [Google Scholar] [CrossRef]
- Cheng, H.; Feng, Y.; Duan, Z.; Duan, X.; Zhao, S.; Wang, Y.; Gong, Z.; Wang, L. Toxicities of Microplastic Fibers and Granules on the Development of Zebrafish Embryos and Their Combined Effects with Cadmium. Chemosphere 2021, 269, 128677. [Google Scholar] [CrossRef] [PubMed]
- De Marco, G.; Conti, G.O.; Giannetto, A.; Cappello, T.; Galati, M.; Iaria, C.; Pulvirenti, E.; Capparucci, F.; Mauceri, A.; Ferrante, M.; et al. Embryotoxicity of Polystyrene Microplastics in Zebrafish Danio rerio. Environ. Res. 2022, 208, 112552. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, H.; He, C.; Jin, Y.; Fu, Z. Polystyrene Nanoparticles Trigger the Activation of P38 MAPK and Apoptosis via Inducing Oxidative Stress in Zebrafish and Macrophage Cells. Environ. Pollut. 2021, 269, 116075. [Google Scholar] [CrossRef] [PubMed]
- Brun, N.R.; van Hage, P.; Hunting, E.R.; Haramis, A.-P.G.; Vink, S.C.; Vijver, M.G.; Schaaf, M.J.M.; Tudorache, C. Polystyrene Nanoplastics Disrupt Glucose Metabolism and Cortisol Levels with a Possible Link to Behavioural Changes in Larval Zebrafish. Commun. Biol. 2019, 2, 382. [Google Scholar] [CrossRef]
- Cheng, H.; Duan, Z.; Wu, Y.; Wang, Y.; Zhang, H.; Shi, Y.; Zhang, H.; Wei, Y.; Sun, H. Immunotoxicity Responses to Polystyrene Nanoplastics and Their Related Mechanisms in the Liver of Zebrafish (Danio Rerio) Larvae. Environ. Int. 2022, 161, 107128. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gundlach, M.; Yang, S.; Jiang, J.; Velki, M.; Yin, D.; Hollert, H. Quantitative Investigation of the Mechanisms of Microplastics and Nanoplastics toward Zebrafish Larvae Locomotor Activity. Sci. Total. Environ. 2017, 584–585, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Heng, X.; Chu, W. Polystyrene Nano/Microplastics Induce Microbiota Dysbiosis, Oxidative Damage, and Innate Immune Disruption in Zebrafish. Microb. Pathog. 2022, 163, 105387. [Google Scholar] [CrossRef]
- Yuan, Y.; Sepúlveda, M.S.; Bi, B.; Huang, Y.; Kong, L.; Yan, H.; Gao, Y. Acute Polyethylene Microplastic (PE-MPs) Exposure Activates the Intestinal Mucosal Immune Network Pathway in Adult Zebrafish (Danio Rerio). Chemosphere 2023, 311, 137048. [Google Scholar] [CrossRef]
- Xie, S.; Zhou, A.; Wei, T.; Li, S.; Yang, B.; Xu, G.; Zou, J. Nanoplastics Induce More Serious Microbiota Dysbiosis and Inflammation in the Gut of Adult Zebrafish than Microplastics. Bull. Environ. Contam. Toxicol. 2021, 107, 640–650. [Google Scholar] [CrossRef]
- Dimitriadi, A.; Papaefthimiou, C.; Genizegkini, E.; Sampsonidis, I.; Kalogiannis, S.; Feidantsis, K.; Bobori, D.C.; Kastrinaki, G.; Koumoundouros, G.; Lambropoulou, D.A.; et al. Adverse Effects Polystyrene Microplastics Exert on Zebrafish Heart - Molecular to Individual Level. J. Hazard. Mater. 2021, 416, 125969. [Google Scholar] [CrossRef]
- Mak, C.W.; Ching-Fong Yeung, K.; Chan, K.M. Acute Toxic Effects of Polyethylene Microplastic on Adult Zebrafish. Ecotoxicol. Environ. Saf. 2019, 182, 109442. [Google Scholar] [CrossRef] [PubMed]
- Limonta, G.; Mancia, A.; Benkhalqui, A.; Bertolucci, C.; Abelli, L.; Fossi, M.C.; Panti, C. Microplastics Induce Transcriptional Changes, Immune Response and Behavioral Alterations in Adult Zebrafish. Sci. Rep. 2019, 9, 15775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, D.; Yang, Y.; Zhang, H.; Zhu, J.; Liu, J.; Bu, X.; Li, E.; Qin, J.; Yu, N.; et al. Combined Effects of Polystyrene Microplastics and Copper on Antioxidant Capacity, Immune Response and Intestinal Microbiota of Nile Tilapia (Oreochromis Niloticus). Sci. Total. Environ. 2022, 808, 152099. [Google Scholar] [CrossRef]
- Jang, F.H.; Wong, C.; Choo, J.; Aun Sia, E.S.; Mujahid, A.; Müller, M. Increased Transfer of Trace Metals and Vibrio Sp. from Biodegradable Microplastics to Catfish Clarias gariepinus. Environ. Pollut. 2022, 298, 118850. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Vieira, L.R.; Guilhermino, L. Single and Combined Effects of Microplastics and Mercury on Juveniles of the European Seabass (Dicentrarchus Labrax): Changes in Behavioural Responses and Reduction of Swimming Velocity and Resistance Time. Environ. Pollut. 2018, 236, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Luzio, A.; Félix, L.; Bellas, J.; Monteiro, S.M. Oxidative Stress, Apoptosis and Serotonergic System Changes in Zebrafish (Danio Rerio) Gills after Long-Term Exposure to Microplastics and Copper. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 258, 109363. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Jin, S.-R.; Chen, Z.-Z.; Gao, J.-Z.; Liu, Y.-N.; Liu, J.-H.; Feng, X.-S. Single and Combined Effects of Microplastics and Cadmium on the Cadmium Accumulation, Antioxidant Defence and Innate Immunity of the Discus Fish (Symphysodon Aequifasciatus). Environ. Pollut. 2018, 243, 462–471. [Google Scholar] [CrossRef]
- Luís, L.G.; Ferreira, P.; Fonte, E.; Oliveira, M.; Guilhermino, L. Does the Presence of Microplastics Influence the Acute Toxicity of Chromium(VI) to Early Juveniles of the Common Goby (Pomatoschistus Microps)? A Study with Juveniles from Two Wild Estuarine Populations. Aquat. Toxicol. 2015, 164, 163–174. [Google Scholar] [CrossRef]
- Arshad, K.; Aqeel, M.; Noman, A.; Nazir, A.; Mahmood, A.; Rizvi, Z.F.; Sarfraz, W.; Hyder, S.; Zaka, S.; Khalid, N. Ecological Health Risk Assessment of Microplastics and Heavy Metals in Sediments, Water, Hydrophytes (Alternanthera Philoxeroides, Typha Latifolia, and Ipomoea Carnea), and Fish (Labeo Rohita) in Marala Wetlands in Sialkot, Pakistan. Environ. Sci. Pollut. Res. Int. 2023, 30, 41272–41285. [Google Scholar] [CrossRef]
- Santos, D.; Perez, M.; Perez, E.; Cabecinha, E.; Luzio, A.; Félix, L.; Monteiro, S.M.; Bellas, J. Toxicity of Microplastics and Copper, Alone or Combined, in Blackspot Seabream (Pagellus Bogaraveo) Larvae. Environ. Toxicol. Pharmacol. 2022, 91, 103835. [Google Scholar] [CrossRef]
- Santos, D.; Félix, L.; Luzio, A.; Parra, S.; Cabecinha, E.; Bellas, J.; Monteiro, S.M. Toxicological Effects Induced on Early Life Stages of Zebrafish (Danio Rerio) after an Acute Exposure to Microplastics Alone or Co-Exposed with Copper. Chemosphere 2020, 261, 127748. [Google Scholar] [CrossRef] [PubMed]
- Miranda, T.; Vieira, L.R.; Guilhermino, L. Neurotoxicity, Behavior, and Lethal Effects of Cadmium, Microplastics, and Their Mixtures on Pomatoschistus Microps Juveniles from Two Wild Populations Exposed under Laboratory Conditions―Implications to Environmental and Human Risk Assessment. Int. J. Environ. Res. Public Health 2019, 16, 2857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, M.; Chen, X.; Yang, C.; Wu, L. Combined Toxicity of Microplastics and Cadmium on the Zebrafish Embryos (Danio Rerio). Sci. Total. Environ. 2020, 743, 140638. [Google Scholar] [CrossRef] [PubMed]
- Assan, D.; Huang, Y.; Mustapha, U.F.; Addah, M.N.; Li, G.; Chen, H. Fish Feed Intake, Feeding Behavior, and the Physiological Response of Apelin to Fasting and Refeeding. Front. Endocrinol. 2021, 12, 798903. [Google Scholar] [CrossRef] [PubMed]
- Melvin, S.D.; Wilson, S.P. The Utility of Behavioral Studies for Aquatic Toxicology Testing: A Meta-Analysis. Chemosphere 2013, 93, 2217–2223. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, J.; Kim, N.; Han, G.C. Behavioral Changes of Zebrafish According to Cisplatin-Induced Toxicity of the Balance System. Hum. Exp. Toxicol. 2014, 33, 1167–1175. [Google Scholar] [CrossRef]
- Zuo, Z.; Wang, Q.; Zhang, C.; Zou, J. Single and Combined Effects of Microplastics and Cadmium on Juvenile Grass Carp (Ctenopharyngodon Idellus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 261, 109424. [Google Scholar] [CrossRef]
- Santos, D.; Luzio, A.; Matos, C.; Bellas, J.; Monteiro, S.M.; Félix, L. Microplastics Alone or Co-Exposed with Copper Induce Neurotoxicity and Behavioral Alterations on Zebrafish Larvae after a Subchronic Exposure. Aquat. Toxicol. 2021, 235, 105814. [Google Scholar] [CrossRef]
- Yin, L.; Chen, B.; Xia, B.; Shi, X.; Qu, K. Polystyrene Microplastics Alter the Behavior, Energy Reserve and Nutritional Composition of Marine Jacopever (Sebastes Schlegelii). J. Hazard. Mater. 2018, 360, 97–105. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Figueiredo, N.; Carvalho, F.; Carvalho, C.; Guilhermino, L. Microplastics Cause Neurotoxicity, Oxidative Damage and Energy-Related Changes and Interact with the Bioaccumulation of Mercury in the European Seabass, Dicentrarchus Labrax (Linnaeus, 1758). Aquat. Toxicol. 2018, 195, 49–57. [Google Scholar] [CrossRef]
- Teng, M.; Zhao, X.; Wu, F.; Wang, C.; Wang, C.; White, J.C.; Zhao, W.; Zhou, L.; Yan, S.; Tian, S. Charge-Specific Adverse Effects of Polystyrene Nanoplastics on Zebrafish (Danio Rerio) Development and Behavior. Environ. Int. 2022, 163, 107154. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Carvalho, C.; Guilhermino, L. Microplastics Increase Mercury Bioconcentration in Gills and Bioaccumulation in the Liver, and Cause Oxidative Stress and Damage in Dicentrarchus Labrax Juveniles. Sci. Rep. 2018, 8, 15655. [Google Scholar] [CrossRef]
- Evariste, L.; Barret, M.; Mottier, A.; Mouchet, F.; Gauthier, L.; Pinelli, E. Gut Microbiota of Aquatic Organisms: A Key Endpoint for Ecotoxicological Studies. Environ. Pollut. 2019, 248, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Hamid, N.; Deng, S.; Jia, P.-P.; Pei, D.-S. Individual and Combined Toxicogenetic Effects of Microplastics and Heavy Metals (Cd, Pb, and Zn) Perturb Gut Microbiota Homeostasis and Gonadal Development in Marine Medaka (Oryzias Melastigma). J. Hazard. Mater. 2020, 397, 122795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ye, L.; Wang, C.; Xiong, X.; Li, Y.; Li, P.; Zhang, X.; Yu, H. Toxic Effect of Combined Exposure of Microplastics and Copper on Goldfish (Carassius Auratus): Insight from Oxidative Stress, Inflammation, Apoptosis and Autophagy in Hepatopancreas and Intestine. Bull. Environ. Contam. Toxicol. 2022, 109, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, L.; Gu, W.; Liu, S.; Wu, B. Heterogeneity Effects of Nanoplastics and Lead on Zebrafish Intestinal Cells Identified by Single-Cell Sequencing. Chemosphere 2022, 289, 133133. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhu, Z.; Xie, Y.; Zheng, C.; Zhou, Z.; Zhu, T.; Zhang, Y. Comparison of the Combined Toxicity of Polystyrene Microplastics and Different Concentrations of Cadmium in Zebrafish. Aquat. Toxicol. 2022, 250, 106259. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.; Félix, L.; Luzio, A.; Parra, S.; Bellas, J.; Monteiro, S.M. Single and Combined Acute and Subchronic Toxic Effects of Microplastics and Copper in Zebrafish (Danio Rerio) Early Life Stages. Chemosphere 2021, 277, 130262. [Google Scholar] [CrossRef] [PubMed]
- Jinhui, S.; Sudong, X.; Yan, N.; Xia, P.; Jiahao, Q.; Yongjian, X. Effects of Microplastics and Attached Heavy Metals on Growth, Immunity, and Heavy Metal Accumulation in the Yellow Seahorse, Hippocampus Kuda Bleeker. Mar. Pollut. Bull. 2019, 149, 110510. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kurobe, T.; Flores, I.; Teh, S.J. Early Warning Signs of Endocrine Disruption in Adult Fish from the Ingestion of Polyethylene with and without Sorbed Chemical Pollutants from the Marine Environment. Sci. Total. Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef]
- Bobori, D.C.; Feidantsis, K.; Dimitriadi, A.; Datsi, N.; Ripis, P.; Kalogiannis, S.; Sampsonidis, I.; Kastrinaki, G.; Ainali, N.M.; Lambropoulou, D.A.; et al. Dose-Dependent Cytotoxicity of Polypropylene Microplastics (PP-MPs) in Two Freshwater Fishes. Int. J. Mol. Sci. 2022, 23, 13878. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.T.B.; Estrela, F.N.; de Rodrigues, A.S.L.; Chagas, T.Q.; Pereira, P.S.; Silva, F.G.; Malafaia, G. Nanopolystyrene Particles at Environmentally Relevant Concentrations Causes Behavioral and Biochemical Changes in Juvenile Grass Carp (Ctenopharyngodon Idella). J. Hazard. Mater. 2021, 403, 123864. [Google Scholar] [CrossRef]
- Tan, F.; Wang, M.; Wang, W.; Lu, Y. Comparative Evaluation of the Cytotoxicity Sensitivity of Six Fish Cell Lines to Four Heavy Metals in Vitro. Toxicol. Vitro 2008, 22, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Peng, L.-B.; Wang, D.; Zhu, Q.-L.; Zheng, J.-L. Combined Effects of Polystyrene Microplastics and Cadmium on Oxidative Stress, Apoptosis, and GH/IGF Axis in Zebrafish Early Life Stages. Sci. Total. Environ. 2022, 813, 152514. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of Microplastics on the Accumulation and Chronic Toxic Effects of Cadmium in Zebrafish (Danio Rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef]
- Andriantsitohaina, R.; Duluc, L.; García-Rodríguez, J.C.; Gil-del Valle, L.; Guevara-Garcia, M.; Simard, G.; Soleti, R.; Su, D.-F.; Velásquez-Pérez, L.; Wilson, J.X.; et al. Systems Biology of Antioxidants. Clin. Sci. 2012, 123, 173–192. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics Induce Intestinal Inflammation, Oxidative Stress, and Disorders of Metabolome and Microbiome in Zebrafish. Sci. Total. Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Xie, Y.; Ma, Y.; Zhang, J.; Wei, H.; Abdou, A.I.E. Physiological Response and Oxidative Stress of Grass Carp (Ctenopharyngodon Idellus) under Single and Combined Toxicity of Polystyrene Microplastics and Cadmium. Ecotoxicol. Environ. Saf. 2022, 245, 114080. [Google Scholar] [CrossRef]
- Pan, Y.-X.; Luo, Z.; Zhuo, M.-Q.; Wei, C.-C.; Chen, G.-H.; Song, Y.-F. Oxidative Stress and Mitochondrial Dysfunction Mediated Cd-Induced Hepatic Lipid Accumulation in Zebrafish Danio rerio. Aquat. Toxicol. 2018, 199, 12–20. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Khosraviani, K.; Hosseinpour Delavar, F.; Arghideh, M.; Zavvar, F.; Hoseinifar, S.H.; Van Doan, H.; Zabihi, E.; Reverter, M. Hepatic Transcriptomic and Histopathological Responses of Common Carp, Cyprinus Carpio, to Copper and Microplastic Exposure. Mar. Pollut. Bull. 2022, 175, 113401. [Google Scholar] [CrossRef]
- Qin, L.; Duan, Z.; Cheng, H.; Wang, Y.; Zhang, H.; Zhu, Z.; Wang, L. Size-Dependent Impact of Polystyrene Microplastics on the Toxicity of Cadmium through Altering Neutrophil Expression and Metabolic Regulation in Zebrafish Larvae. Environ. Pollut. 2021, 291, 118169. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, Q.; Lin, C.; He, L.; Wei, L. Histological Alterations, Oxidative Stress, and Inflammatory Response in the Liver of Swamp Eel (Monopterus Albus) Acutely Exposed to Copper. Fish. Physiol. Biochem. 2021, 47, 1865–1878. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, S.; Wang, Z.; Zhang, C.; Pan, Z.; Sun, D.; Xu, G.; Zou, J. Single and Combined Effects of Microplastics and Cadmium on the Cadmium Accumulation and Biochemical and Immunity of Channa argus. Biol. Trace Elem. Res. 2022, 200, 3377–3387. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Nishimura, N.; Shimada, Y. Toxicological Interactions of Microplastics/Nanoplastics and Environmental Contaminants: Current Knowledge and Future Perspectives. J. Hazard. Mater. 2021, 405, 123913. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Yu, Y.-B.; Choi, J.-H. Toxic Effects on Bioaccumulation, Hematological Parameters, Oxidative Stress, Immune Responses and Neurotoxicity in Fish Exposed to Microplastics: A Review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef]
- Banaee, M.; Soltanian, S.; Sureda, A.; Gholamhosseini, A.; Haghi, B.N.; Akhlaghi, M.; Derikvandy, A. Evaluation of Single and Combined Effects of Cadmium and Micro-Plastic Particles on Biochemical and Immunological Parameters of Common Carp (Cyprinus Carpio). Chemosphere 2019, 236, 124335. [Google Scholar] [CrossRef]
- Da Acosta, D.S.; Danielle, N.M.; Altenhofen, S.; Luzardo, M.D.; Costa, P.G.; Bianchini, A.; Bonan, C.D.; da Silva, R.S.; Dafre, A.L. Copper at Low Levels Impairs Memory of Adult Zebrafish (Danio Rerio) and Affects Swimming Performance of Larvae. Comp. Biochem. Physiol. Part. C: Toxicol. Pharmacol. 2016, 185–186, 122–130. [Google Scholar] [CrossRef]
- Santos, D.; Luzio, A.; Bellas, J.; Monteiro, S.M. Microplastics- and Copper-Induced Changes in Neurogenesis and DNA Methyltransferases in the Early Life Stages of Zebrafish. Chem. Biol. Interact. 2022, 363, 110021. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Cheng, H.; Wang, Y.; Fang, Y.; Wang, L.; Duan, Z. Polystyrene Microplastics Inhibit the Neurodevelopmental Toxicity of Mercury in Zebrafish (Danio Rerio) Larvae with Size-Dependent Effects. Environ. Pollut. 2022, 314, 120216. [Google Scholar] [CrossRef]
- Santos, D.; Luzio, A.; Félix, L.; Cabecinha, E.; Bellas, J.; Monteiro, S.M. Microplastics and Copper Induce Apoptosis, Alter Neurocircuits, and Cause Behavioral Changes in Zebrafish (Danio Rerio) Brain. Ecotoxicol. Environ. Safety 2022, 242, 113926. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio Rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Ling, X.; Yan, Z.; Wu, D.; Liu, J.; Lu, G. Effects of Nanoplastics and Butyl Methoxydibenzoylmethane on Early Zebrafish Embryos Identified by Single-Cell RNA Sequencing. Environ. Sci. Technol. 2021, 55, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kong, H.; Ji, X.; Gao, Y.; Jin, M. Zebrafish Behavioral Phenomics Applied for Phenotyping Aquatic Neurotoxicity Induced by Lead Contaminants of Environmentally Relevant Level. Chemosphere 2019, 224, 445–454. [Google Scholar] [CrossRef]
- Torres-Ruiz, M.; De la Vieja, A.; de Alba Gonzalez, M.; Esteban Lopez, M.; Castaño Calvo, A.; Cañas Portilla, A.I. Toxicity of Nanoplastics for Zebrafish Embryos, What We Know and Where to Go Next. Sci. Total. Environ. 2021, 797, 149125. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.F.; Meyer, D.N.; Petriv, A.-M.V.; Soto, A.L.; Shields, J.N.; Akemann, C.; Baker, B.B.; Tsou, W.-L.; Zhang, Y.; Baker, T.R. Nanoplastics Impact the Zebrafish (Danio Rerio) Transcriptome: Associated Developmental and Neurobehavioral Consequences. Environ. Pollut. 2020, 266, 115090. [Google Scholar] [CrossRef]




| Types of Microplastics | Particle Size | Exposure Concentration | Exposure Time | Toxic Effect | References |
|---|---|---|---|---|---|
| Embryo (0–5 d) | |||||
| PET | 150 mm | 20 mg/L | 3 d | Heart rate increases, blood flow rate increases, hatching rate decreases | [41] |
| PS | 10 μm | 200 particles/mL | 5 d | Oxidative stress, developmental deformities, and decreased survival rate | [42] |
| PS | 42 nm | 10 mg/mL | 5 d | Oxidative stress, inflammation, and apoptosis increased | [43] |
| Juvenile (5–90 d) | |||||
| PS | 25 nm | 20 mg/L | 2 d | Glucose metabolism is disturbed, and behavior is inhibited | [44] |
| PS | 50 nm, 100 nm | 0.1 mg/L, 0.5 mg/L, 2 mg/L, 10 mg/L | 3 d | Oxidative stress, liver inflammation, liver immunotoxicity | [45] |
| PE | 50 nm, 45 μm | 1 mg/L | 120 h | Oxidative stress, inhibited behavior, and shortened body length | [46] |
| Adult fish | |||||
| PE | 50 μm, 100 nm | 100 μg/L | 14 d | Oxidative damage to the central nervous system, intestinal flora imbalance, stimulate immune response | [47] |
| PE | 30 μm | 1000 μg/L | 7 d | Immunotoxicity, intestinal inflammation | [48] |
| PE | 80 nm, 8 μm | 10 μg/L, 1 mg/L | 21 d | Intestinal flora disorder, intestinal inflammation | [49] |
| PE | 3–12 µm | 260 mg/L | 21 d | Oxidative stress, metabolic changes, DNA damage | [50] |
| PE | 10–22 μm, 45–53 μm, 90–106 μm, 212–250 μm, 500–600 μm | 2 mg/L | 4 d | Intestinal injury, neurotoxicity | [51] |
| PS | 90% < 90 μm, 10% < 25 μm | 100 μg/L 1000 μg/L | 20 d | Immunotoxicity, lipid metabolism changes | [52] |
| Species of Fish | Types of MPs | Particle Size | MPs Concentration | Types of HMs | HMs Concentration | Exposure Duration | The Effects of Biological Toxicity | References |
|---|---|---|---|---|---|---|---|---|
| Nile tilapia (Oreochromis niloticus) | PS | 0.1 μm | 1 mg/L | Cu | 0.5 mg/L, 1 mg/L, 2 mg/L | 24 h | 1. Caused serious pathological changes in the internal organs. 2. Increased the number of harmful bacteria in the gut of Nile tilapia and decreased the immunity. | [53] |
| Catfish (Clarias gariepinus) | Polyamide 12, PLA | N.A. | N.A. | Cu | 0.050 mg/L | 3 months | 1. The concentration of Cu and Pb in gills was the highest, followed by that in the liver and intestine. 2. The microflora become disordered, reducing the immunity and causing potential vibrio infection. | [54] |
| Pb | 0.060 mg/L | |||||||
| European seabass (Dicentrarchus labrax) | Polymer microspheres | 1–5 μm | 5–28% | Hg | 45–53% | 96 h | 1. Affects the behavioral responses. 2. Increases the bioconcentration of Hg in the gills and bioaccumulation in the liver. | [55] |
| Zebrafish (Danio rerio) | PS | 1–5 μm | 2 mg/L | Cu | 25 μg/L | 30 d | Resulting in increased membrane lipid oxidation, SOD activity decreased, DNA damage and so on. | [56] |
| Discus fish (Symphysodon aequifasciatus) | PS | 32–40 μm | 50 μg/L, 500 μg/L | Cd | 50 μg/L | 30 d | Severe oxidative stress was observed. | [57] |
| Goby (Pomatoschistus microps) | PE | 1–5 μm | 0.184 mg/L | Cr | 5.6 mg/L, 8.4 mg/L, 12.6 mg/L, 18.9 mg/L, 28.4 mg/L | 48 h | Inhibited 31% of the acetylcholinesterase activity. | [58] |
| Species of Fish | Types of MPs | Particle Size | MPs Concentration | Types of HMs | HMs Concentration | Exposure Time | Effects on Fish Behavior | References |
|---|---|---|---|---|---|---|---|---|
| Zebrafish (Danio rerio) | polymer microspheres | 1–5 µm | 2 mg/L | Cu | 60 μg/L, 125 μg/L | 14 d | Decreased the average speed, travel distance, and absolute turning angle. Adversely affected their swimming ability. Affected the avoidance behavior. Larvae did not respond to adverse stimuli. | [68] |
| European seabass (Dicentrarchus labrax) | polymer microspheres | 1–5 µm | 5–28% | Hg | 45–53% | 96 h | Reduced swimming speed and survival time. | [55] |
| Grass carp (Ctenopharyngodon idellus) | PS | 5 μm | 700 μg/L | Cd | 100 μg/L | 24 h, 48 h | The swimming speed increased first and then decreased. The behavioral mechanisms were altered. Reduce their exposure to pollutants by reducing their activity levels and metabolic rates. | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Zhao, H.; Liu, Y.; Jin, L.; Peng, R. Factors Affecting the Adsorption of Heavy Metals by Microplastics and Their Toxic Effects on Fish. Toxics 2023, 11, 490. https://doi.org/10.3390/toxics11060490
Chen Q, Zhao H, Liu Y, Jin L, Peng R. Factors Affecting the Adsorption of Heavy Metals by Microplastics and Their Toxic Effects on Fish. Toxics. 2023; 11(6):490. https://doi.org/10.3390/toxics11060490
Chicago/Turabian StyleChen, Qianqian, Haiyang Zhao, Yinai Liu, Libo Jin, and Renyi Peng. 2023. "Factors Affecting the Adsorption of Heavy Metals by Microplastics and Their Toxic Effects on Fish" Toxics 11, no. 6: 490. https://doi.org/10.3390/toxics11060490
APA StyleChen, Q., Zhao, H., Liu, Y., Jin, L., & Peng, R. (2023). Factors Affecting the Adsorption of Heavy Metals by Microplastics and Their Toxic Effects on Fish. Toxics, 11(6), 490. https://doi.org/10.3390/toxics11060490