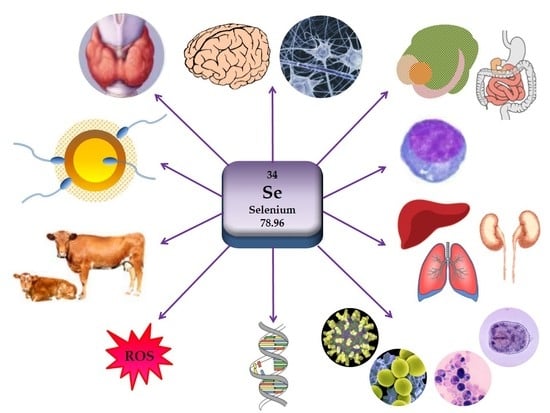
A Summary of New Findings on the Biological Effects of Selenium in Selected Animal Species—A Critical Review
Abstract
:1. Methodology of the Review
2. Biochemistry of Selenium
2.1. Importance of Selenium for Animal Health
2.2. Biochemical and Molecular Biological Activities of Selenium in Organism
2.2.1. Role of Selenium in Oxidative Stress
2.2.2. Relationship of Selenium to Cancer
2.2.3. Antioxidative Role of Selenium against the Toxic Effect of Heavy Metals
2.2.4. Epigenetic Effects of Selenium and Their Implications for Prevention of Carcinogenic Process
2.3. Health Disorders of Animals Associated with Selenium Deficiency
2.3.1. Described Diseases Associated with Selenium Deficiency
2.3.2. Effect of Selenium on Female Reproduction
2.3.3. Effect of Selenium on Male Reproduction
2.3.4. Effect of Selenium on Reduction of Intramammary Infection and Milk Quality
2.3.5. Effect of Selenium on Rumen Fermentation
2.3.6. Effect of Selenium on Hair Production
3. Selenium Status Assessment in Animals
3.1. Selenium Status Assessment
3.2. Total Selenium Concentration
3.3. Enzymatic Methods of Assessment of Selenium Status
4. Dietary Addition of Selenium
4.1. Intake Recommendations for Selenium in Animals
4.2. Dietary Forms of Selenium
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAS | atomic absorption spectroscopy |
| ABTS+ | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| ADF | acid detergent fiber |
| AdoHcy | S-Adenosyl-homocysteine |
| Aebp2 | AE binding protein 2 gene |
| ALP | alkaline phosphatase |
| aNDF | amylase-treated neutral detergent fiber |
| ApoER2 | apolipoprotein E receptor 2 |
| AST | aspartate aminotransferase |
| ATP | adenosine triphosphate |
| Bax | pro-apoptotic protein Bax, Bcl-2-associated X protein |
| Bcl-2 | anti-apoptotic protein Bcl-2 (B-cell lymphoma 2) |
| BHT | butylated hydroxytoluene |
| BW | body weight |
| Caco-2 | caco-2 cell line—heterogeneous human epithelial colorectal adenocarcinoma cells |
| CAT | catalase |
| CAT | catalase gene |
| cGPx | cellular glutathione peroxidase; GPx1 |
| CH3Se | methylselenol |
| CNS | central nervous system |
| Cu | copper |
| Cu/Zn-SOD | copper/zinc superoxide dismutase; SOD1 |
| D1,2,3 | deionidase 1,2,3 |
| DM | dry matter |
| DNA | deoxyribonucleic acid |
| DTNB | 5-5′-dithiobis[2-nitrobenzoic acid] |
| ECG | electrocardiography |
| EDTA | ethylenediaminetetraacetic acid |
| ELISA | enzyme-linked immunosorbent assay |
| ESCs | embryonic stem cells |
| Ex/Em | excitation/emission |
| FIA-GF-AAS | flow injection analysis-graphite furnace-atomic absorption spectrometry |
| FOX | xylenol orange |
| GDH | glutamate dehydrogenase |
| GGT | gamma-glutamyl transferase |
| GH | growth hormone |
| GIT | gastrointestinal tract |
| GPx | glutathione peroxidase |
| GPx1,2,3,4 | glutathione peroxidase 1,2,3,4 |
| GPx1,2,3,4 | glutathione peroxidase 1,2,3,4 genes |
| GR | glutathione reductase |
| Grx | glutaredoxin |
| GSH | reduced glutathione |
| GS-SeH | glutathioselenol |
| GSSG | oxidized glutathione |
| GS-TNB | glutathione adduct of GSH |
| Hb | hemoglobin |
| HCl | hydrochloric acid |
| Hcy | homocysteine |
| HDAC | histone deacetylase |
| Hg | mercury (hydrargyrum) |
| HG-AAS | hydride generation-atomic absorption spectrometry |
| HNO3 | nitric acid |
| H2O2 | hydrogen peroxide |
| HPLC | high-performance liquid chromatography |
| HPLC-ICP-MS | high-performance liquid chromatography-inductively coupled plasma-mass spectrometry |
| HSe− | hydrogen selenide ion |
| H2Se | hydrogen selenide |
| HT | hematein |
| HTH2 | hematoxylin |
| ICP | inductively coupled plasma |
| ICP-MS | inductively coupled plasma-mass spectrometry |
| ICP-OES | inductively coupled plasma-optical emission spectrometry |
| IEC | ion exchange chromatography |
| IGF-1 | insulin-like growth factor 1 |
| IGF-1R | insulin-like growth factor 1 receptor |
| INT | 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride |
| IRE | iron responsive element |
| IRP | iron regulatory protein |
| LC | liquid chromatography |
| LC-ICP-MS | liquid chromatography-inductively coupled plasma-mass spectrometry |
| LR | linear regression |
| MCSeP | mitochondrial capsule selenoprotein |
| MDA | malondialdehyde |
| MDA-TBA2 | adduct formed by reaction of MDA with TBA |
| MeSeCys | Se-methylselenocysteine |
| Met | methionine |
| min–max | minimum‒maximum |
| miRNA | microRNA—a small non-coding RNA molecule |
| Mn | manganese |
| MnSOD | manganese superoxide dismutase; SOD2 |
| mRNA | messenger ribonucleic acid |
| MTs | metallothioneins |
| MT-I | metallothionein-I gene |
| MT-II | metallothionein-II gene |
| NaBH4 | sodium borohydride |
| NAD | nicotinamide adenine dinucleotide |
| NADP+ | nicotinamide adenine dinucleotide phosphate |
| NADPH | reduced form of NADP+ |
| NaOH | sodium hydroxide |
| Na2SeO3 | sodium selenite |
| NBT | nitroblue tetrazolium |
| NH3 | ammonia |
| NMD | nutritional muscular dystrophy |
| O2 | dioxygen |
| O2− | superoxide anion |
| OD | optical density |
| OS | oxidative stress |
| p53 | p53 gene encoding the tumor suppressor protein p53 |
| PCV | packed cell volume |
| PDGF-A | platelet-derived growth factor A |
| PDGF-B | platelet-derived growth factor B |
| pH | potential of hydrogen (pondus hydrogenia) |
| PHGPx | phospholipid-hydroperoxide GPx |
| PI3K/Akt pathway | phosphatidylinositol 3′-kinase/protein kinase B (serine/threonine-protein kinase) pathway |
| PMN | polymorphonuclear leucocytes |
| PO43− | phosphate ion |
| PR | polynomial regression |
| PRDX1–6 | peroxiredoxin 1–6 genes |
| Prickle2 | prickle homolog 2 gene |
| PUFAs | polyunsaturated fatty acids |
| r | correlation coefficient |
| R2, r2 | coefficient of determination (r2 for simple linear regression) |
| RDI | recommended daily intake |
| RNA | ribonucleic acid |
| Rnd2 | Rho family GTPase (guanosine triphosphatase) 2 gene |
| ROH | lipid hydroxide |
| ROOH | lipid hydroperoxide |
| ROS | reactive oxygen species |
| RPC | reversed-phase chromatography |
| RTK | receptor tyrosine kinase |
| RV | reference value |
| S | sulfur |
| SAM | S-adenosylmethionine |
| SCC | somatic cell count |
| SCFAs | short-chain fatty acids |
| SCS | somatic cell score |
| SD | standard deviation |
| SE | standard error |
| Se | selenium |
| Se0 | elemental selenium |
| Se+IV | selenite |
| Se+VI | selenate |
| SECIS | selenocysteine insertion sequence |
| SeCys | selenocysteine |
| SeCys2 | selenocystine |
| SeGPx | selenium-dependent glutathione peroxidase |
| SeH4 | tetrahydridoselenonium dication |
| SELENBP1 | selenium binding protein 1 gene |
| SEM | standard error of mean |
| SeMet | selenomethionine |
| SeO32− | selenite |
| SeO42− | selenate |
| SEP15 | selenoprotein 15 gene |
| SEPP | selenoprotein P |
| SEPP1 | selenoprotein P gene |
| SO42− | sulfate ion |
| SOD | superoxide dismutase |
| SOD1,2,3 | superoxide dismutase 1,2,3 |
| SOD1,2,3 | superoxide dismutase 1–3 genes |
| SPS-2 | selenophosphate synthase-2 |
| SPS-2 | selenophosphate synthase-2 gene |
| T3 | triiodothyronine |
| T4 | thyroxine |
| TBA | thiobarbituric acid |
| TBARS | thiobarbituric acid reactive substances |
| TBH | tertiary butyl hydroperoxide |
| TNB | 5-thio-2-nitrobenzoic acid |
| Trx | thioredoxin |
| TRXNRD1–2 | thioredoxin reductase 1/2 genes |
| TrxR | thioredoxin reductase |
| TSP | transsulfuration pathway |
| UGA | nucleotide triplet UGA encoding selenocysteine |
| 3′ UTR | 3′ untranslated region |
| UV | ultraviolet |
| VESD | vitamin E/selenium deficiency |
| VFA | volatile fatty acid |
| VG-ICP-MS | vapor generation-inductively coupled plasma-mass spectrometry |
| WMD | white muscle disease |
| x | mean |
| XOD | xanthine oxidase |
| Zn | zinc |
Appendix A
| Animals | Selenium Concentration | GPx Activity | SOD Activity | CAT Activity | MDA Level | Reference |
|---|---|---|---|---|---|---|
| Specification (Region, Breed, Sex, Age, Weight) | Units | Units | Units | Units | Units | |
| Pigs | ||||||
| --- | RV: 0.12–0.30 μg∙mL−1 (in serum) | RV: 100–200 μmol∙min−1 at 37 °C∙g−1 Hb (erythrocyte GPx) | --- | --- | --- | [304] |
| Pigs - age of < 1 day - 1–9 days - 10–29 days - 30–70 days - 71–180 days - 181–300 days - 301–700 days - > 700 days | RV: 70–90 70–120 70–120 100–160 140–190 180–220 180–220 180–220 ng∙mL−1 (in serum) | --- | --- | --- | --- | [314] |
| 7-day-old piglets (Duroc × Landrace)—control group | --- | ~222 U∙mg−1 protein (in liver) *1 | ~265 U∙mg−1 protein (in liver) *1 | --- | ~2.4 nmol∙mg−1 protein (in liver) *1 | [361] |
| Piglets from crossbred pregnant sows (Large White × Landrace) on day 107 of gestation—control animals | --- | 621.69 ± 24.93 mmol∙L−1 (x ± SEM) (in serum) *2 | --- | 7.38 ± 0.27 U∙mL−1 (x ± SEM) (in serum) *2 | --- | [343] |
| Crossbred (Yorkshire × Landrace × Duroc) weaned pigs (28 ± 2 days of age) | --- | 0.13 U∙g−1 Hb (erythrocyte GPx) *3 | 443.3 U∙g−1 Hb (erythrocyte Cu/Zn-SOD) *3 | 1.74 U∙g−1 Hb (erythrocyte CAT) *3 | 4.29 μM (in plasma) *3 | [342] |
| Cattle | ||||||
| --- | RV: 0.08–0.30 μg∙mL−1 (in serum) | RV: 19–36 μmol∙min−1 at 37 °C∙g−1 Hb (erythrocyte GPx) | --- | --- | --- | [304] |
| Cattle - age of <1 day - 1–9 days - 10–29 days - 30–300 days - 301–700 days - >700 days | RV: 50–70 50–70 55–75 60–80 65–90 70–100 ng∙mL−1 (in serum) | --- | --- | --- | --- | [314] |
| Holstein-Frisian cows 12 h postpartum—control group | 129.0 ± 18.0 ng∙mL−1 (x ± SD) (in blood) *4 | 90.6 ± 16.1 μkat∙L−1 (x ± SD) (in whole blood) *4 | --- | --- | 5.71 ± 0.94 μM (x ± SD) (in serum) *4 | [270] |
| Cattle—control group | --- | 172.5 ± 30.7 U∙g−1 Hb (x ± SD) (erythrocyte GPx); 24.3 ± 4.8 U∙g−1 protein (x ± SD) (hepatic GPx) *5 | --- | --- | --- | [357] |
| Cattle (dairy cows, bulls, heifers) in Czech Republic | 78.25 ± 46.67 (1.33–212.40) μg∙L−1 (x ± SD; min–max) (in whole blood) *6 | 525.51 ± 335.56 (0.41–1521.1) μkat∙L−1 (x ± SD; min–max) (in whole blood) *6 RV of GPx activity: 472.20–665.40 * μkat∙L−1 | --- | --- | --- | [267] |
| Cattle—(a) bulls (b) heifers (c) cows | 56.9 ± 43.2 39.0 ± 20.8 83.2 ± 20.0 μg∙L−1 (x ± SD) (in whole blood) *6 | 368.7 ± 343.4 227.4 ± 130.8 741.7 ± 233.5 μkat∙L−1 (x ± SD) (in whole blood) *6 RV of GPx activity: 760.23 ** μkat∙L−1 | --- | --- | --- | [313] |
| Sheep | ||||||
| --- | RV: 0.08–0.50 μg∙mL−1 (in serum) | 60–180 μmol∙min−1 at 37 °C∙g−1 Hb (erythrocyte GPx) | --- | --- | --- | [304] |
| Sheep - age of < 1 day - 1–9 days - 10–29 days - 30–70 days - 71–180 days - 181–300days - 301–700 days - >700 days | RV - 50–80 - 60–90 - 70–100 - 80–110 - 80–110 - 80–110 - 90–120 - 120–160 ng∙mL−1 (in serum) | --- | --- | --- | --- | [314] |
| Iranian fat-tailed sheep | --- | RV: 191.67–196.52 U∙g−1 Hb (in blood) *** *7 | RV: 948.65–1011.50 U∙g−1 Hb (in blood) *** *7 | RV: 1834.29–1915.63 U∙g−1 Hb (in blood) *** *7 | RV: 0.53–0.60 μmol∙L−1 (in blood) *** *7 | [346] |
| Sheep in the Czech Republic (Suffolk or Merinolandschaft breeds) | 123.42 ± 57.84 μg∙L−1 (x ± SD) (in blood) *8 | 814.34 ± 463.64 μkat∙L−1 (x ± SD) (in blood) RV: >637 μkat∙L−1 (LR), resp. > 677 μkat∙L−1 in whole blood (PR) **** *8 | --- | --- | --- | [271] |
| Grazing ewes in Serbia (Wirtenberg × Cigaja crossbred sheep)—control group | --- | 157.4 ± 61.9 μkat∙L−1 (in whole blood) *9 | --- | --- | --- | [393] |
| ½ Dorper (♂) × ½ Small thin-tailed (♀) crossed ram lambs (4 months old, 25 ± 1 kg) (a) in free-range conditions (b) in individual stalls | --- | (a) 84.01 ± 4.33 (b) 71.56 ± 2.06 U∙mg−1 (x ± SEM) (GPx4 in testes) *10 | (a) 6.05 ± 0.03 (b) 5.88 ± 0.12 U∙mg−1 (x ± SEM) (in testes) *10 | (a) 5.28 ± 0.11 (b) 4.29 ± 0.08 U∙mg−1 (x ± SEM) (in testes) *10 | (a) ~0.65 (b) ~1.2 nM∙mg−1 (in testes) *10 | [363] |
| Akkaraman sheep, weight 20–25 kg, age 6–12 months—control group | --- | 18.71 ± 1.11 U∙mg −1 protein (x ± SD) (in liver) *11 | 5.00 ± 0.21 U∙mg−1 protein (x ± SD) (Cu/Zn-SOD in liver) *11 | 849.24 ± 23.83 k∙g−1 (x ± SD) (in liver) *11 | 45.26 ± 1.15 nmol∙g −1 (x ± SD) (in liver) *11 | [394] |
| Goats | ||||||
| Goats - age of <1 day - 1–9 days - 10–29 days - 30–70 days - 71–180 days - 181–300 days - 301–700 days - >700 days | RV - 50–80 - 60–90, - 70–100 - 80–110 - 80–110 - 80–110 - 90–120 - 120–160 ng∙mL−1 (in serum) | --- | --- | --- | --- | [314] |
| Red Sokoto goats of about 1-year-old, weighing 10–14 kg—control group | --- | ~54 IU∙L−1 (in serum) *12 | ~2.4 IU∙L−1 (in serum) *12 | ~47.4 IU∙L−1 (in serum) *12 | ~ 1.25 nmol∙L−1 (in serum) *12 | [344] |
| Weanling Boer goat bucks (2 months old) from selenium deficiency region in central China—control group | 0.6491 mg∙kg−1 (in testes) *13 | 13.55 ± 3.15 U∙mL−1 (x ± SD) (in semen); 65.20 ± 5.89 U∙mg−1 (x ± SD) (testicular GPx) *13 | --- | --- | --- | [362] |
| Cashmere goats, aged 3-year-old and weighing 34.35 ± 0.94 kg from selenium deficiency region in China—control group | 85.24 ng∙mL−1 (in serum); 32.6 ng∙mL−1 (in skin) *14 | 264.82 U∙ml−1 (in serum); 113.89 U∙mL−1 (in skin) *14 | 72 U∙mL−1 (in serum); 9.29 U∙mL−1 (in skin) *14 | 2.31 nmol∙ml−1 (in serum) 0.46 nmol∙ml−1 (in skin) *14 | [102] | |
| Horses | ||||||
| --- | RV: 0.14–0.25 μg∙mL−1 (in serum) | RV: 30–150 μmol∙min−1 at 37 °C∙g−1 Hb (erythrocyte GPx) | --- | --- | --- | [304] |
| Horses - age of < 1 day - 1–9 days - 10–29 days - 30–70 days - 71–180 days - 181–300 days - 301–700 days - >700 days | RV: 70–90 70–90 80–110 90–110 90–110 90–110 100–130 130–160 ng∙mL−1 (in serum) | --- | --- | --- | --- | [314] |
| Arabian mares—healthy (control group), age of 15 ± 1.5 months | --- | 32.07 ± 5.10 U∙g−1 Hb (x ± SE) (erythrocyte GPx) *15 | --- | --- | 1.50 ± 0.13 nmol∙mL−1 (x ± SE) (in plasma) *15 | [395] |
| Standardbred horses (mares, geldings)—control group | ~0.052 ppm (in plasma); 0.15 ppm (in red blood cells) *16 | ~ 100 U∙g−1 Hb (in whole blood) *16 | --- | --- | --- | [306] |
| Polish Sztumski, Polish Lidzbark, and Sokolski horses (geldings and mares), age: 4–10 years | --- | 36 ± 14 (9–67) U∙g−1 Hb (x ± SD; min–max) *17 | --- | --- | --- | [359] |
| Italian Saddle horses from herd in Piacenza province (Italy), age: 13.6 ± 4.8 years—control group | 174.7 ng∙g−1 (in blood); 87.7 ng∙g−1 (in plasma) *18 | 23,085 U∙L−1 178.0 U∙g−1 Hb (GPx1 in blood); 839.6 U∙L−1 (GPx3 in plasma) *18 | --- | --- | --- | [303] |
| Horses under maintenance care (females, Arabians, ~380 kg, ~14 years) and athlete animals (both genders, Mangalarga Marchador, ~365 kg, ~7 years)—values before test | --- | 328.37 ± 10.29 UL∙g−1 Hb (x ± SD) (in blood) *19 | 1983.05 ± 140.84 UL∙g−1 Hb (x ± SD) (in blood) *19 | --- | --- | [396] |
| Slovenian warm-blooded horses (both genders), age of 2–10 years, body weight of 389.7 ± 126.1 kg | --- | 53.2 ± 1.4 U∙g−1 Hb (x ± SE) (in whole blood) *20 | 1330.3 ± 20.8 U∙g−1 Hb (x ± SE) (in whole blood) *20 | --- | --- | [358] |
| Arabian mares (4–6 years old)—control group | --- | --- | --- | --- | 1.006 ± 0.078 (0.870–1.100) μmol∙L−1 (x ± SD; min−max) (in blood) *21 | [397] |
| Male Arabian horses (4–6 years old)—control (healthy) group | --- | --- | 110.00 ± 6.26 U∙mL−1 (x ± SE) (in erythrocyte hemolysate) *22 | 1480.66 ± 543.00 U∙mL−1 (x ± SE) (in erythrocyte hemolysate) *22 | 1.00 ± 0.12 μmol∙L−1 (x ± SE) (in erythrocyte hemolysate) *22 | [398] |
| Standardbreds trotters (mares, stallions), age 16–20 months—healthy animals | --- | 51.2 ± 1.93 U∙g−1 Hb (x ± SEM) (in whole blood) *23 | --- | --- | --- | [399] |
| 18-month-old horses (fillies, geldings) of American Quarter Horse, American Paint Horse, and grade-stock type horses—control group | 0.108 μg∙mL−1 (in plasma) | 10.0 mU∙mg−1 protein (GPx3 in plasma); 233 mU∙mg−1 Hb (GPx1 in red blood cells) *24 | --- | --- | --- | [360] |
| Donkeys | ||||||
| Female donkeys, 2–5 years of age and 130–190 kg in weight—control group | 120.62 ± 4.07 (mg∙kg−1) (x ± SEM) (in serum) *25 | --- | --- | --- | --- | [305,400] |
References
- Lu, J.; Holmgren, A. Selenoproteins. J. Biol. Chem. 2009, 284, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Abuelo, A.; Alves-Nores, V.; Hernandez, J.; Muiño, R.; Benedito, J.L.; Castillo, C. Effect of parenteral antioxidant supplementation during the dry period on postpartum glucose tolerance in dairy cows. J. Vet. Int. Med. 2016, 30, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Battin, E.E.; Brumaghim, J.L. Antioxidant activity of sulfur and selenium: A review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem. Biophys. 2009, 55, 1–23. [Google Scholar] [CrossRef] [PubMed]
- De Camargo, E.V.; Lopes, S.T.; Costa, M.M.; Paim, F.; Barbosa, C.S.; Leal, M.L. Neutrophil oxidative metabolism and haemogram of sheep experimentally infected with Haemonchus contortus and supplemented with selenium and vitamin E. J. Anim. Physiol. Anim. Nutr. (Berl.) 2010, 94, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Zrieq, R.; Al-Quraishy, S.; Abdel Moneim, A.E. Selenium nanoparticles attenuate oxidative stress and testicular damage in streptozotocin-induced diabetic rats. Molecules 2016, 21, 1517. [Google Scholar] [CrossRef] [PubMed]
- Hasanvand, A.; Abbaszadeh, A.; Darabi, S.; Nazari, A.; Gholami, M.; Kharazmkia, A. Evaluation of selenium on kidney function following ischemic injury in rats; protective effects and antioxidant activity. J. Ren. Inj. Prev. 2016, 6, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid. Redox Signal. 2000, 2, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.L.; de Camargo, E.V.; Ross, D.H.; Molento, M.B.; Lopes, S.T.; da Rocha, J.B. Effect of selenium and vitamin E on oxidative stress in lambs experimentally infected with Haemonchus contortus. Vet. Res. Commun. 2010, 34, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, E.; Yin, S.; Fan, L.; Hu, H. Methylseleninic Acid prevents patulin-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress and inactivation of p53 and MAPKs. J. Agric. Food Chem. 2017, 65, 5299–5305. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Li, X.; Li, Z.; Wu, G.R.; Fu, X.F.; Yang, X.M.; Zhang, X.Q.; Gao, X.B. The effect of selenium supplementation on coronary heart disease: A systematic review and meta-analysis of randomized controlled trials. J. Trace Elem. Med. Biol. 2017, 44, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, S.T.; Dobrachinski, F.; de Carvalho, N.R.; Amaral, G.P.; Barcelos, R.P.; Oliveira, V.A.; Oliveira, C.S.; Giordani, C.F.; Pereira, M.E.; Rodrigues, O.E.; et al. Free radical scavenging in vitro and biological activity of diphenyl diselenide-loaded nanocapsules: DPDS-NCS Antioxidant and toxicological effects. Int. J. Nanomed. 2015, 10, 5663–5670. [Google Scholar] [CrossRef]
- Traulsen, H.; Steinbrenner, H.; Buchczyk, D.P.; Klotz, L.O.; Sies, H. Selenoprotein P protects low-density lipoprotein against oxidation. Free Radic. Res. 2004, 38, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Xu, H. ROS responsive selenium-containing polymers. Nanomedicine 2016, 12, 465. [Google Scholar] [CrossRef]
- Aaseth, J.; Alexander, J.; Bjørklund, G.; Hestad, K.; Dusek, P.; Roos, P.M.; Alehagen, U. Treatment strategies in Alzheimer’s disease: A review with focus on selenium supplementation. Biometals 2016, 29, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.F.; Esworthy, R.S.; Doroshow, J.H. Role of Se-dependent glutathione peroxidases in gastrointestinal inflammation and cancer. Free Radic. Biol. Med. 2004, 36, 1481–1495. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H. Selenium and inflammation: Underlying anti-inflammatory mechanisms. Horm. Metab. Res. 2009, 41, 443–447. [Google Scholar] [CrossRef] [PubMed]
- El-Ghazaly, M.A.; Fadel, N.; Rashed, E.; El-Batal, A.; Kenawy, S.A. Anti-inflammatory effect of selenium nanoparticles on the inflammation induced in irradiated rats. Can. J. Physiol. Pharmacol. 2017, 95, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, Z.; Xing, H.; Yu, J.; Zhang, N.; Xu, S. Selenium Deficiency-induced inflammation and increased expression of regulating inflammatory cytokines in the chicken gastrointestinal tract. Biol. Trace Elem. Res. 2016, 173, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Leyck, S.; Parnham, M.J. Acute antiinflammatory and gastric effects of the seleno-organic compound ebselen. Agents Actions 1990, 30, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Y.; Zeng, X.; Bo, L.; Jiang, S.; Du, X.; Xie, Y.; Jiang, R.; Zhao, J.; Song, W. Investigation of selenium pretreatment in the attenuation of lung injury in rats induced by fine particulate matters. Environ. Sci. Pollut. Res. Int. 2017, 24, 4008–4017. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Welling, M.N.; Mantri, S.B.; Desai, K. In vitro and in vivo antioxidant, cytotoxic, and anti-chronic inflammatory arthritic effect of selenium nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Rooke, J.A.; Robinson, J.J.; Arthur, J.R. Effects of vitamin E and selenium on the performance and immune status of ewes and lambs. J. Agric. Sci. 2004, 142, 153–262. [Google Scholar] [CrossRef]
- Speckmann, B.; Steinbrenner, H. Selenium and selenoproteins in inflammatory bowel diseases and experimental colitis. Inflamm. Bowel Dis. 2014, 20, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Vunta, H.; Belda, B.J.; Arner, R.J.; Channa Reddy, C.; Vanden Heuvel, J.P.; Sandeep Prabhu, K. Selenium attenuates pro-inflammatory gene expression in macrophages. Mol. Nutr. Food Res. 2008, 52, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Guo, X.; Li, Z.; Li, C.; Wang, C.; Lv, W.; Wang, J.; Xiao, F.; Kamal, M.A.; Yuan, C. Antimutagenic effects of selenium-enriched polysaccharides from pyracantha fortuneana through suppression of cytochrome P450 1A subfamily in the mouse liver. Molecules 2016, 21, 1731. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. Effects of selenium and low levels of lead on mammary tumor development and growth in MMTV-infected female mice. Biol. Trace Elem. Res. 2008, 125, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.; Yasser, M.M.; Sholkamy, E.N.; Ali, A.M.; Mehanni, M.M. Anticancer activity of biostabilized selenium nanorods synthesized by Streptomyces bikiniensis strain Ess_amA-1. Int. J. Nanomed. 2015, 10, 3389–3401. [Google Scholar] [CrossRef]
- Hassan, C.E.; Webster, T.J. The effect of red-allotrope selenium nanoparticles on head and neck squamous cell viability and growth. Int. J. Nanomed. 2016, 11, 3641–3654. [Google Scholar] [CrossRef]
- Kong, L.; Yuan, Q.; Zhu, H.; Li, Y.; Guo, Q.; Wang, Q.; Bi, X.; Gao, X. The suppression of prostate LNCaP cancer cells growth by Selenium nanoparticles through Akt/Mdm2/AR controlled apoptosis. Biomaterials 2011, 32, 6515–6522. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, C.; Sampath, K.S.; Arunkumar, P.; Kumar, M.S.; Sujatha, V.; Premkumar, K.; Thirunavukkarasu, C. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst. Eng. 2013, 36, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Stolzoff, M.; Webster, T.J. Reducing bone cancer cell functions using selenium nanocomposites. J. Biomed. Mater. Res. A 2016, 104, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.A.; Sarin, L.; Hurt, R.H.; Webster, T.J. Differential effects of nanoselenium doping on healthy and cancerous osteoblasts in coculture on titanium. Int. J. Nanomed. 2010, 5, 351–358. [Google Scholar]
- Tran, P.; Webster, T.J. Enhanced osteoblast adhesion on nanostructured selenium compacts for anti-cancer orthopedic applications. Int. J. Nanomed. 2008, 3, 391–396. [Google Scholar]
- Vekariya, K.K.; Kaur, J.; Tikoo, K. ERα signaling imparts chemotherapeutic selectivity to selenium nanoparticles in breast cancer. Nanomedicine 2012, 8, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tang, Q.; Zhong, X.; Bai, Y.; Chen, T.; Zhang, Y.; Li, Y.; Zheng, W. Surface decoration by Spirulina polysaccharide enhances the cellular uptake and anticancer efficacy of selenium nanoparticles. Int. J. Nanomed. 2012, 7, 835–844. [Google Scholar] [CrossRef]
- Zheng, J.S.; Zheng, S.Y.; Zhang, Y.B.; Yu, B.; Zheng, W.; Yang, F.; Chen, T. Sialic acid surface decoration enhances cellular uptake and apoptosis-inducing activity of selenium nanoparticles. Colloids Surf. B Biointerfaces 2011, 83, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F.J.; Gray, W.P. Chemopreventive agents: Selenium. Pharmacol. Ther. 1998, 79, 179–192. [Google Scholar] [CrossRef]
- Lü, J.; Jiang, C. Selenium and cancer chemoprevention: Hypotheses integrating the actions of selenoproteins and selenium metabolites in epithelial and non-epithelial target cells. Antioxid. Redox Signal. 2005, 7, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine (Lond.) 2017, 12, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Puschner, B.; Prolla, T.A. Gene expression profiling of low selenium status in the mouse intestine: Transcriptional activation of genes linked to DNA damage, cell cycle control and oxidative stress. J. Nutr. 2001, 131, 3175–3181. [Google Scholar] [PubMed]
- Sinha, R.; El-Bayoumy, K. Apoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compounds. Curr. Cancer Drug Targets 2004, 4, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Taylor, E.W.; Wang, Y.; Wan, X.; Zhang, J. Encapsulated nanoepigallocatechin-3-gallate and elemental selenium nanoparticles as paradigms for nanochemoprevention. Int. J. Nanomed. 2012, 7, 1711–1721. [Google Scholar] [CrossRef]
- Zheng, S.; Li, X.; Zhang, Y.; Xie, Q.; Wong, Y.S.; Zheng, W.; Chen, T. PEG-nanolized ultrasmall selenium nanoparticles overcome drug resistance in hepatocellular carcinoma HepG2 cells through induction of mitochondria dysfunction. Int. J. Nanomed. 2012, 7, 3939–3949. [Google Scholar] [CrossRef]
- Cihalova, K.; Chudobova, D.; Michalek, P.; Moulick, A.; Guran, R.; Kopel, P.; Adam, V.; Kizek, R. Staphylococcus aureus and MRSA Growth and Biofilm Formation after Treatment with Antibiotics and SeNPs. Int. J. Mol. Sci. 2015, 16, 24656–24672. [Google Scholar] [CrossRef] [PubMed]
- Guisbiers, G.; Wang, Q.; Khachatryan, E.; Mimun, L.C.; Mendoza-Cruz, R.; Larese-Casanova, P.; Webster, T.J.; Nash, K.L. Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. Int. J. Nanomed. 2016, 11, 3731–3736. [Google Scholar] [CrossRef] [PubMed]
- Chudobova, D.; Cihalova, K.; Dostalova, S.; Ruttkay-Nedecky, B.; Rodrigo, M.A.; Tmejova, K.; Kopel, P.; Nejdl, L.; Kudr, J.; Gumulec, J.; et al. Comparison of the effects of silver phosphate and selenium nanoparticles on Staphylococcus aureus growth reveals potential for selenium particles to prevent infection. FEMS Microbiol. Lett. 2014, 351, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Larese-Casanova, P.; Webster, T.J. Inhibition of various gram-positive and gram-negative bacteria growth on selenium nanoparticle coated paper towels. Int. J. Nanomed. 2015, 10, 2885–2894. [Google Scholar] [CrossRef]
- Guisbiers, G.; Lara, H.H.; Mendoza-Cruz, R.; Naranjo, G.; Vincent, B.A.; Peralta, X.G.; Nash, K.L. Inhibition of Candida albicans biofilm by pure selenium nanoparticles synthesized by pulsed laser ablation in liquids. Nanomedicine 2017, 13, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.; Salari Mohazab, N.; Ayatollahi Mousavi, S.A. Antifungal Activity of Selenium Nanoparticles Synthesized by Bacillus species Msh-1 Against Aspergillus fumigatus and Candida albicans. Jundishapur J. Microbiol. 2015, 8, e26381. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, N.; Soflaei, S.; Shakibaie, M.; Yazdi, M.H.; Ghaffarifar, F.; Dalimi, A.; Shahverdi, A.R. Efficacy of biogenic selenium nanoparticles against Leishmania major: In vitro and in vivo studies. J. Trace Elem. Med. Biol. 2013, 27, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Bauomy, A.A.; Diab, M.S.M.; Al-Quraishy, S. Protective role of selenium nanoparticles against Schistosoma mansoni induced hepatic injury in mice. Biomed. Res. 2016, 27, 214–219. [Google Scholar]
- Mahmoudvand, H.; Harandi, M.F.; Shakibaie, M.; Aflatoonian, M.R.; ZiaAli, N.; Sadat Makki, M.S.; Jahanbakhsh, S. Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int. J. Surg. 2014, 12, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Pascual, A.; Aranda, A. Thyroid hormone receptors, cell growth and differentiation. Biochim. Biophys. Acta 2013, 1830, 3908–3916. [Google Scholar] [CrossRef] [PubMed]
- Hefnawy, A.E.G.; Tórtora-Pérez, J.L. The importance of selenium and the effects of its deficiency in animal health. Small Rumin. Res. 2010, 89, 185–192. [Google Scholar] [CrossRef]
- Arthur, J.R. The glutathione peroxidases. Cell Mol. Life Sci. 2000, 57, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Mangiapane, E.; Pessione, A.; Pessione, E. Selenium and selenoproteins: An overview on different biological systems. Curr. Protein. Pept. Sci. 2014, 15, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F. Selenium in Nutrition and Health; Nottingham University Press: Nottingham, UK, 2006; pp. 487–587. [Google Scholar]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.P.; Shah, R.; Copeland, P.R. Regulation of selenocysteine incorporationinto the selenium transport protein, selenoprotein P. J. Biol. Chem. 2014, 289, 25317–25326. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef] [PubMed]
- Allmang, C.; Krol, A. Selenoprotein synthesis: UGA Does not end the story. Biochimie 2006, 88, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.E.; Berry, M.J. Eukaryotic selenoprotein synthesis: Mechanistic insight incorporating new factors and new functions for old factors. IUBMB Life 2008, 60, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.J.; Banu, L.; Chen, Y.Y.; Mandel, S.J.; Kieffer, J.D.; Harney, J.W.; Larsen, P.R. Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3′ untranslated region. Nature 1991, 353, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, J.L.; Miniard, A.C.; Driscoll, D.M. Characterization of the UGA-recoding and SECIS-binding activities of SECIS-binding protein 2. RNA Biol. 2014, 11, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, M.; Ahmadpour, F.; Chahardoli, B.; Malekpour-Dehkordi, Z.; Nourbakhsh, M.; Hosseini-Fard, S.R.; Doustimotlagh, A.; Golestani, A.; Razzaghy-Azar, M. Selenium and its relationship with selenoprotein P and glutathioneperoxidase in children and adolescents with Hashimoto’s thyroiditisand hypothyroidism. J. Trace Elem. Med. Biol. 2016, 34, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Duntas, L.H.; Benvenga, S. Selenium an element for life. Endocrine 2015, 48, 756–775. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.L.; Wang, C.W.; Tan, S.R.; Liang, Y.; Yao, H.D.; Zhang, Z.W.; Xu, S.W. Selenium deficiency inhibits the conversion of thyroidal thyroxine (T4) to triiodothyronine (T3) in chicken thyroids. Biol. Trace Elem. Res. 2014, 161, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Rowntree, J.E.; Hill, G.M.; Hawkins, D.R.; Link, J.E.; Rincker, M.J.; Bednar, G.W.; Kreft, R.A., Jr. Effect of Se on selenoprotein activity and thyroid hormone metabolism in beef and dairy cows and calves. J. Anim. Sci. 2004, 82, 2995–3005. [Google Scholar] [CrossRef] [PubMed]
- Guyot, H.; Rollin, F. The diagnosis of selenium and iodine deficiencies in cattle. Ann. Med. Vet. 2007, 151, 166–191. [Google Scholar]
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2002, 23, 38–89. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.R.; Zavacki, A.M. The role of the iodothyronine deiodinases in the physiology and pathophysiology of thyroid hormone action. Eur. Thyroid J. 2012, 1, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Dentice, M.; Marsili, A.; Zavacki, A.; Larsen, P.R.; Salvatore, D. The deiodinases and the control of intracellular thyroid hormone signaling during cellular differentiation. Biochim. Biophys. Acta 2013, 1830, 3937–3945. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, L.A.; Pachucki, J.; St Germain, D.L. Thyroid hormones inhibit type 2 iodothyronine deiodinase in the rat cerebral cortex by both pre- and posttranslational mechanisms. Endocrinology 1997, 138, 5231–5237. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Shinde, P.L.; Dass, R.S.; Garg, A.K. Effect of vitamin E and selenium supplementation on haematology, blood chemistry and thyroid hormones in male buffalo (Bubalus bubalis) calves. J. Anim. Feed Sci. 2009, 18, 241–256. [Google Scholar] [CrossRef]
- Sethy, K.; Dass, R.S.; Garg, A.K.; Sahu, S.; Gogoi, S. Effect of different selenium sources (Selenium yeast and Sodium selenite) on haematology, blood chemistry and thyroid hormones in male goats (Capra hircus). Indian J. Anim. Res. 2015, 49, 788–792. [Google Scholar] [CrossRef]
- Mittag, J.; Behrends, T.; Hoefig, C.S.; Vennström, B.; Schomburg, L. Thyroid hormones regulate selenoprotein expression and selenium status in mice. PLoS ONE 2010, 5, e12931. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J.; Gärtner, R. Selenium and thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Dercksen, D.P.; Counotte, G.H.; Hazebroek, M.K.; Arts, W.; van Rijn, T. Selenium requirements of dairy goats [Article in Dutch]. Tijdschr Diergeneeskd 2007, 132, 468–471. [Google Scholar] [PubMed]
- Effraimidis, G.; Wiersinga, W.M. Mechanisms in endocrinology: Autoimmunethyroid disease: Old and new players. Eur. J. Endocrinol. 2014, 170, R241–R252. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L. Selenium, selenoproteins and the thyroid gland: Interactions in health and disease. Nat. Rev. Endocrinol. 2012, 8, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Radostits, O.M.; Gay, C.C.; Hinchcliff, K.W.; Constable, P.D. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 10th ed.; Saunders: Madrid, Spain, 2007; pp. 552–557. [Google Scholar]
- Wang, C.; Liu, Q.; Yang, W.Z.; Dong, Q.; Yang, X.M.; He, D.C.; Zhang, P.; Dong, K.H.; Huang, Y.X. Effects of selenium yeast on rumen fermentation, lactation performance and feed digestibilities in lactating dairy cows. Livest. Sci. 2009, 126, 239–244. [Google Scholar] [CrossRef]
- Aghwan, Z.A.; Sazili, A.Q.; Kadhim, K.K.; Alimon, A.R.; Goh, Y.M.; Adeyemi, K.D. Effects of dietary supplementation of selenium and iodine on growth performance, carcass characteristics and histology of thyroid gland in goats. Anim. Sci. J. 2016, 87, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Alhidary, I.A.; Shini, S.; Al Jassim, R.A.; Abudabos, A.M.; Gaughan, J.B. Effects of selenium and vitamin E on performance, physiological response, and selenium balance in heat-stressed sheep. J. Anim. Sci. 2015, 93, 576–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alimohamady, R.; Aliarabi, H.; Bahari, A.; Dezfoulian, A.H. Influence of different amounts and sources of selenium supplementation on performance, some blood parameters, and nutrient digestibility in lambs. Biol. Trace Elem. Res. 2013, 154, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Calvo, L.; Toldrá, F.; Rodríguez, A.I.; López-Bote, C.; Rey, A.I. Effect of dietary selenium source (organic vs. mineral) and muscle pH on meat quality characteristics of pigs. Food Sci. Nutr. 2016, 5, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Downs, K.M.; Hess, J.B.; Bilgili, S.F. Selenium source effect on broiler carcass characteristics, meat quality and drip loss. J. Appl. Anim. Res. 2000, 18, 61–72. [Google Scholar] [CrossRef]
- Habibian, M.; Sadeghi, G.; Ghazi, S.; Moeini, M.M. Selenium as a feed supplement for heat-stressed poultry: A review. Biol. Trace Elem. Res. 2015, 165, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, M.; Zhan, X.; Li, X.; Zhao, R. Effect of different selenium sources on productive performance, serum and milk Se concentrations, and antioxidant status of sows. Biol. Trace Elem. Res. 2011, 142, 471–480. [Google Scholar] [CrossRef] [PubMed]
- James, B.W.; Goodband, R.D.; Unruh, J.A.; Tokach, M.D.; Nelssen, J.L.; Dritz, S.S.; O’Quinn, P.R.; Andrews, B.S. Effects of creatine monohydrate on finishing pig growth performance, carcass characteristics and meat quality. Anim. Feed Sci. Technol. 2002, 96, 135–145. [Google Scholar] [CrossRef]
- Lv, C.H.; Wang, T.; Regmi, N.; Chen, X.; Huang, K.; Liao, S.F. Effects of dietary supplementation of selenium-enriched probiotics on production performance and intestinal microbiota of weanling piglets raised under high ambient temperature. J. Anim. Physiol. Anim. Nutr. (Berl.) 2015, 99, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Mateo, R.D.; Spallholz, J.E.; Elder, R.; Yoon, I.; Kim, S.W. Efficacy of dietary selenium sources on growth and carcass characteristics of growing-finishing pigs fed diets containing high endogenous selenium. J. Anim. Sci. 2007, 85, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Revilla-Vázquez, A.; Ramírez-Bribiesca, E.; López-Arellano, R.; Hernández-Calva, L.M.; Tórtora-Pérez, J.; García-García, E.; Cruz, M.R.G. Supplement of selenium with intraruminal bolus of sodium selenite in sheep. Agrociencia 2008, 42, 629–635. [Google Scholar]
- Song, Y.X.; Hou, J.X.; Zhang, L.; Wang, J.G.; Liu, X.R.; Zhou, Z.Q.; Cao, B.Y. Effect of dietary selenomethionine supplementation on growth performance, tissue Se concentration, and blood glutathione peroxidase activity in kid boer goats. Biol. Trace Elem. Res. 2015, 167, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Suchý, P.; Straková, E.; Herzig, I. Selenium in poultry nutrition: A review. Czech J. Anim. Sci. 2014, 59, 495–503. [Google Scholar]
- Tufarelli, V.; Laudadio, V. Dietary supplementation with selenium and vitamin E improves milk yield, composition and rheological properties of dairy Jonica goats. J. Dairy Res. 2011, 78, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yao, J.; Yang, Z.; Yue, W.; Ren, Y.; Zhang, C.; Liu, X.; Wang, H.; Zhao, X.; Yuan, S.; et al. Improved fetal hair follicle development by maternal supplement of selenium at nano size (Nano-Se). Livest. Sci. 2011, 142, 270–275. [Google Scholar] [CrossRef]
- Zhan, X.A.; Wang, M.; Zhao, R.Q.; Li, W.F.; Xu, Z.R. Effects of different selenium source on selenium distribution, loin quality and antioxidant status in finishing pigs. Anim. Feed Sci. Technol. 2007, 132, 202–211. [Google Scholar] [CrossRef]
- Kommisrud, E.; Osterås, O.; Vatn, T. Blood selenium associated with health and fertility in Norwegian dairy herds. Acta Vet. Scand. 2005, 46, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, U.; Kamran, Z.; Raza, I.; Ahmad, S.; Babar, W.; Riaz, M.H.; Iqbal, Z. Role of selenium in male reproduction—A review. Anim. Reprod. Sci. 2014, 146, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bourne, N.; Wathes, D.C.; Lawrence, K.E.; McGowan, M.; Laven, R.A. The effect of parenteral supplementation of vitamin E with selenium on the health and productivity of dairy cattle in the UK. Vet. J. 2008, 177, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Cerny, K.L.; Anderson, L.; Burris, W.R.; Rhoads, M.; Matthews, J.C.; Bridges, P.J. Form of supplemental selenium fed to cycling cows affects systemic concentrations of progesterone but not those of estradiol. Theriogenology 2016, 85, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Chandra, G.; Aggarwal, A.; Singh, A.; Singh, A.K.; Kumar, M.; Kushwaha, R.; Singh, Y.K. Oxidative stress in sperm biology—A review. Agric. Rev. 2012, 33, 54–61. [Google Scholar]
- El-Sharawy, M.; Eid, E.; Darwish, S.; Abdel-Razek, I.; Islam, M.R.; Kubota, K.; Yamauchi, N.; El-Shamaa, I. Effect of organic and inorganic selenium supplementation on semen quality and blood enzymes in buffalo bulls. Anim. Sci. J. 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Flohé, L.; Garolla, A.; Roveri, A.; Ursini, F.; Maiorino, M. Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol. Reprod. 2002, 67, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Giadinis, N.D.; Loukopoulos, P.; Petridou, E.J.; Panousis, N.; Konstantoudaki, K.; Filioussis, G.; Tsousis, G.; Brozos, C.; Koutsoumpas, A.T.; Chaintoutis, S.C.; et al. Abortions in three beef cattle herds attributed to selenium deficiency. Pak. Vet. J. 2016, 36, 145–148. [Google Scholar]
- Grazul-Bilska, A.T.; Neville, T.L.; Borowczyk, E.; Sharma, A.; Reynolds, L.P.; Caton, J.S.; Redmer, D.A.; Vonnahme, K.A. Ovarian and uterine characteristics and onset of puberty in adolescent offspring: Effects of maternal diet and selenium supplementation in sheep. Theriogenology 2014, 81, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, R.G. The influences of dietary intakes and supplementation with selenium and vitamin E on reproduction diseases and reproductive efficiency in cattle and sheep. Vet. Res. Commun. 2003, 27, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Mahan, D.C.; Peters, J.C. Long-term effects of dietary organic and inorganic selenium sources and levels on reproducing sows and their progeny. J. Anim. Sci. 2004, 82, 1343–1358. [Google Scholar] [CrossRef] [PubMed]
- Marin-Guzman, J.; Mahan, D.C.; Pate, J.L. Effect of dietary selenium and vitamin E on spermatogenic development in boars. J. Anim. Sci. 2000, 78, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Dufrasne, I. Selenium in Cattle: A Review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef] [PubMed]
- Moeini, M.M.; Karami, H.; Mikaeili, E. Effect of selenium and vitamin E supplementation during the late pregnancy on reproductive indices and milk production in heifers. Anim. Reprod. Sci. 2009, 114, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Patterson, H.H.; Adams, D.C.; Klopfenstein, T.J.; Clark, R.T.; Teichert, B. Supplementation to meet metabolizable protein requirements of primiparous beef heifers: II. Pregnancy and economics. J. Anim. Sci. 2003, 81, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yue, W.; Zhang, C.; Ren, Y.; Zhu, X.; Wang, Q.; Shi, L.; Lei, F. Effects of maternal and dietary selenium (Se-enriched yeast) on oxidative status in testis and apoptosis of germ cells during spermatogenesis of their offspring in goats. Anim. Reprod. Sci. 2010, 119, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Song, R.; Yao, X.; Ren, Y. Effects of selenium on the proliferation, apoptosis and testosterone production of sheep Leydig cells in vitro. Theriogenology 2017, 93, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Spears, J.W.; Weiss, W.P. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet. J. 2008, 176, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Speight, S.M.; Estienne, M.J.; Harper, A.F.; Crawford, R.J.; Knight, J.W.; Whitaker, B.D. Effects of dietary supplementation with an organic source of selenium on characteristics of semen quality and in vitro fertility in boars. J. Anim. Sci. 2012, 90, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Fisinin, V.I. Selenium in Pig Nutrition and reproduction: Boars and semen quality—A Review. Asian-Australas. J. Anim. Sci. 2015, 28, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, D.J.; Talukdar, P.; Ahmed, K. Minerals and its impact on fertility of livestock: A review. Agric. Rev. 2016, 37, 333–337. [Google Scholar] [CrossRef]
- Zubair, M.; Ali, M.; Ahmad, M.; Sajid, S.M.; Ahmad, I.; Gul, S.T. Effect of Selenium and Vitamin E on cryopreservation of semen and reproductive performance of animals (a review). J. Entomol. Zool. Stud. 2015, 3, 82–86. [Google Scholar]
- Bao, R.K.; Zheng, S.F.; Wang, X.Y. Selenium protects against cadmium-induced kidney apoptosis in chickens by activating the PI3K/AKT/Bcl-2 signaling pathway. Environ. Sci. Pollut. Res. Int. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Aaseth, J.; Ajsuvakova, O.P.; Nikonorov, A.A.; Skalny, A.V.; Skalnaya, M.G.; Tinkov, A.A. Molecular interaction between mercury and selenium in neurotoxicity. Coord. Chem. Rev. 2017, 332, 30–37. [Google Scholar] [CrossRef]
- Dai, X.; Thongchot, S.; Dokduang, H.; Loilome, W.; Khuntikeo, N.; Titapun, A.; Ungarreevittaya, P.; Yongvanit, P.; Techasen, A.; Namwat, N. Potential of selenium compounds as new anticancer agents for cholangiocarcinoma. Anticancer Res. 2016, 36, 5981–5988. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, J.; Song, X.; Zhang, J.; Wang, X.; Jing, H.; Ren, Z.; Li, S.; Zhang, C.; Jia, L. Antioxidative, anti-inflammation and lung-protective effects of mycelia selenium polysaccharides from Oudemansiella radicata. Int. J. Biol. Macromol. 2017, 104, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.X.; Wen, Z.S.; Xiang, X.W.; Ma, L.; Wang, X.B.; Ma, J.Y.; Qu, Y.L. Immunomodulatory effect of low molecular-weight seleno-aminopolysaccharides in intestinal epithelial cells. Int. J. Biol. Macromol. 2017, 99, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Guastamacchia, E.; Giagulli, V.A.; Licchelli, B.; Triggiani, V. Selenium and iodine in autoimmune thyroiditis. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Hegedüs, V.; Prokisch, J.; Fébel, H.; Kleiner, D.; Ditrói, K.; Szijártó, A.; Blázovics, A. Nanoselenium treatment in fatty liver. Z. Gastroenterol. 2012, 50. [Google Scholar] [CrossRef]
- Lee, J.M.; Chun, H.J.; Choi, H.S.; Kim, E.S.; Seo, Y.S.; Jeen, Y.T.; Lee, H.S.; Um, S.H.; Kim, C.H.; Sul, D. Selenium administration attenuates 5-flurouracil-induced intestinal mucositis. Nutr. Cancer 2017, 69, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Orct, T.; Lazarus, M.; Ljubojević, M.; Sekovanić, A.; Sabolić, I.; Blanuša, M. Metallothionein, essential elements and lipid peroxidation in mercury-exposed suckling rats pretreated with selenium. Biometals 2015, 28, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.P.; Webster, T. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomed. 2011, 6, 1553–1558. [Google Scholar]
- Wu, C.; Xu, Z.; Huang, K. Effects of Dietary Selenium on inflammation and hydrogen sulfide in the gastrointestinal tract in chickens. Biol. Trace Elem. Res. 2016, 174, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Jiang, L.; Chu, Y.; Zhang, Y.S. Protective effect of selenium against cisplatin-induced nasopharyngeal cancer in male albino rats. Oncol. Lett. 2016, 12, 5068–5074. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Darke, A.K.; Penney, K.L.; Tangen, C.M.; Goodman, P.J.; Lee, G.S.; Sun, T.; Peisch, S.; Tinianow, A.M.; Rae, J.M.; et al. Selenium- or vitamin E-related gene variants, interaction with supplementation, and risk of high-grade prostate cancer in SELECT. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Wallenberg, M.; Gandin, V.; Misra, S.; Tisato, F.; Marzano, C.; Rigobello, M.P.; Kumar, S.; Björnstedt, M. Methylselenol formed by spontaneous methylation of selenide is a superior selenium substrate to the thioredoxin and glutaredoxin systems. PLoS ONE 2012, 7, e50727. [Google Scholar] [CrossRef] [PubMed]
- Weekley, C.M.; Jeong, G.; Tierney, M.E.; Hossain, F.; Maw, A.M.; Shanu, A.; Harris, H.H.; Witting, P.K. Selenite-mediated production of superoxide radical anions in A549 cancer cells is accompanied by a selective increase in SOD1 concentration, enhanced apoptosis and Se-Cu bonding. J. Biol. Inorg. Chem. 2014, 19, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, J.H.; Chi, G.Y.; Kim, G.Y.; Chang, Y.C.; Moon, S.K.; Nam, S.W.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. Induction of apoptosis and autophagy by sodium selenite in A549 human lung carcinoma cells through generation of reactive oxygen species. Toxicol. Lett. 2012, 212, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.M.; Yang, C.F.; Ding, W.X.; Liu, J.; Ong, C.N. Superoxide radical-initiated apoptotic signalling pathway in selenite-treated HepG(2) cells: Mitochondria serve as the main target. Free Radic. Biol. Med. 2001, 30, 9–21. [Google Scholar] [CrossRef]
- Wang, H.T.; Yang, X.L.; Zhang, Z.H.; Lu, J.L.; Xu, H.B. Reactive oxygen species from mitochondria mediate SW480 cells apoptosis induced by Na2SeO3. Biol. Trace Elem. Res. 2002, 85, 241–254. [Google Scholar] [CrossRef]
- Zhong, W.; Oberley, T.D. Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer Res. 2001, 61, 7071–7078. [Google Scholar] [PubMed]
- Zhu, Y.; Xu, H.; Huang, K. Mitochondrial permeability transition and cytochrome c release induced by selenite. J. Inorg. Biochem. 2002, 90, 43–50. [Google Scholar] [CrossRef]
- Husbeck, B.; Nonn, L.; Peehl, D.M.; Knox, S.J. Tumor-selective killing by selenite in patient-matched pairs of normal and malignant prostate cells. Prostate 2006, 66, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Nilsonne, G.; Sun, X.; Nyström, C.; Rundlöf, A.K.; Potamitou Fernandes, A.; Björnstedt, M.; Dobra, K. Selenite induces apoptosis in sarcomatoid malignant mesothelioma cells through oxidative stress. Free Radic. Biol. Med. 2006, 41, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Ip, C.; Ganther, H.E. Activity of methylated forms of selenium in cancer prevention. Cancer Res. 1990, 50, 1206–1211. [Google Scholar] [PubMed]
- Xiang, N.; Zhao, R.; Zhong, W. Sodium selenite induces apoptosis by generation of superoxide via the mitochondrial-dependent pathway in human prostate cancer cells. Cancer Chemother. Pharmacol. 2009, 63, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zuo, L.; Shen, T.; Xu, C.M.; Zhang, Z.N. Induction of apoptosis by sodium selenite in human acute promyelocytic leukemia NB4 cells: Involvement of oxidative stress and mitochondria. J. Trace Elem. Med. Biol. 2003, 17, 19–26. [Google Scholar] [CrossRef]
- Kim, E.H.; Sohn, S.; Kwon, H.J.; Kim, S.U.; Kim, M.J.; Lee, S.J.; Choi, K.S. Sodium selenite induces superoxide-mediated mitochondrial damage and subsequent autophagic cell death in malignant glioma cells. Cancer Res. 2007, 67, 6314–6324. [Google Scholar] [CrossRef] [PubMed]
- Björnstedt, M.; Kumar, S.; Holmgren, A. Selenodiglutathione is a highly efficient oxidant of reduced thioredoxin and a substrate for mammalian thioredoxin reductase. J. Biol. Chem. 1992, 267, 8030–8034. [Google Scholar] [PubMed]
- Ganther, H.E. Reduction of the selenotrisulfide derivative of glutathione to a persulfide analog by glutathione reductase. Biochemistry 1971, 10, 4089–4098. [Google Scholar] [CrossRef] [PubMed]
- Horky, P.; Jancikova, P.; Sochor, J.; Hynek, D.; Chavis, G.J.; Ruttkay-Nedecky, B.; Cernei, N.; Zitka, O.; Zeman, L.; Adam, V.; et al. Effect of organic and inorganic form of selenium on antioxidant status of breeding boars ejaculate revealed by electrochemistry. Int. J. Electrochem. Sci. 2012, 7, 9643–9657. [Google Scholar]
- Kim, J.H.; Wang, S.Y.; Kim, I.C.; Ki, J.S.; Raisuddin, S.; Lee, J.S.; Han, K.N. Cloning of a river pufferfish (Takifugu obscurus) metallothionein cDNA and study of its induction profile in cadmium-exposed fish. Chemosphere 2008, 71, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Raisuddin, S.; Tewari, S.; Goel, S.K.; Raizada, R.B.; Behari, J.R. Evaluation of comparative effect of pre- and posttreatment of selenium on mercury-induced oxidative stress, histological alterations, and metallothionein mRNA expression in rats. J. Biochem. Mol. Toxicol. 2010, 24, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G. Selenium as an antidote in the treatment of mercury intoxication. Biometals 2015, 28, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E. Selenoprotein P-expression, functions, and roles in mammals. Biochim. Biophys. Acta 2009, 1790, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Bjørklund, G.; Tinkov, A.A.; Skalny, A.V.; Al Dera, H. Relationship between selenium, lead, and mercury in red blood cells of Saudi autistic children. Metab. Brain Dis. 2017, 32, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E.; Motley, A.K.; Winfrey, V.P.; Kurokawa, S.; Mitchell, S.L.; Zhang, W. Selenoprotein P and apolipoprotein E receptor-2 interact at the blood-brain barrier and also within the brain to maintain an essential selenium pool that protects against neurodegeneration. FASEB J. 2014, 28, 3579–3588. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, K.M.; Abd El-Kawi, S.H.; Hashem, K.S. The prospective protective effect of selenium nanoparticles against chromium-induced oxidative and cellular damage in rat thyroid. Int. J. Nanomed. 2013, 8, 1713–1720. [Google Scholar]
- Hao, P.; Zhu, Y.; Wang, S.; Wan, H.; Chen, P.; Wang, Y.; Cheng, Z.; Liu, Y.; Liu, J. Selenium Administration Alleviates Toxicity of Chromium(VI) in the Chicken Brain. Biol. Trace Elem. Res. 2017, 178, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Zhu, Y.; Chen, P.; Wang, Y.; Hao, P.; Cheng, Z.; Liu, Y.; Liu, J. Effect of various selenium doses on chromium(IV)-induced nephrotoxicity in a male chicken model. Chemosphere 2017, 174, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.J.; Horgan, K.A.; White, B.; Walls, D. Selenium source impacts protection of porcine jejunal epithelial cells from cadmium-induced DNA damage, with maximum protection exhibited with yeast-derived selenium compounds. Biol. Trace Elem. Res. 2017, 176, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bao, R.; Fu, J. The antagonistic effect of selenium on cadmium-induced damage and mRNA levels of selenoprotein genes and inflammatory factors in chicken kidney Tissue. Biol. Trace Elem. Res. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.M.; Lebda, M.A.; Abouzed, T.K.; Nasr, S.M.; Shoukry, M. Neuro- and nephrotoxicity of subchronic cadmium chloride exposure and the potential chemoprotective effects of selenium nanoparticles. Metab. Brain Dis. 2017, 28. [Google Scholar] [CrossRef] [PubMed]
- Özkan-Yilmaz, F.; Özlüer-Hunt, A.; Gündüz, S.G.; Berköz, M.; Yalin, S. Effects of dietary selenium of organic form against lead toxicity on the antioxidant system in Cyprinus carpio. Fish Physiol. Biochem. 2014, 40, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Danzeisen, R.; Achsel, T.; Bederke, U.; Cozzolino, M.; Crosio, C.; Ferri, A.; Frenzel, M.; Gralla, E.B.; Huber, L.; Ludolph, A.; et al. Superoxide dismutase 1 modulates expression of transferrin receptor. J. Biol. Inorg. Chem. 2006, 11, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.N.; Enns, C.A. Iron homeostasis: New tales from the crypt. Blood 2000, 96, 4020–4027. [Google Scholar] [PubMed]
- Bartfay, W.J. Selenium status and the pathogenesis of iron-overload cardiomyopathies: Cause or Consequence. Queen’s Health Sci. J. 2003, 6, 40–46. [Google Scholar]
- Bartfay, W.J.; Bartfay, E. Decreasing effects of iron toxicosis on selenium and glutathione peroxidase activity. West. J. Nurs. Res. 2002, 24, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.A.; Kouzarides, T. Cancer epigenetics: From mechanism to therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, B.; Grune, T. Epigenetic effects of selenium and their implications for health. Epigenetics 2015, 10, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Dozmorov, M.G.; Wren, J.D.; Alarcón-Riquelme, M.E. Epigenomic elements enriched in the promoters of autoimmunity susceptibility genes. Epigenetics 2014, 9, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Häsler, R.; Feng, Z.; Bäckdahl, L.; Spehlmann, M.E.; Franke, A.; Teschendorff, A.; Rakyan, V.K.; Down, T.A.; Wilson, G.A.; Feber, A.; et al. A functional methylome map of ulcerative colitis. Genome Res. 2012, 22, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Jansson, P.A.; Perfilyev, A.; Volkov, P.; Pedersen, M.; Svensson, M.K.; Poulsen, P.; Ribel-Madsen, R.; Pedersen, N.L.; Almgren, P.; et al. Altered DNA methylation and differential expression of genes influencing metabolism and inflammation in adipose tissue from subjects with type 2 diabetes. Diabetes 2014, 63, 2962–2976. [Google Scholar] [CrossRef] [PubMed]
- Whayne, T.F. Epigenetics in the development, modification, and prevention of cardiovascular disease. Mol. Biol. Rep. 2015, 42, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Arai, E.; Kanai, Y. DNA methylation profiles in precancerous tissue and cancers: Carcinogenetic risk estimation and prognostication based on DNA methylation status. Epigenomics 2010, 2, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Timp, W.; Feinberg, A.P. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nat. Rev. Cancer 2013, 13, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Jakupoglu, C.; Moreno, S.G.; Lippl, S.; Banjac, A.; Schneider, M.; Beck, H.; Hatzopoulos, A.K.; Just, U.; Sinowatz, F.; et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol. Cell. Biol. 2004, 24, 9414–9423. [Google Scholar] [CrossRef] [PubMed]
- Bösl, M.R.; Takaku, K.; Oshima, M.; Nishimura, S.; Taketo, M.M. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp). Proc. Natl. Acad. Sci. USA 1997, 94, 5531–5534. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D.; Uthus, E.O.; Finley, J.W. Dietary selenium and arsenic affect DNA methylation in vitro in Caco-2 cells and in vivo in rat liver and colon. J. Nutr. 2000, 130, 2903–2909. [Google Scholar] [PubMed]
- Uthus, E.O.; Ross, S.A.; Davis, C.D. Differential effects of dietary selenium (Se) and folate on methyl metabolism in liver and colon of rats. Biol. Trace Elem. Res. 2006, 109, 201–214. [Google Scholar] [CrossRef]
- Armstrong, K.M.; Bermingham, E.N.; Bassett, S.A.; Treloar, B.P.; Roy, N.C.; Barnett, M.P. Global DNA methylation measurement by HPLC using low amounts of DNA. Biotechnol. J. 2011, 6, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Zhao, R.; Song, G.; Zhong, W. Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogenesis 2008, 29, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Ohgane, J.; Yagi, S.; Ito, R.; Iwasaki, Y.; Saito, K.; Akutsu, K.; Takatori, S.; Ishii, R.; Hayashi, R.; et al. Epigenetic assessment of environmental chemicals detected in maternal peripheral and cord blood samples. J. Rep. Dev. 2011, 57, 507–517. [Google Scholar] [CrossRef]
- Zeng, H.; Yan, L.; Cheng, W.H.; Uthus, E.O. Dietary selenomethionine increases exon-specific DNA methylation of the p53 gene in rat liver and colon mucosa. J. Nutr. 2011, 141, 1464–1468. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Patel, D.J. Structural biology-based insights into combinatorial readout and crosstalk among epigenetic marks. Biochim. Biophys. Acta 2014, 1839, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, K.J.; Johnstone, R.W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat. Rev. Drug Discov. 2014, 13, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Gowda, R.; Madhunapantula, S.V.; Desai, D.; Amin, S.; Robertson, G.P. Selenium-containing histone deacetylase inhibitors for melanoma management. Cancer Biol. Ther. 2012, 13, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Kassam, S.; Goenaga-Infante, H.; Maharaj, L.; Hiley, C.T.; Juliger, S.; Joel, S.P. Methylseleninic acid inhibits HDAC activity in diffuse large B-cell lymphoma cell lines. Cancer Chemother. Pharmacol. 2011, 68, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.; Salli, U.; Vrana, K.E.; Amin, S. SelSA, selenium analogs of SAHA as potent histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 2044–2047. [Google Scholar] [CrossRef] [PubMed]
- Maciel-Dominguez, A.; Swan, D.; Ford, D.; Hesketh, J. Selenium alters miRNA profile in an intestinal cell line: Evidence that miR-185 regulates expression of GPX2 and SEPSH2. Mol. Nutr. Food Res. 2013, 57, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, J.X.; He, Y.Q.; Feng, C.; Zhang, X.J.; Sheng, J.Q.; Li, P.F. MicroRNA-185 regulates chemotherapeutic sensitivity in gastric cancer by targeting apoptosis repressor with caspase recruitment domain. Cell Death Dis. 2014, 5, e1197. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Cui, X.; Hong, Y.; Wang, J.; Li, Y.; Chen, L.; Liu, Y.; Gao, Y.; Xu, D.; Wang, Q. MicroRNA-185 suppresses proliferation, invasion, migration, and tumorigenicity of human prostate cancer cells through targeting androgen receptor. Mol. Cell Biochem. 2013, 377, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F., Jr. The Vitamins: Fundamental Aspects in Nutrition and Health, 3rd ed.; Elsevier Academic Press: Cambridge, MA, USA, 2008; p. 583. ISBN 513: 978-580-512-183493-183497. [Google Scholar]
- Au Yeung, K.J.; Smith, A.; Zhao, A.; Madden, K.B.; Elfrey, J.; Sullivan, C.; Levander, O.; Urban, J.F.; Shea-Donohue, T. Impact of vitamin E or selenium deficiency on nematode-induced alterations in murine intestinal function. Exp. Parasitol. 2005, 109, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Madden, K.B.; Yeung, K.J.; Zhao, A.; Elfrey, J.; Finkelman, F.; Levander, O.; Shea-Donohue, T.; Urban, J.F., Jr. Deficiencies in selenium and/or vitamin E lower the resistance of mice to Heligmosomoides polygyrus infections. J. Nutr. 2005, 135, 830–836. [Google Scholar] [PubMed]
- Abutarbush, S.M.; Radostits, O.M. Congenital nutritional muscular dystrophy in a beef calf. Can. Vet. J. 2003, 44, 738–739. [Google Scholar] [PubMed]
- Radostits, O.M.; Gay, C.C.; Blood, D.C.; Hinchcliff, K.W. Veterinary Medicine: A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses, 9th ed.; WB Saunders: London, UK, 2000. [Google Scholar]
- Streeter, R.M.; Divers, T.J.; Mittel, L.; Korn, A.E.; Wakshlag, J.J. Selenium deficiency associations with gender, breed, serum vitamin E and creatine kinase, clinical signs and diagnoses in horses of different age groups: A retrospective examination 1996–2011. Equine Vet. J. Suppl. 2012, 44, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Cardona, Á.J.; Reza, G.L. Esteatosis en un burro (Equus asinus). Primer reporte en Colombia (Steatosis in donkey (Equus asinus). First report in Colombia). Rev. MVZ Cordoba 2011, 16, 2793–2798. [Google Scholar] [CrossRef]
- Sobiech, P.; Kuleta, Z. Levels of selected biochemical indicators of serum and blood during subclinical form of nutritional muscular dystrophy in lambs. Pol. J. Vet. Sci. 1999, 2, 37–41. [Google Scholar]
- Żarczyńska, K.; Sobiech, P.; Radwińska, J.; Rękawek, W. Effects of selenium on animal health. J. Elementol. 2013, 18, 329–340. [Google Scholar] [CrossRef]
- Kojouri, G.A.; Rezakhani, A.; Ahmadi, H. Arrhythmias in advance stiff lamb disease. Small Rumin. Res. 2009, 84, 65–69. [Google Scholar] [CrossRef]
- Van Loon, G.; Lefère, L.; Bauwens, C.; Kleyn, K.; Broux, B.; De Clercq, D.; Deprez, P. Yellow fat disease (steatitis): Description of 20 cases with emphasis on typical ultrasonographic findings. Equine Vet. J. 2015, 47. [Google Scholar] [CrossRef] [PubMed]
- Bruijn, C.; Veldhuis, E.; Sloet, M. Yellow fat disease in equides. Equine Vet. Educ. 2006, 18, 38–44. [Google Scholar] [CrossRef]
- Fajt, Z.; Svoboda, M.; Drábek, J.; Dubanský, V. Selen a jeho význam pro zdravotní stav prasat—Review. Veterinarstvi 2009, 59, 221–224. [Google Scholar]
- Kamada, H.; Nonaka, I.; Takenouchi, N.; Amari, M. Effects of selenium supplementation on plasma progesterone concentrations in pregnant heifers. Anim. Sci. J. 2014, 85, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, M.; Kitahara, G.; Sameshima, H.; Osawa, T. Serum selenium and liposoluble vitamins in Japanese Black cows that had stillborn calves. J. Vet. Med. Sci. 2016, 78, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Underwood, E.J.; Suttle, N.F. Selenium. In The Mineral Nutrition of Livestock, 3rd ed.; CABI Publishing: Wallingford, UK, 2004; pp. 421–475. [Google Scholar]
- Kamada, H. Effects of selenium-rich yeast supplementation on the plasma progesterone levels of postpartum dairy cows. Asian-Australas. J. Anim. Sci. 2017, 30, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wilde, D. Influence of macro and micro minerals in the peri-parturient period on fertility in dairy cattle. Anim. Reprod. Sci. 2006, 96, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Ceko, M.J.; Hummitzsch, K.; Hatzirodos, N.; Bonner, W.M.; Aitken, J.B.; Russell, D.L.; Lane, M.; Rodgers, R.J.; Harris, H.H. X-ray fluorescence imaging and other analyses identify selenium and GPX1 as important in female reproductive function. Metallomics 2015, 7, 66–77. [Google Scholar]
- Davis, C.D.; Tsuji, P.A.; Milner, J.A. Selenoproteins and cancer prevention. Annu. Rev. Nutr. 2012, 32, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [CrossRef] [PubMed]
- Badade, Z.G.; More, K.; Narshetty, J. Oxidative stress adversely affects spermatogenesis in male infertility. Biomed. Res. 2011, 22, 323–328. [Google Scholar]
- Brouwers, J.F.; Gadella, B.M. In situ detection and localization of lipid peroxidation in individual bovine sperm cells. Free Radic. Biol. Med. 2003, 35, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Cerolini, S.; Maldjian, A.; Surai, P.; Noble, R. Viability, susceptibility to peroxidation and fatty acid composition of boar semen during liquid storage. Anim. Reprod. Sci. 2000, 58, 99–111. [Google Scholar] [CrossRef]
- Kemal Duru, N.; Morshedi, M.; Oehninger, S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil. Steril. 2000, 74, 1200–1207. [Google Scholar] [CrossRef]
- Kefer, J.C.; Agarwal, A.; Sabanegh, E. Role of antioxidants in the treatment of male infertility. Int. J. Urol. 2009, 16, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Nallella, K.P.; Allamaneni, S.S.; Said, T.M. Role of antioxidants in treatment of male infertility: An overview of the literature. Reprod. Biomed. Online 2004, 8, 616–627. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Youssef, H.A.A.; Elshazly, M.I.; Rashed, L.A.; Sabry, I.M.; Ibrahim, E.K. Thiobarbituric acid reactive substance (TBARS) a marker of oxidative stress in obstructive sleep apnea. Egypt. J. Chest Dis. Tuberc. 2014, 63, 119–124. [Google Scholar] [CrossRef]
- Bhutia, R.D.; Upadhyay, B.; Maneesh, M. Association of plasma level of thiobarbituric acid reactive substances with extent of hepatocellular injury in preterm infants with cholestatic jaundice. Indian J. Clin. Biochem. 2006, 21, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.J.; Li, K.; Ye, Z.Q.; Chen, Y.G.; Yu, X.; Huang, Y.F. Analysis of lipid peroxidative levels in seminal plasma of infertile men by high-performance liquid chromatography. Arch. Androl. 2004, 50, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Tavilani, H.; Goodarzi, M.T.; Vaisi-Raygani, A.; Salimi, S.; Hassanzadeh, T. Activity of antioxidant enzymes in seminal plasma and their relationship with lipid peroxidation of spermatozoa. Int. Braz. J. Urol. 2008, 34, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Breininger, E.; Beorlegui, N.B.; O’Flaherty, C.M.; Beconi, M.T. Alpha-tocopherol improves biochemical and dynamic parameters in cryopreserved boar semen. Theriogenology 2005, 63, 2126–2135. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, A.; Kadirvel, G.; Bujarbaruah, K.M.; Bardoloi, R.K.; Das, A.; Kumar, S.; Naskar, S. Preservation of boar semen at 18 degrees C induces lipid peroxidation and apoptosis like changes in spermatozoa. Anim. Reprod. Sci. 2009, 110, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Fernández, J.; Gómez-Izquierdo, E.; Tomás, C.; Mocé, E.; de Mercado, E. Is sperm freezability related to the post-thaw lipid peroxidation and the formation of reactive oxygen species in boars? Reprod. Domest. Anim. 2013, 48, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Giadinis, N.D.; Panousis, N.; Petridou, E.J.; Siarkou, V.I.; Lafi, S.Q.; Pourliotis, K.; Hatzopoulou, E.; Fthenakis, G.C. Selenium, vitamin E and vitamin A blood concentrations in dairy sheep flocks with increased or low clinical mastitis incidence. Small Rumin. Res. 2011, 95, 193–196. [Google Scholar] [CrossRef]
- Meschy, F. Nutrition Minérale des Ruminants; Editions Quae: Versaille, France, 2010; p. 208. [Google Scholar]
- Sordillo, L.M. Selenium-dependent regulation of oxidative stress and immunity in periparturient dairy cattle. Vet. Med. Int. 2013, 2013, 154045. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.; Khol-Parisini, A.; Schafft, H.; Lahrssen-Wiederholt, M.; Hulan, H.W.; Dinse, D.; Zentek, J. The role of dietary selenium in bovine mammary gland health and immune function. Anim. Health Res. Rev. 2009, 10, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Passchyn, P.; Piepers, S.; De Vliegher, S. Pathogen group-specific risk factors for intramammary infection in treated and untreated dairy heifers participating in a prepartum antimicrobial treatment trial. J. Dairy Sci. 2014, 97, 6260–6270. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Márquez, A.; Barkema, H.W.; Stryhn, H.; Dohoo, I.R.; Keefe, G.P.; Wichtel, J.J. Bulk tank milk selenium and its association with milk production parameters in Canadian dairy herds. Can. Vet. J. 2012, 53, 51–56. [Google Scholar] [PubMed]
- Erskine, R.J.; Eberhart, R.J.; Grasso, P.J.; Scholz, R.W. Induction of Escherichia coli mastitis in cows fed selenium-deficient or selenium-supplemented diets. Am. J. Vet. Res. 1989, 50, 2093–2100. [Google Scholar] [PubMed]
- Erskine, R.J.; Eberhart, R.J.; Scholz, R.W. Experimentally induced Staphylococcus aureus mastitis in selenium-deficient and selenium-supplemented dairy cows. Am. J. Vet. Res. 1990, 51, 1107–1111. [Google Scholar] [PubMed]
- Ali-Vehmas, T.; Vikerpuur, M.; Fang, W.; Sandholm, M. Giving selenium supplements to dairy cows strengthens the inflammatory response to intramammary infection and induces a growth-suppressing effect on mastitis pathogens in whey. Zentralbl. Veterinarmed. A 1997, 44, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; O’Boyle, N.; Gandy, J.C.; Corl, C.M.; Hamilton, E. Shifts in thioredoxin reductase activity and oxidant status in mononuclear cells obtained from transition dairy cattle. J. Dairy Sci. 2007, 90, 1186–1192. [Google Scholar] [CrossRef]
- Ceballos, A.; Kruze, J.; Barkema, H.W.; Dohoo, I.R.; Sanchez, J.; Uribe, D.; Wichtel, J.J.; Wittwer, F. Barium selenate supplementation and its effect on intramammary infection in pasture-based dairy cows. J. Dairy Sci. 2010, 93, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Marquez, A.; Barkema, H.W.; Stryhn, H.; Wichtel, J.J.; Neumann, J.; Mella, A.; Kruze, J.; Espindola, M.S.; Wittwer, F. The effect of selenium supplementation before calving on early-lactation udder health in pastured dairy heifers. J. Dairy Sci. 2010, 93, 4602–4612. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.S.; Bicalho, M.L.; Pereira, R.V.; Caixeta, L.S.; Knauer, W.A.; Oikonomou, G.; Gilbert, R.O.; Bicalho, R.C. Effect of an injectable trace mineral supplement containing selenium, copper, zinc, and manganese on the health and production of lactating Holstein cows. Vet. J. 2013, 197, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Wu, X.; Shen, X.; Zhang, K.; Ren, F.; Huang, K. Effects of selenium form on blood and milk selenium concentrations, milk component and milk fatty acid composition in dairy cows. J. Sci. Food Agric. 2010, 90, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Naveiro, O.; Domínguez-González, R.; Bermejo-Barrera, A.; Cocho, J.A.; Fraga, J.M.; Bermejo-Barrera, P. Determination of total selenium and selenium distribution in the milk phases in commercial cow’s milk by HG-AAS. Anal. Bioanal. Chem. 2005, 381, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Eulogio, G.L.J.; Hugo, C.V.; Antonio, C.N.; Alejandro, C.-I.; Juan, M.Q. Effects of the selenium and vitamin E in the production, physicochemical composition and somatic cell count in milk of Ayrshire cows. J. Anim. Vet. Adv. 2012, 11, 687–691. [Google Scholar]
- Kim, J.; Van Soest, P.J.; Combs, G.F., Jr. Studies on the effects of selenium on rumen microbial fermentation in vitro. Biol. Trace Elem. Res. 1997, 56, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994; p. 476. [Google Scholar]
- Macfarlane, G.T.; Gibson, G.R.; Beatty, E.; Cummings, J.H. Estimation of shortchain fatty production from protein by human intestinal bacteria on branched-chain fatty acid measurements. FEMS Microbiol. Ecol. 1992, 101, 81–88. [Google Scholar]
- Galbraith, M.L.; Vorachek, W.R.; Estill, C.T.; Whanger, P.D.; Bobe, G.; Davis, T.Z.; Hall, J.A. Rumen microorganisms decrease bioavailability of inorganic selenium supplements. Biol. Trace Elem. Res. 2016, 171, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Van Saun, R.J.; Bobe, G.; Stewart, W.C.; Vorachek, W.R.; Mosher, W.D.; Nichols, T.; Forsberg, N.E.; Pirelli, G.J. Organic and inorganic selenium: I. Oral bioavailability in ewes. J. Anim. Sci. 2012, 90, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Hidiroglou, M.; Jenkins, K.J. Fate of Se-75-selenomethionine in gastrointestinal-tract of sheep. Can. J. Anim. Sci. 1973, 53, 527–536. [Google Scholar] [CrossRef]
- Turner, R.J.; Weiner, J.H.; Taylor, D.E. Selenium metabolism in Escherichia coli. Biometals 1998, 11, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.S.; Davis, T.Z.; Vera, J.M.; Miller, D.N.; Panter, K.E.; ZoBell, D.R. Addition of high concentration of inorganic selenium in orchardgrass (Dactylis glomerata L.) hay diet does not interfere with microbial fermentation in mixed ruminal microorganisms in continuous cultures. Prof. Anim. Sci. 2013, 29, 39–45. [Google Scholar] [CrossRef]
- Mihaliková, K.; Grešáková, Ľ.; Boldižárová, K.; Faix, Š.; Leng, Ľ.; Kišidayová, S. The effects of organic selenium supplementation on the rumen ciliate population in sheep. Folia Microbiol. 2005, 50, 353–356. [Google Scholar] [CrossRef]
- Faixová, Z.; Piešová, E.; Maková, Z.; Čobanová, K.; Faix, Š. Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on ruminal enzyme activities and blood chemistry in sheep. Acta Vet. Brno 2016, 85, 185–194. [Google Scholar] [CrossRef]
- Karl, J.P.; Alemany, J.A.; Koenig, C.; Kraemer, W.J.; Frystyk, J.; Flyvbjerg, A.; Young, A.J.; Nindl, B.C. Diet, body composition, and physical fitness influences on IGF-I bioactivity in women. Growth Horm. IGF Res. 2009, 19, 491–496. [Google Scholar] [CrossRef] [PubMed]
- McElwee, K.; Hoffmann, R. Growth factors in early hair follicle morphogenesis. Eur. J. Dermatol. 2000, 10, 341–350. [Google Scholar] [PubMed]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Lindner, G.; Botchkarev, V.A.; Botchkareva, N.V.; Ling, G.; van der Veen, C.; Paus, R. Analysis of apoptosis during hair follicle regression (catagen). Am. J. Pathol. 1997, 151, 1601–1617. [Google Scholar] [PubMed]
- Ahn, S.Y.; Pi, L.Q.; Hwang, S.T.; Lee, W.S. Effect of IGF-I on hair growth is related to the anti-apoptotic Effect of IGF-I and up-regulation of PDGF-A and PDGF-B. Ann. Dermatol. 2012, 24, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Kamp, H.; Geilen, C.C.; Sommer, C.; Blume-Peytavi, U. Regulation of PDGF and PDGF receptor in cultured dermal papilla cells and follicular keratinocytes of the human hair follicle. Exp. Dermatol. 2003, 12, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, A.; Schlessinger, J. Signal transduction by receptors with tyrosine kinase activity. Cell 1990, 61, 203–212. [Google Scholar] [CrossRef]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar] [CrossRef] [PubMed]
- Ludvíková, E.; Pavlata, L.; Vyskočil, M.; Jahn, P. Selenium status of horses in the Czech Republic. Acta Vet. Brno 2005, 74, 369–375. [Google Scholar] [CrossRef]
- Pavlata, L.; Pechová, A.; Illek, J. Direct and indirect assessment of selenium status in cattle—A comparison. Acta Vet. Brno 2000, 69, 281–287. [Google Scholar] [CrossRef]
- Slavík, P.; Illek, J.; Rajmon, R.; Zelený, T.; Jílek, F. Selenium dynamics in the blood of beef cows and calves fed diets supplemented with organic and inorganic selenium sources and the effect on reproduction. Acta Vet. Brno 2008, 77, 11–15. [Google Scholar] [CrossRef]
- Harapin, I.; Bauer, M.; Bedrica, L.; Potočnjak, D. Correlation between glutathione peroxidase activity and the quantity of selenium in the whole blood of beef calves. Acta Vet. Brno 2000, 69, 87–92. [Google Scholar] [CrossRef]
- Jovanović, B.I.V.M.; Veličković, M.; Milanović, S.; Valčić, O.; Gvozdić, D.; Vranješ-Đurić, S. Supplemental selenium reduces the levels of biomarkers of oxidative and general stress in peripartum dairy cows. Acta Vet. Beograd 2015, 65, 191–201. [Google Scholar]
- Pavlata, L.; Misurova, L.; Pechova, A.; Husakova, T.; Dvorak, R. Direct and indirect assessment of selenium status in sheep—A comparison. Vet. Med. 2012, 57, 219–223. [Google Scholar]
- Pechova, A.; Pavlata, L.; Illek, J. Blood and tissue selenium determination by hydride generation atomic absorption spectrophotometry. Acta Vet. Brno 2005, 74, 483–490. [Google Scholar] [CrossRef]
- Slavík, P.; Illek, J.; Zelený, T. Selenium status in heifers, late pregnancy cows and their calves in the Šumava Region, Czech Republic. Acta Vet. Brno 2007, 76, 519–524. [Google Scholar] [CrossRef]
- Chung, J.Y.; Kim, J.H.; Ko, Y.H.; Jang, I.S. Effects of dietary supplemented inorganic and organic selenium on antioxidant defense systems in the intestine, serum, liver and muscle of Korean native goats. Asian Australas. J. Anim. 2007, 20, 52–59. [Google Scholar] [CrossRef]
- Stockdale, C.R.; Gill, H.S. Effect of duration and level of supplementation of diets of lactating dairy cows with selenized yeast on selenium concentrations in milk and blood after the withdrawal of supplementation. J. Dairy Sci. 2011, 94, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Balán, J.; Vosmanská, M.; Száková, J.; Mestek, O. Speciační analýza selenu v odtučněném řepkovém šrotu. Chem. Listy 2014, 108, 256–263. [Google Scholar]
- Juniper, D.T.; Phipps, R.H.; Jones, A.K.; Bertin, G. Selenium supplementation of lactating dairy cows: Effect on selenium concentration in blood, milk, urine, and feces. J. Dairy Sci. 2006, 89, 3544–3551. [Google Scholar] [CrossRef]
- Konvičná, J.; Vargová, M.; Paulíková, I.; Kováč, G.; Kostecká, Z. Oxidative stress and antioxidant status in dairy cows during prepartal and postpartal periods. Acta Vet. Brno 2015, 84, 133–140. [Google Scholar] [CrossRef]
- Kralik, Z.; Kralik, G.; Biazik, E.; Straková, E.; Suchý, P. Effects of organic selenium in broiler feed on the content of selenium and fatty acid profile in lipids of thigh muscle tissue. Acta Vet. Brno 2013, 82, 277–282. [Google Scholar] [CrossRef]
- Faixová, Z.; Faix, Š.; Bořutová, R.; Leng, Ľ. Efficacy of dietary selenium to counteract toxicity of deoxynivalenol in growing broiler chickens. Acta Vet. Brno 2007, 76, 349–356. [Google Scholar] [CrossRef]
- Kuricová, S.; Boldižárová, K.; Grešáková, Ľ.; Levkut, M.; Leng, Ľ. Chicken selenium satus when fed a diet supplemented with Se-yeast. Acta Vet. Brno 2003, 72, 339–346. [Google Scholar] [CrossRef]
- Liesegang, A.; Staub, T.; Wichert, B.; Wanner, M.; Kreuzer, M. Effect of vitamin E supplementation of sheep and goats fed diets supplemented with polyunsaturated fatty acids and low in Se. J. Anim. Physiol. Anim. Nutr. (Berl.) 2008, 92, 292–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldmann, J.; Salaün, P.; Lombi, E. Critical review perspective: Elemental speciation analysis methods in environmental chemistry—Moving towards methodological integration. Environ. Chem. 2009, 6, 275–289. [Google Scholar] [CrossRef]
- Sanz-Medel, A. Trace element analytical speciation in biological systems: Importance, challenges and trends. Spectrochim. Acta Part B Atomic Spectrosc. 1998, 53, 197–211. [Google Scholar] [CrossRef]
- Szpunar, J.; McSheehy, S.; Połeć, K.; Vacchina, V.; Mounicou, S.; Rodriguez, I.; Łobiński, R. Gas and liquid chromatography with inductively coupled plasma mass spectrometry detection for environmental speciation analysis—Advances and limitations. Spectrochim. Acta Part B Atomic Spectrosc. 2000, 55, 779–793. [Google Scholar] [CrossRef]
- Michalke, B. The coupling of LC to ICP-MS in element speciation—Part II: Recent trends in application. Trends Anal. Chem. 2002, 21, 154–165. [Google Scholar] [CrossRef]
- Takahashi, K.; Suzuki, N.; Ogra, Y. Bioavailability Comparison of Nine Bioselenocompounds In Vitro and In Vivo. Int. J. Mol. Sci. 2017, 18, 506. [Google Scholar] [CrossRef] [PubMed]
- Kahakachchi, C.; Boakye, H.T.; Uden, P.C.; Tyson, J.F. Chromatographic speciation of anionic and neutral selenium compounds in Se-accumulating Brassica juncea (Indian mustard) and in selenized yeast. J. Chromatogr. A 2004, 1054, 303–312. [Google Scholar] [CrossRef]
- Kotrebai, M.; Birringer, M.; Tyson, J.F.; Block, E.; Uden, P.C. Selenium speciation in enriched and natural samples by HPLC-ICP-MS and HPLC-ESI-MS with perfluorinated carboxylic acid ion-pairing agents. Analyst 2000, 125, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Lombi, E.; Stroud, J.L.; McGrath, S.P.; Zhao, F.J. Selenium speciation in soil and rice: Influence of water management and Se fertilization. J. Agric. Food Chem. 2010, 58, 11837–11843. [Google Scholar] [CrossRef] [PubMed]
- McSheehy, S.; Kelly, J.; Tessier, L.; Mester, Z. Identification of selenomethionine in selenized yeast using two-dimensional liquid chromatography-mass spectrometry based proteomic analysis. Analyst 2005, 130, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Montes-Bayón, M.; Molet, M.J.; González, E.B.; Sanz-Medel, A. Evaluation of different sample extraction strategies for selenium determination in selenium-enriched plants (Alliumsativum and Brassicajuncea) and Se speciation by HPLC-ICP-MS. Talanta 2006, 68, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Slekovec, M.; Goessler, W. Accumulation of selenium in natural plants and selenium supplemented vegetable and selenium speciation by HPLC-ICPMS. Chem. Speciat. Bioavailab. 2005, 17, 63–73. [Google Scholar] [CrossRef]
- Cuderman, P.; Kreft, I.; Germ, M.; Kovacevic, M.; Stibilj, V. Selenium species in selenium-enriched and drought-exposed potatoes. J. Agric. Food Chem. 2008, 56, 9114–9120. [Google Scholar] [CrossRef] [PubMed]
- Cuderman, P.; Ožbolt, L.; Kreft, I.; Stibilj, V. Extraction of Se species in buckwheat sprouts grown from seeds soaked in various Se solutions. Food Chem. 2010, 123, 941–948. [Google Scholar] [CrossRef]
- Pedrero, Z.; Encinar, J.R.; Madrid, Y.; Cámara, C. Identification of selenium species in selenium-enriched Lens esculenta plants by using two-dimensional liquid chromatography-inductively coupled plasma mass spectrometry and [77Se]selenomethionine selenium oxide spikes. J. Chromatogr. A 2007, 1139, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Smrkolj, P.; Stibilj, V.; Kreft, I.; Germ, M. Selenium species in buckwheat cultivated with foliar addition of Se(VI) and various levels of UV-B radiation. Food Chem. 2006, 96, 675–681. [Google Scholar] [CrossRef]
- Montes-Bayón, M.; LeDuc, D.L.; Terry, N.; Caruso, J.A. Selenium speciation in wild-type and genetically modified Se accumulating plants with HPLC separation and ICP-MS/ES-MS detection. J. Anal. Atomic Spectrom. 2002, 17, 872–879. [Google Scholar] [CrossRef]
- Van Saun, R.J. Rational approach to selenium supplementation essential. Feedstuffs 1990, 15, 15–17. [Google Scholar]
- National Research Council (NRC). Selenium in Nutrition, Revised ed.; National Academy Press: Washington, DC, USA, 1983; pp. 107–113. [Google Scholar]
- Tracy, M.L.; Möller, G. Continuous flow vapor generation for inductively coupled argon plasma spectrometric analysis. Part 1: Selenium. J. Assoc. Off. Anal. Chem. 1990, 73, 404–410. [Google Scholar] [PubMed]
- Koh, T.S. Interlaboratory study of blood selenium determinations. J. Assoc. Off. Anal. Chem. 1987, 70, 664–667. [Google Scholar] [PubMed]
- Calamari, L.; Ferrari, A.; Bertin, G. Effect of selenium source and dose on selenium status of mature horses. J. Anim. Sci. 2009, 87, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats, 11th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; p. 2356. ISBN 2978-2350-7020-7057-2350. [Google Scholar]
- Kojouri, G.A.; Sharifi, S. Preventing effects of nano-selenium particles on serum concentration of blood urea nitrogen, creatinine, and total protein during intense exercise in donkey. J. Equine Vet. Sci. 2013, 33, 597–600. [Google Scholar] [CrossRef]
- Montgomery, J.B.; Wichtel, J.J.; Wichtel, M.G.; McNiven, M.A.; McClure, J.T.; Markham, F.; Horohov, D.W. Effects of selenium source on measures of selenium status and immune function in horses. Can. J. Vet. Res. 2012, 76, 281–291. [Google Scholar] [PubMed]
- Maas, J.; Galey, F.D.; Peauroi, J.R.; Case, J.T.; Littlefield, E.S.; Gay, C.C.; Koller, L.D.; Crisman, R.O.; Weber, D.W.; Warner, D.W.; et al. The correlation between serum selenium and blood selenium in cattle. J. Vet. Diagn. Investig. 1992, 4, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Chadio, S.E.; Kotsampasi, B.M.; Menegatos, J.G.; Zervas, G.P.; Kalogiannis, D.G. Effect of selenium supplementation on thyroid hormone levels and selenoenzyme activities in growing lambs. Biol. Trace Elem. Res. 2006, 109, 145–154. [Google Scholar] [CrossRef]
- Fortier, M.E.; Audet, I.; Giguère, A.; Laforest, J.P.; Bilodeau, J.F.; Quesnel, H.; Matte, J.J. Effect of dietary organic and inorganic selenium on antioxidant status, embryo development, and reproductive performance in hyperovulatory first-parity gilts. J. Anim. Sci. 2012, 90, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Mahan, D.C. Comparative effects of high dietary levels of organic and inorganic selenium on selenium toxicity of growing-finishing pigs. J. Anim. Sci. 2001, 79, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.A.; Sato, I.; Yamagushi, K. Comparison of selenium level and glutathione peroxidase activity in tissues of vitamin B6 deficient rats fed sodium selenite or dl selenomethionine. J. Nutr. Biochem. 1992, 3, 633–639. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Mahan, D.C. Prolonged feeding of high dietary levels of organic and inorganic selenium to gilts from 25 kg body weight through one parity. J. Anim. Sci. 2001, 79, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Pavlata, L.; Pechová, A.; Bečvář, O.; Illek, J. Selenium status in cattle at slaughter: Analyses of blood, skeletal muscle, and liver. Acta Vet. Brno 2001, 70, 277–284. [Google Scholar] [CrossRef]
- Stowe, H.D.; Herdt, T.H. Clinical assessment of selenium status of livestock. J. Anim. Sci. 1992, 70, 3928–3933. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.R.; Jim, G.K.; Booker, C.W.; Guichon, P.T. A survey of the selenium status of beef cows in Alberta. Can. Vet. J. 1995, 36, 698–702. [Google Scholar] [PubMed]
- Gunter, S.A.; Beck, P.A.; Phillips, J.K. Effects of supplementary selenium source on the performance and blood measurements in beef cows and their calves. J. Anim. Sci. 2003, 81, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Premarathna, H.L.; McLaughlin, M.J.; Kirby, J.K.; Hettiarachchi, G.M.; Beak, D.; Stacey, S.; Chittleborough, D.J. Potential availability of fertilizer selenium in field capacity and submerged soils. Soil. Sci. Soc. Am. J. 2010, 74, 1589–1596. [Google Scholar] [CrossRef]
- Roca-Perez, L.; Gil, C.; Cervera, M.L.; Gonzálvez, A.; Ramos-Miras, J.; Pons, V.; Bech, J.; Boluda, R. Selenium and heavy metals content in some Mediterranean soils. J. Geochem. Explor. 2010, 107, 110–116. [Google Scholar] [CrossRef]
- Zachara, B.A.; Pawluk, H.; Bloch-Boguslawska, E.; Sliwka, K.M.; Korenkiewicz, J.; Skok, Z.; Ryć, K. Tissue level, distribution, and total body selenium content in healthy and diseased humans in Poland. Arch. Environ. Health 2001, 56, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.C.; Gupta, S.C. Selenium in soils and crops, its deficiencies in livestock and humans: Implications for management. Commun. Soil Sci. Plant Anal. 2000, 31, 1791–1807. [Google Scholar] [CrossRef]
- Delesalle, C.; de Bruijn, M.; Wilmink, S.; Vandendriessche, H.; Mol, G.; Boshuizen, B.; Plancke, L.; Grinwis, G. White muscle disease in foals: Focus on selenium soil content. A case series. BMC Vet. Res. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef] [PubMed]
- Supriatin, S.; Weng, L.; Comans, R.N. Selenium speciation and extractability in Dutch agricultural soils. Sci. Total Environ. 2015, 532, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.T.; Li, Z.; Tran, T.A.T.; Wang, D.; Liang, D. Role of organic acids on the bioavailability of selenium in soil: A review. Chemosphere 2017, 184, 618–635. [Google Scholar] [CrossRef] [PubMed]
- Awadeh, F.T.; Kincaid, R.L.; Johnson, K.A. Effect of level and source of dietary selenium on concentrations of thyroid hormones and immunoglobulins in beef cows and calves. J. Anim. Sci. 1998, 76, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M.; Roveri, A. Phospholipid hydroperoxide glutathione peroxidase (PHGPx): More than an antioxidant enzyme? Biomed. Environ. Sci. 1997, 10, 327–332. [Google Scholar] [PubMed]
- Maas, J.; Peauroi, J.R.; Tonjes, T.; Karlonas, J.; Galey, F.D.; Han, B. Intramuscular selenium administration in selenium-deficient cattle. J. Vet. Int. Med. 1993, 7, 342–348. [Google Scholar] [CrossRef]
- Hogan, J.S.; Smith, K.L.; Weiss, W.P.; Todhunter, D.A.; Schockey, W.L. Relationships among vitamin E, selenium, and bovine blood neutrophils. J. Dairy Sci. 1990, 73, 2372–2378. [Google Scholar] [CrossRef]
- Ellison, R.S. A review of copper and selenium reference ranges in cattle and sheep. Vet. Contin. Educ. Massey Univ. 1992, 145, 3–27. [Google Scholar]
- Juniper, D.T.; Phipps, R.H.; Givens, D.I.; Jones, A.K.; Green, C.; Bertin, G. Tolerance of ruminant animals to high dose in-feed administration of a selenium-enriched yeast. J. Anim. Sci. 2008, 86, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, E.N.; Hesketh, J.E.; Sinclair, B.R.; Koolaard, J.P.; Roy, N.C. Selenium-enriched foods are more effective at increasing glutathione peroxidase (GPx) activity compared with selenomethionine: A meta-analysis. Nutrients 2014, 6, 4002–4031. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Xie, S.; Liu, M.; Xiao, X.; Liu, H.; Zhu, X.; Yang, Y. The effects of dietary selenium on growth performances, oxidative stress and tissue selenium concentration of gibel carp (Carassius auratus gibelio). Aquac. Nutr. 2011, 17, e741–e749. [Google Scholar] [CrossRef]
- Dalto, B.D.; Tsoi, S.; Audet, I.; Dyck, M.K.; Foxcroft, G.R.; Matte, J.J. Gene expression of porcine blastocysts from gilts fed organic or inorganic selenium and pyridoxine. Reproduction 2015, 149, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Muhammad, N.; Naeem, M.; Deobald, A.M.; Kamdem, J.P.; Rocha, J.B. In vitro evaluation of glutathione peroxidase (GPx)-like activity and antioxidant properties of an organoselenium compound. Toxicol. In Vitro 2015, 29, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Méplan, C. Selenium and chronic diseases: A nutritional genomics perspective. Nutrients 2015, 7, 3621–3651. [Google Scholar] [CrossRef] [PubMed]
- Acda, S.P.; Chae, B.J. A review on the applications of organic trace minerals in pig nutrition. Pak. J. Nutr. 2002, 1, 25–30. [Google Scholar]
- Schoonheere, N.; Dotreppe, O.; Pincemail, J.; Istasse, L.; Hornick, J.L. Dietary incorporation of feedstuffs naturally high in organic selenium for racing pigeons (Columba livia): Effects on plasma antioxidant markers after a standardised simulation of a flying effort. J. Anim. Physiol. Anim. Nutr. (Berl.) 2009, 93, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Ficek, R.; Drabek, J. Efficacy of selenium from Se-enriched yeast on selenium transfer from sows to piglets. Acta Vet. Brno 2008, 77, 515–521. [Google Scholar] [CrossRef]
- Todd, S.E.; Thomas, D.G.; Hendriks, W.H. Selenium balance in the adult cat in relation to intake of dietary sodium selenite and organically bound selenium. J. Anim. Physiol. Anim. Nutr. (Berl.) 2012, 96, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Ashton, K.; Hooper, L.; Harvey, L.J.; Hurst, R.; Casgrain, A.; Fairweather-Tait, S.J. Methods of assessment of selenium status in humans: A systematic review. Am. J. Clin. Nutr. 2009, 89, 2025S–2039S. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.N.; Hung, Y.S.; Yang, T.S.; Lin, J.H.; Weng, C.F. Aspergillus awamori-fermented mung bean seed coats enhance the antioxidant and immune responses of weaned pigs. J. Anim. Physiol. Anim. Nutr. (Berl.) 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, X.; Su, G.; Shi, B.; Shan, A. High concentration of vitamin E supplementation in sow diet during the last week of gestation and lactation affects the immunological variables and antioxidative parameters in piglets. J. Dairy Res. 2017, 84, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Ocheja, O.B.; Ayo, J.O.; Aluwong, T.; Minka, N.S. Effects of L-glutamine on rectal temperature and some markers of oxidative stress in Red Sokoto goats during the hot-dry season. Trop. Anim. Health Prod. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Dalto, D.B.; Lapointe, J.; Matte, J.J. Assessment of antioxidative and selenium status by seleno-dependent glutathione peroxidase activity in different blood fractions using a pig model: Issues for clinical nutrition and research. J. Anim. Physiol. Anim. Nutr. (Berl.) 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Nazifi, S.; Ghafari, N.; Farshneshani, F.; Rahsepar, M.; Razavi, S.M. Reference values of oxidative stress parameters in adult Iranian fat-tailed sheep. Pak. Vet. J. 2010, 30, 13–16. [Google Scholar]
- Esworthy, R.S.; Chu, F.-F.; Doroshow, J.H. Analysis of glutathione-related enzymes. In Current Protocols in Toxicology; Item 7.1.17; Costa, E., Hodgson, E., Lawrence, D.A., Reed, D.J., GreenLee, W.F., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- King, J.C. Physiology of pregnancy and nutrient metabolism. Am. J. Clin. Nutr. 2000, 71, 1218S–1225S. [Google Scholar] [PubMed]
- Gerloff, B.J. Effect of selenium supplementation on dairy cattle. J. Anim. Sci. 1992, 70, 3934–3940. [Google Scholar] [CrossRef] [PubMed]
- Scholz, R.W.; Hutchinson, L.J. Distribution of glutathione peroxidase activity and selenium in the blood of dairy cows. Am. J. Vet. Res. 1979, 40, 245–249. [Google Scholar] [PubMed]
- Cowell, R.L. Veterinary Clinical Pathology Secrets; Questions and Answers Reveal the Secrets of Veterinary Clinical Pathology; Elsevier, Inc.: Amsterdam, The Netherlands, 2004; p. 408. ISBN 401-56053-56633-56050. [Google Scholar]
- Scholz, R.W.; Cook, L.S.; Todhunter, D.A. Distribution of selenium-dependent and nonselenium-dependent glutathione peroxidase activity in tissues of young cattle. Am. J. Vet. Res. 1981, 42, 1724–1729. [Google Scholar] [PubMed]
- Dalto, D.B.; Audet, I.; Lapointe, J.; Matte, J.J. The importance of pyridoxine for the impact of the dietary selenium sources on redox balance, embryo development, and reproductive performance in gilts. J. Trace Elem. Med. Biol. 2016, 34, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Dalto, D.B.; Roy, M.; Audet, I.; Palin, M.F.; Guay, F.; Lapointe, J.; Matte, J.J. Interaction between vitamin B6 and source of selenium on the response of the selenium-dependent glutathione peroxidase system to oxidative stress induced by oestrus in pubertal pig. J. Trace Elem. Med. Biol. 2015, 32, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Dalto, D.B.; Matte, J.J. Pyridoxine (vitamin B6) and the glutathione peroxidase system; a link between one-carbon metabolism and antioxidation. Nutrients 2017, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Abd Ellah, M.R.; Niishimori, K.; Goryo, M.; Okada, K.; Yasuda, J. Glutathion peroxidase and glucose-6-phosphate dehydrogenase activities in bovine blood and liver. J. Vet. Med. Sci. 2004, 66, 1219–1221. [Google Scholar] [CrossRef] [PubMed]
- Nemec Svete, A.; Čebulj-Kadunc, N.; Frangež, R.; Kruljc, P. Serum cortisol and haematological, biochemical and antioxidant enzyme variables in horse blood sampled in a slaughterhouse lairage, immediately before stunning and during exsanguination. Animal 2012, 6, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiedź, A.; Nicpoń, J.; Zawadzki, M.; Służewska-Niedźwiedź, M.; Januszewska, L. The influence of road transport on the activities of glutathione reductase, glutathione peroxidase, and glutathione-S-transferase in equine erythrocytes. Vet. Clin. Pathol. 2012, 41, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Siciliano, P.D.; Engle, T.E.; Larson, C.K.; Ward, T.L. Effect of selenium supplementation and source on the selenium status of horses. J. Anim. Sci. 2006, 84, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhu, W.; Guo, Q.; Luo, W.; Zhang, J.; Xu, W.; Xu, J. Weaning induced hepatic oxidative stress, apoptosis, and aminotransferases through MAPK signaling pathways in piglets. Oxid. Med. Cell. Longev. 2016, 2016, 4768541. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.G.; Yang, R.J.; Yue, W.B.; Xun, W.J.; Zhang, C.X.; Ren, Y.S.; Shi, L.; Lei, F.L. Effect of elemental nano-selenium on semen quality, glutathione peroxidase activity, and testis ultrastructure in male Boer goats. Anim. Reprod. Sci. 2010, 118, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jin, Y.; Du, M.; Liu, W.; Ren, Y.; Zhang, C.; Zhang, J. The effect of dietary grape pomace supplementation on epididymal sperm quality and testicular antioxidant ability in ram lambs. Theriogenology 2017, 97, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Hough, C.D.; Cho, K.R.; Zonderman, A.B.; Schwartz, D.R.; Morin, P.J. Coordinately up-regulated genes in ovarian cancer. Cancer Res. 2001, 61, 3869–3876. [Google Scholar] [PubMed]
- Brigelius-Flohé, R. Tissue-specific functions of individual glutathione peroxidases. Free Radic. Biol. Med. 1999, 27, 951–965. [Google Scholar] [CrossRef]
- Humann-Ziehank, E.; Renko, K.; Mueller, A.S.; Roehrig, P.; Wolfsen, J.; Ganter, M. Comparing functional metabolic effects of marginal and sufficient selenium supply in sheep. J. Trace Elem. Med. Biol. 2013, 27, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.F.; Esworthy, R.S.; Doroshow, J.H.; Doan, K.; Liu, X.F. Expression of plasma glutathione peroxidase in human liver in addition to kidney, heart, lung, and breast in humans and rodents. Blood 1992, 79, 3233–3238. [Google Scholar] [PubMed]
- Miranda, S.G.; Wang, Y.J.; Purdie, N.G.; Osborne, V.R.; Coomber, B.L.; Cant, J.P. Selenomethionine stimulates expression of glutathione peroxidase 1 and 3 and growth of bovine mammary epithelial cells in primary culture. J. Dairy Sci. 2009, 92, 2670–2683. [Google Scholar] [CrossRef] [PubMed]
- Bruzelius, K.; Hoac, T.; Sundler, R.; Onning, G.; Akesson, B. Occurrence of selenoprotein enzyme activities and mRNA in bovine mammary tissue. J. Dairy Sci. 2007, 90, 918–927. [Google Scholar] [CrossRef]
- Naiki-Ito, A.; Asamoto, M.; Hokaiwado, N.; Takahashi, S.; Yamashita, H.; Tsuda, H.; Ogawa, K.; Shirai, T. Gpx2 is an overexpressed gene in rat breast cancers induced by three different chemical carcinogens. Cancer Res. 2007, 67, 11353–11358. [Google Scholar] [CrossRef] [PubMed]
- Bickhardt, K.; Ganterm, M.; Sallmann, P. Investigation of the manifestation of vitamin E and selenium deficiency in sheep and goats. Dtsch.-Tierarztliche-Wochenschr. 1999, 106, 242–247. [Google Scholar]
- Fraga, C.G.; Ariass, R.F.; Llesuy, S.F. Effect of vitamin E and Se deficiency on rat liver chemiluminescence. Biochem. G 1987, 242, 383–392. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Watkins, C.A.; Bayne, C.W. Contribution of respiratory burst activity to innate immune function and the effects of disease status and agent on chemiluminescence responses by ruminant phagocytes in vitro. Vet. Immunol. Immunopathol. 2006, 112, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Ivancic, J.J.; Weiss, W.P. Effect of dietary sulfur and selenium concentrations on selenium balance of lactating Holstein cows. J. Dairy Sci. 2001, 84, 225–232. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Selenium and selenium-antagonistic elements in nutritional cancer prevention. Crit. Rev. Biotechnol. 2009, 29, 10–17. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Dairy Cattle, 7th Revised ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Suttle, N.F. Mineral Nutrition of Livestock, 4th ed.; British Library: London, UK, 2010. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Sheep, 6th ed.; National Academy Press: Washington, DC, USA, 1985. [Google Scholar]
- Papazafeiriou, A.Z.; Lakis, C.; Stefanou, S.; Yiakoulaki, M.; Mpokos, P.; Papanikolaou, K. Trace elements content of plant material growing on alkaline organix soils and its suitabilty for small ruminant extensive farming. Bulg. J. Agric. Sci. 2016, 22, 733–739. [Google Scholar]
- National Research Council (NRC). Minerals. In Nutrient Requirements of Horses, 6th ed.; National Research Council, Ed.; National Academies Press: Washington, DC, USA, 2007; pp. 94–97. [Google Scholar]
- Pagan, J.D.; Karnezos, P.; Kennedy, M.A.P.; Currier, T.; Hoekstra, K.E. Effect of selenium source on selenium digestibility and retention in exercised Thoroughbreds. In Proceedings of the Equine Nutrition and Physiology Society, Raleigh, NC, USA, 2–5 June 1999; pp. 135–140. [Google Scholar]
- Geor, R.J.; Coenen, M.; Harris, P. Equine Applied and Clinical Nutrition E-Book: Health, Welfare and Performance; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; p. 592. ISBN 978-590-7020-3422-7020. [Google Scholar]
- Guyot, H.; Spring, P.; Andrieu, S.; Rollin, F. Comparative responses to sodium selenite and organic selenium supplements in Belgium Blue cows and calves. Livest. Sci. 2007, 111, 259–263. [Google Scholar]
- National Research Council (NRC). Mineral Tolerances of Animals, 2nd Revised ed.; National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- Hill, G.M.; Link, J.E.; Meyer, L.; Fritsche, K.L. Effect of vitamin E and selenium on iron utilization in neonatal pigs. J. Anim. Sci. 1999, 77, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Mahan, D.C. Effect of organic and inorganic selenium sources and levels on sow colostrum and milk selenium content. J. Anim. Sci. 2000, 78, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.; McMillan, E. Comparative effects of organic and inorganic selenium on selenium transfer from sows to nursing pigs. J. Anim. Sci. 2006, 84, 1729–1733. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. Selenomethionine: A review of its nutritional significance, metabolism and toxicity. J. Nutr. 2000, 130, 1653–1656. [Google Scholar] [PubMed]
- Knowles, S.O.; Grace, N.D.; Wurms, K.; Lee, J. Significance of amount and form of dietary selenium on blood, milk, and casein selenium concentrations in grazing cows. J. Dairy Sci. 1999, 82, 429–437. [Google Scholar] [CrossRef]
- Svoboda, M.; Kotrbáček, V.; Ficek, R.; Drábek, J. Effect of Organic Selenium from Se-enriched Alga (Chlorella spp.) on Selenium Transfer from Sows to Their Progeny. Acta Vet. Brno 2009, 78, 373–377. [Google Scholar] [CrossRef]
- Trávníček, J.; Racek, J.; Trefil, L.; Rodinová, H.; Kroupová, V.; Illek, J.; Doucha, J.; Písek, L. Activity of glutathione peroxidase (GSH-Px) in the blood of ewes and their lambs receiving the selenium-enriched unicellular alga Chlorella. Czech J. Anim. Sci. 2008, 53, 292–298. [Google Scholar]
- Valčić, O.; Jovanović, I.; Milanović, S.; Gvozdić, D. Selenium status of feedstuffs and grazing ewes in Serbia. Acta Vet. Beaograd 2013, 63, 665–675. [Google Scholar]
- Değer, Y.; Ertekin, A.; Değer, S.; Mert, H. Lipid peroxidation and antioxidant potential of sheep liver infected naturally with distomatosis. Turk. Parazitol. Derg. 2008, 32, 23–26. [Google Scholar]
- Yaralioglu-Gurgoze, S.; Cetin, H.; Cen, O.; Yilmaz, S.; Atli, M.O. Changes in malondialdehyde concentrations and glutathione peroxidase activity in purebred Arabian mares with endometritis. Vet. J. 2005, 170, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Mélo, S.K.; Diniz, A.I.; de Lira, V.L.; de Oliveira Muniz, S.K.; da Silva, G.R.; Manso, H.E.; Manso Filho, H.C. Antioxidant and haematological biomarkers in different groups of horses supplemented with polyunsaturated oil and vitamin E. J. Anim. Physiol. Anim. Nutr. (Berl.) 2016, 100, 852–859. [Google Scholar] [CrossRef] [PubMed]
- El-Bahr, S.M.; El-Deeb, W.M. Acute-phase proteins, oxidative stress biomarkers, proinflammatory cytokines, and cardiac troponin in Arabian mares affected with pyometra. Theriogenology 2016, 86, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, W.M.; El-Bahr, S.M. Investigation of selected biochemical indicators of Equine Rhabdomyolysis in Arabian horses: Pro-inflammatory cytokines and oxidative stress markers. Vet. Res. Commun. 2010, 34, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Janiak, M.; Suska, M.; Dudzińska, W.; Skotnicka, E. Blood glutathione status and activity of glutathione-metabolizing antioxidant enzymes in erythrocytes of young trotters in basic training. J. Anim. Physiol. Anim. Nutr. (Berl.) 2010, 94, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Kojouri, G.A.; Faramarzi, P.; Ahadi, A.M.; Parchami, A. Effect of selenium nanoparticles on expression of HSP90 gene in myocytes after an intense exercise. J. Equine Vet. Sci. 2013, 33, 1054–1056. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Yagi, K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol. Biol. 1998, 108, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Augustin, K.; Blank, R.; Boesch-Saadatmandi, C.; Frank, J.; Wolffram, S.; Rimbach, G. Dietary green tea polyphenols do not affect vitamin E status, antioxidant capacity and meat quality of growing pigs. J. Anim. Physiol. Anim. Nutr. (Berl.) 2008, 92, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Beers, R.F.J.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [PubMed]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef]
- Günzler, W.A.; Kremers, H.; Flohé, L. An improved coupled test procedure for glutathione peroxidase (EC 1-11-1-9-) in blood. Z. Klin. Chem. Klin. Biochem. 1974, 12, 444–448. [Google Scholar] [PubMed]
- Sankari, S.; Atroshi, F. Effect of dietary selenium on erythrocyte glutathione peroxidase and blood selenium in two types of Finnisheep genetically selected for high and low glutathione peroxidase activity. Zbl. Vet. Med. A 1983, 30, 452. [Google Scholar] [CrossRef]
- Jain, S.K.; McVie, R.; Duett, J.; Herbst, J.J. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes 1989, 38, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.P.J.; Dailey, M.; Sugarman, E. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch. Biochem. Biophys. 1987, 255, 329–336. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Tietze, F. Enzymatic method for the quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Botsoglou, N.A. Rapid, sensitive, and specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Jentzsch, A.M.; Bachmann, H.; Fürst, P.; Biesalski, H.K. Improved analysis of malondialdehyde in human body fluids. Free Radic. Biol. Med. 1996, 20, 251–256. [Google Scholar] [CrossRef]
- Gérard-Monnier, D.; Erdelmeier, I.; Régnard, K.; Moze-Henry, N.; Yadan, J.C.; Chaudière, J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Reaner, D.C.; Veillon, C. Elimination of perchloric acid in digestion of biological fluids for fluorometric determination of selenium. Anal. Chem. 1983, 55, 1605–1606. [Google Scholar] [CrossRef] [PubMed]
- Wendel, A. Glutathione peroxidase. Methods Enzymol. 1981, 77, 325–333. [Google Scholar] [PubMed]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [PubMed]
- Wills, E.D. Mechanisms of lipid peroxide formation in animal tissues. Biochem. J. 1966, 99, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E. Glutathione in Red Blood Cell Metabolism. In A Manual of Biochemical Methods; Grunef Strottan: New York, NY, USA, 1975; pp. 112–114. [Google Scholar]
- Satoh, K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar] [PubMed]
- Yagi, K. Assay for blood plasma or serum. Methods Enzymol. 1984, 105, 328–331. [Google Scholar] [PubMed]
- McCord, J.M.; Fridovich, I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J. Biol. Chem. 1969, 244, 6056–6063. [Google Scholar] [PubMed]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Brown, M.W.; Watkinson, J.H. Automated fluorometric method for determination of nanogram quantities of Se. Anal. Chim. Acta 1977, 89, 29–35. [Google Scholar] [CrossRef]
- Beilstein, M.A.; Whanger, P.D. Deposition of dietary organic and inorganic selenium in rat erythrocyte proteins. J. Nutr. 1986, 116, 1701–1710. [Google Scholar] [PubMed]


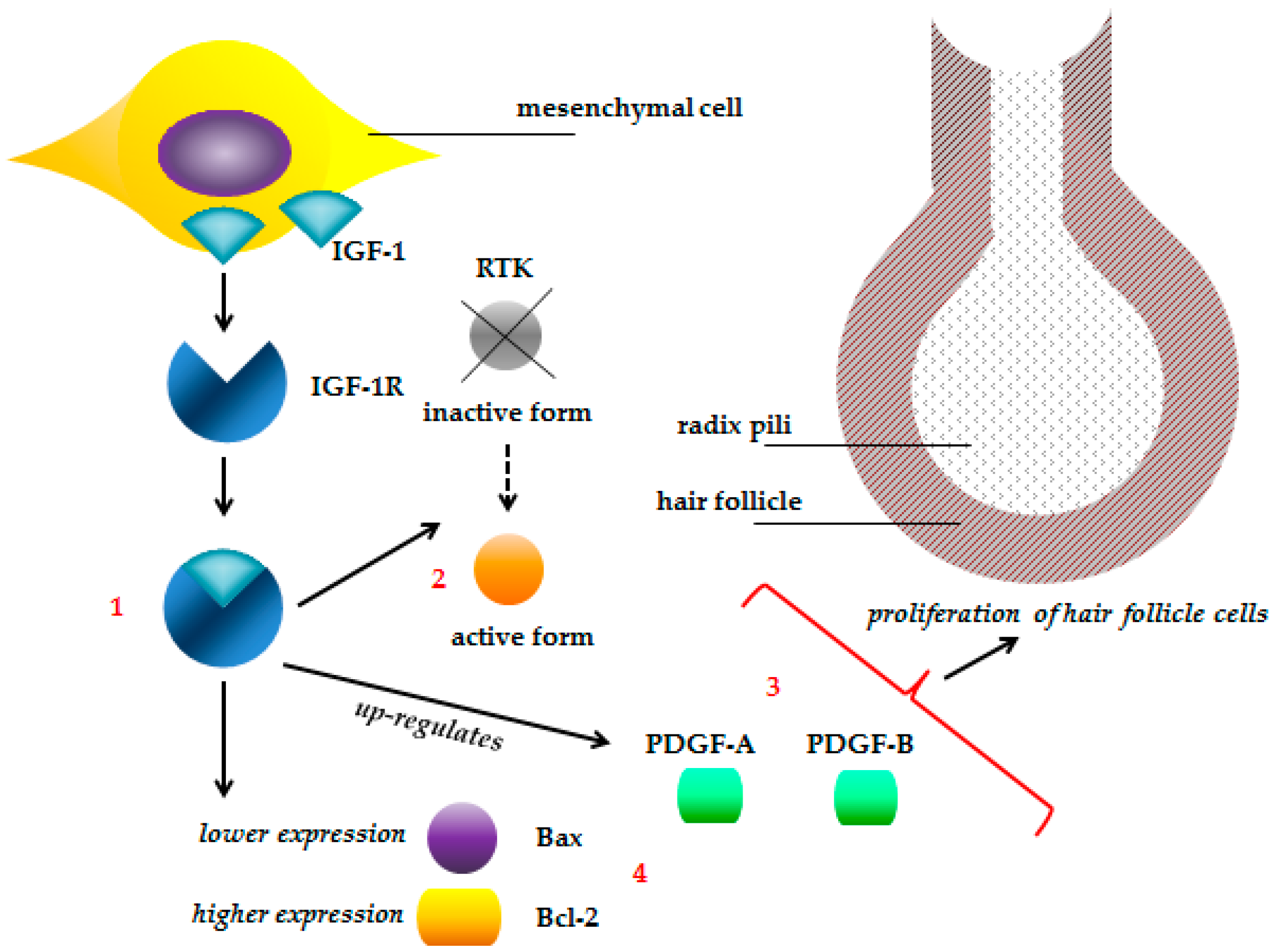
| Animal Species | RDI of Se | Reference |
|---|---|---|
| pigs | 0.15–0.30 mg∙kg−1 of feed | [123] |
| beef cattle (*) | 100 μg∙kg−1 of DM of feed | [376,377] |
| dairy cattle | 300 μg∙kg−1 of DM of feed | [376,377] |
| cattle—calves | 100 μg∙kg−1 of DM of feed | [376,377] |
| sheep | 0.1–0.2 mg∙kg−1 of DM of feed | [378] |
| goats | 0.1 mg∙kg−1 of DM of feed | [379] |
| horses | 0.1 ppm of DM of feed for idle horses | [380,381] |
| 0.3 ppm of DM of feed for exercising horses | [380,381] | |
| donkeys | ~2 mg∙day−1 0.1–0.15 mg∙100 kg−1 BW | [382] [380] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Malevu, T.D.; Sochor, J.; Baron, M.; Melcova, M.; Zidkova, J.; et al. A Summary of New Findings on the Biological Effects of Selenium in Selected Animal Species—A Critical Review. Int. J. Mol. Sci. 2017, 18, 2209. https://doi.org/10.3390/ijms18102209
Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Malevu TD, Sochor J, Baron M, Melcova M, Zidkova J, et al. A Summary of New Findings on the Biological Effects of Selenium in Selected Animal Species—A Critical Review. International Journal of Molecular Sciences. 2017; 18(10):2209. https://doi.org/10.3390/ijms18102209
Chicago/Turabian StyleHosnedlova, Bozena, Marta Kepinska, Sylvie Skalickova, Carlos Fernandez, Branislav Ruttkay-Nedecky, Thembinkosi Donald Malevu, Jiri Sochor, Mojmir Baron, Magdalena Melcova, Jarmila Zidkova, and et al. 2017. "A Summary of New Findings on the Biological Effects of Selenium in Selected Animal Species—A Critical Review" International Journal of Molecular Sciences 18, no. 10: 2209. https://doi.org/10.3390/ijms18102209
APA StyleHosnedlova, B., Kepinska, M., Skalickova, S., Fernandez, C., Ruttkay-Nedecky, B., Malevu, T. D., Sochor, J., Baron, M., Melcova, M., Zidkova, J., & Kizek, R. (2017). A Summary of New Findings on the Biological Effects of Selenium in Selected Animal Species—A Critical Review. International Journal of Molecular Sciences, 18(10), 2209. https://doi.org/10.3390/ijms18102209