Recent Advances in Co3O4-Based Composites: Synthesis and Application in Combustion of Methane
Abstract
:1. Introduction
1.1. Background
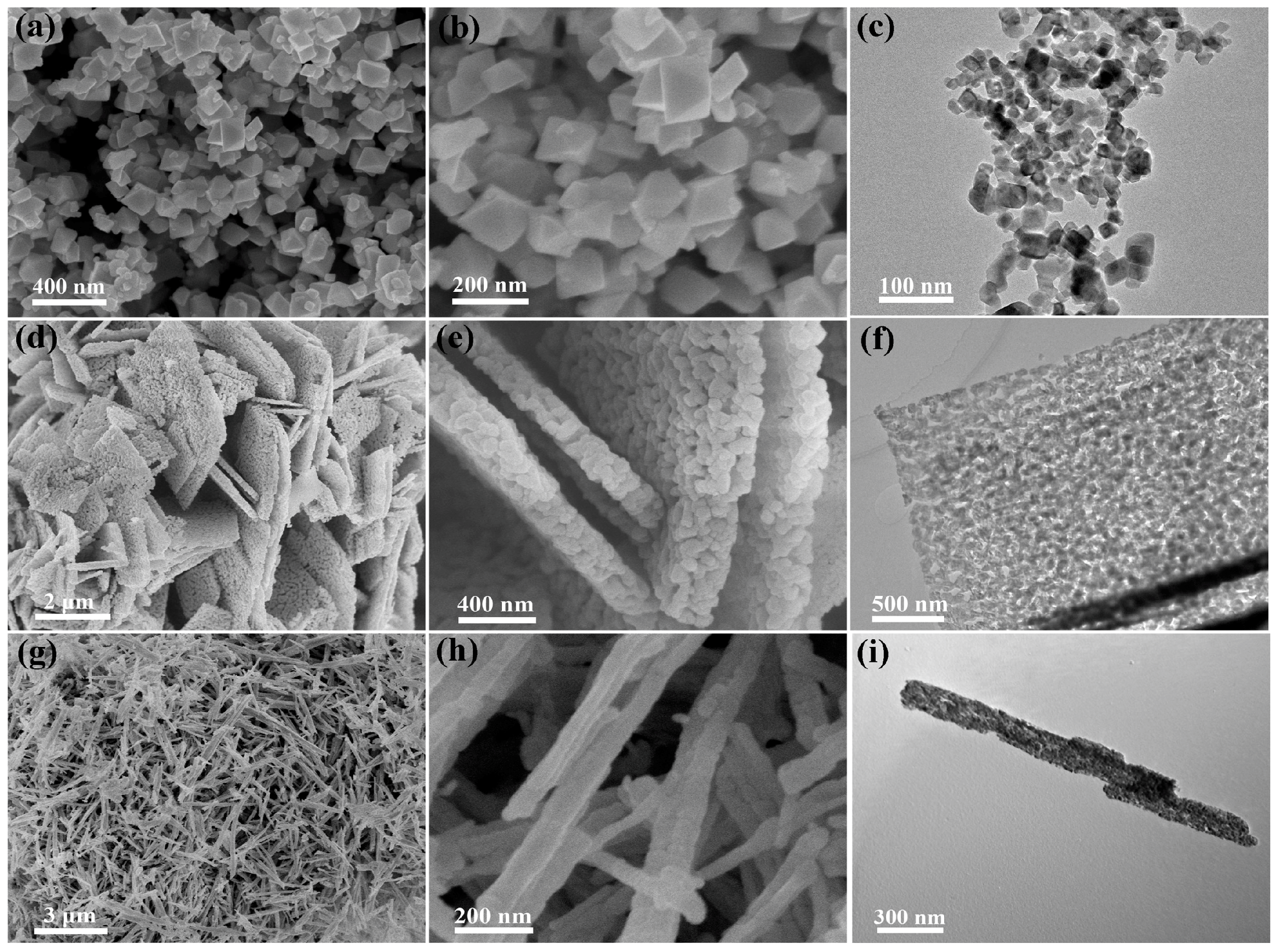
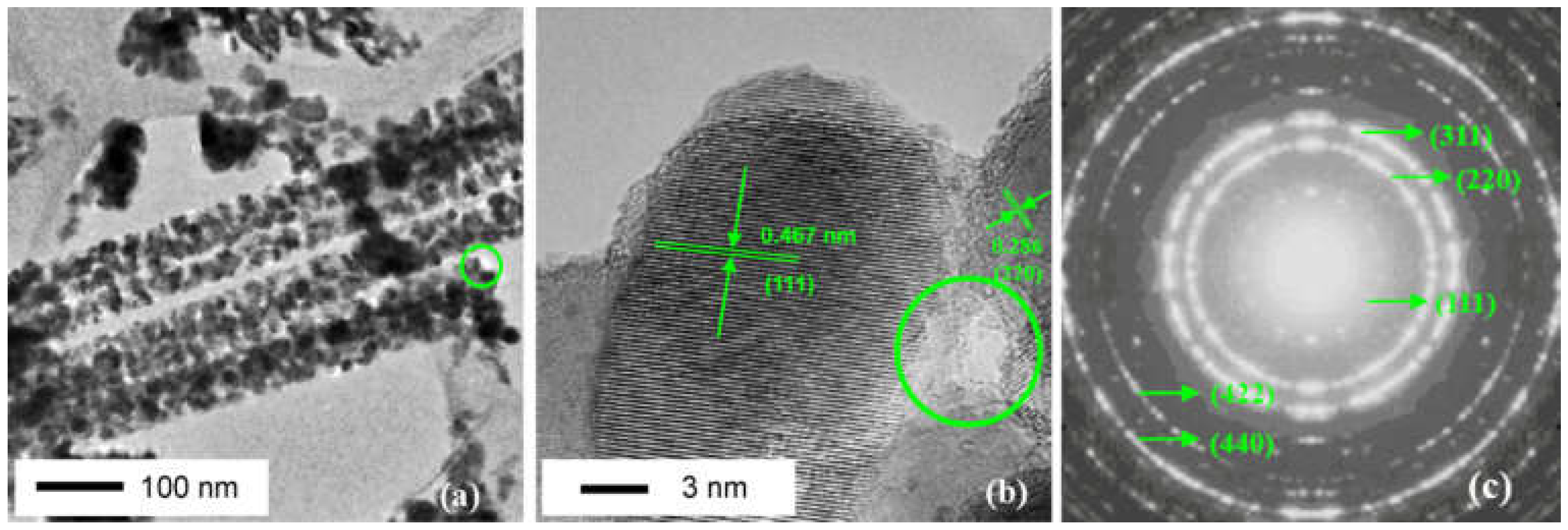
1.2. Co3O4 Catalyst Materials
1.3. Contents of This Review
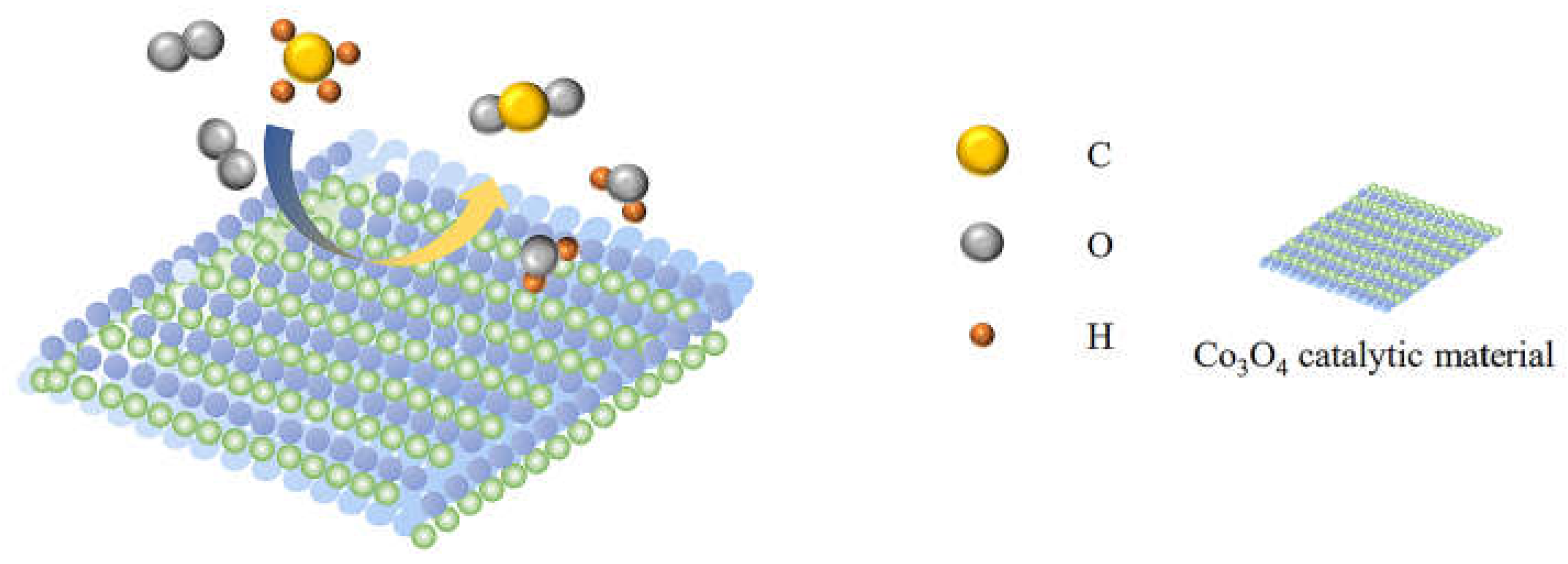
2. Catalytic Oxidation of Methane by Nano-Co3O4
- (1)
- Methane and oxygen adsorption to Co3O4 catalytic material surface.
- (2)
- The formation of active intermediate substances.
- (3)
- Further reaction of active intermediate substance.
3. Co3O4 Composite Catalysts in Combustion of Methane
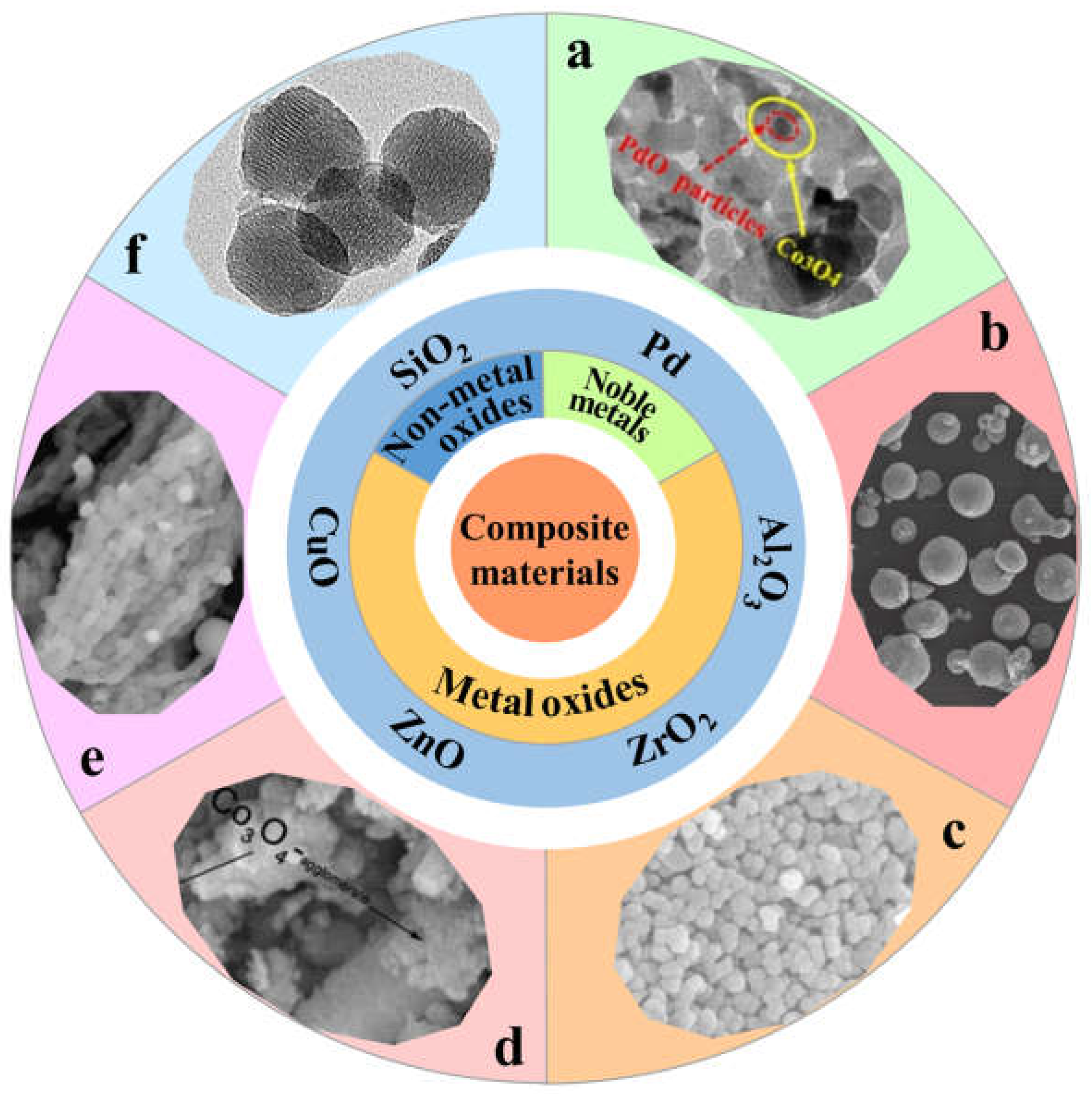
3.1. Noble Metals
3.2. Metal Oxides
3.3. Non Metallic Oxides
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lewandowska, A.; Kocemba, I.; Rynkowski, J. Catalytic Properties of Ag/SnO2 Catalysts Applied in Low-Temperature Methane Oxidation. Pol. J. Environ. Stud. 2008, 17, 433–437. [Google Scholar]
- Liu, K.; Mi, L.; Wang, H.; Xiong, X.; Zhang, K.; Wang, B. Preparation of Ba1-xSrxTiO3 by the sol-gel assisted solid phase method: Study on its formation mechanism and photocatalytic hydrogen production performance. Ceram. Int. 2021, 47, 22055–22064. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, Y.; Zhou, H.; Huang, W.; Pu, Z. Combustion of lean methane over Co3O4 catalysts prepared with different cobalt precursors. RSC Adv. 2020, 10, 4490–4498. [Google Scholar] [PubMed]
- Meisheng, C.; Liangshi, W.; Na, Z.; Zhiqi, L.; Dianqing, L.; Aifan, C. La-Hexaaluminate Catalyst Preparation and Its Performance for Methane Catalytic Combustion. J. Rare Earths 2006, 24, 690–694. [Google Scholar] [CrossRef]
- Kumsung, W.; Chareonpanich, M.; Kongkachuichay, P.; Senkan, S.; Seubsai, A. Single and bimetallic catalyst screenings of noble metals for methane combustion. Catal. Commun. 2018, 110, 83–87. [Google Scholar]
- Khajenoori, M.; Rezaei, M.; Nematollahi, B. Preparation of noble metal nanocatalysts and their applications in catalytic partial oxidation of methane. J. Ind. Eng. Chem. 2013, 19, 981–986. [Google Scholar] [CrossRef]
- Mccarty, J.G.; Wise, H. Perovskite catalysts for methane combustion. Catal. Today 1990, 8, 231–248. [Google Scholar]
- Ordóez, S.; Paredes, J.R.; Díez, F. Sulphur poisoning of transition metal oxides used as catalysts for methane combustion. Appl. Catal. A Gen. 2008, 341, 174–180. [Google Scholar] [CrossRef]
- Cao, X.; Zhou, R.; Rui, N.; Wang, Z.; Wang, J.; Zhou, X.; Liu, C.J. Co3O4/HZSM-5 catalysts for methane combustion: The effect of preparation methodologies. Catal. Today 2017, 297, 219–227. [Google Scholar] [CrossRef]
- Vickers, S.M.; Gholami, R.; Smith, K.J.; Maclachlan, M.J. Mesoporous Mn- and La-doped cerium oxide/cobalt oxide mixed metal catalysts for methane oxidation. ACS Appl. Mater. Interfaces 2015, 7, 11460. [Google Scholar]
- Zhou, M.; Zhang, J.; Liao, L.; Wu, W. Enhanced catalytic methane combustion over Co3O4 nanowire arrays by cation substitution. Mater. Res. Express 2017, 4, 125006. [Google Scholar] [CrossRef]
- Du, Y.; Meng, Q.; Wang, J.; Yan, J.; Fan, H.; Liu, Y.; Dai, H. Three-dimensional mesoporous manganese oxides and cobalt oxides: High-efficiency catalysts for the removal of toluene and carbon monoxide. Microporous Mesoporous Mater. 2012, 162, 199–206. [Google Scholar] [CrossRef]
- Hu, W.; Lan, J.; Guo, Y.; Cao, X.-M.; Hu, P. Origin of Efficient Catalytic Combustion of Methane over Co3O4(110): Active Low-Coordination Lattice Oxygen and Cooperation of Multiple Active Sites. ACS Catal. 2016, 6, 5508–5519. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, W.; Chen, K.; Zhang, X.; Shen, C.; Yuan, L. A rod-like Co3O4 with high efficiency and large specific surface area for lean methane catalytic oxidation. Mol. Catal. 2022, 522, 112229. [Google Scholar] [CrossRef]
- Xiong, X.; Jin, Y.; Wang, H.; He, P.; Xiang, X.; Hu, P.; Liu, K.; Wei, Q.; Wang, B. Study on the hydrogen production properties and electron transfer mechanism of CdS/WO3 composite photocatalyst. Mater. Chem. Phys. 2022, 281, 125824. [Google Scholar] [CrossRef]
- Gong, D.; Zeng, G. Low-temperature combustion of methane over graphene templated Co3O4 defective-nanoplates. Sci. Rep. 2021, 11, 12604. [Google Scholar] [CrossRef]
- Zhao, D.; Duan, Z.; Sun, Y.; Tan, X.; Wu, X. Design of pH-universal NiCoP nanowire electrocatalysts with tunable charge distribution. Mater. Today Chem. 2022, 26, 101203. [Google Scholar] [CrossRef]
- Mu, B.; Zhang, X.; Zhang, Y.; Lu, P.; Hao, J.; Zhang, J. Solution combustion derived oxygen vacancy-rich Co3O4 catalysts for catalytic formaldehyde oxidation at room temperature. RSC Adv. 2022, 12, 9821–9827. [Google Scholar] [CrossRef]
- Zhang, S.; Shan, J.-j.; Zhu, Y.; Frenkel, A.I.; Patlolla, A.; Huang, W.; Yoon, S.J.; Wang, L.; Yoshida, H.; Takeda, S. WGS Catalysis and In Situ Studies of CoO1–x, PtCo n/Co3O4, and Pt m Co m′/CoO1–x Nanorod Catalysts. J. Am. Chem. Soc. 2013, 135, 8283–8293. [Google Scholar]
- Zheng, Y.; Liu, Y.; Zhou, H.; Huang, W.; Pu, Z. Complete combustion of methane over Co3O4 catalysts: Influence of pH values. J. Alloys Compd. 2018, 734, 112–120. [Google Scholar] [CrossRef]
- Choya, A.; Gudyka, S.; de Rivas, B.; Ignacio Gutierrez-Ortiz, J.; Kotarba, A.; Lopez-Fonseca, R. Design, characterization and evaluation of Ce-modified cobalt catalysts supported on alpha alumina in the abatement of methane emissions from natural gas engines. Appl. Catal. A Gen. 2021, 617, 118105. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, H.; Dong, B.; Ni, Y.; Kong, A.; Shan, Y. High Efficient Mesoporous Co3O4 Nanocatalysts For Methane Combustion at Low Temperature. Chemistryselect 2016, 1, 979–983. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Wu, X. Surface Selenization of NiCo-Layered Double Hydroxide Nanosheets for High-Performance Supercapacitors. Batteries 2023, 9, 49. [Google Scholar]
- Zhao, S.; Li, T.; Lin, J.; Wu, P.; Li, Y.; Li, A.; Chen, T.; Zhao, Y.; Chen, G.; Yang, L.; et al. Engineering Co3+-rich crystal planes on Co3O4 hexagonal nanosheets for CO and hydrocarbons oxidation with enhanced catalytic activity and water resistance. Chem. Eng. J. 2021, 420, 130448. [Google Scholar] [CrossRef]
- Chen, K.; Li, W.; Zhou, Z.; Huang, Q.; Liu, Y.; Duan, Q. Hydroxyl groups attached to Co2+ on the surface of Co3O4: A promising structure for propane catalytic oxidation. Catal. Sci. Technol. 2020, 10, 2573–2582. [Google Scholar] [CrossRef]
- Bao, L.; Zhu, S.; Chen, Y.; Wang, Y.; Meng, W.; Xu, S.; Lin, Z.; Li, X.; Sun, M.; Guo, L. Anionic defects engineering of Co3O4 catalyst for toluene oxidation. Fuel 2022, 314, 122774. [Google Scholar] [CrossRef]
- Chai, G.; Pan, S.; Guo, Y.; Zhan, W.; Wang, L.; Guo, Y.; Wang, H. Insight into the Surface-Tuned Activity and Cl-2/HCl Selectivity in the Catalytic Oxidation of Vinyl Chloride over Co3O4(110) versus (001): A DFT Study. J. Phys. Chem. C 2021, 125, 16975–16983. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, C.; Li, X.Y.; Wang, C.; Wang, X.X.; Cao, H.J.; Zhao, M.C.; Wu, G.L.; Su, W.G.; Ma, T.T.; et al. N-doping activated defective Co3O4 as an efficient catalyst for low-temperature methane oxidation. Appl. Catal. B Environ. 2020, 269, 118757. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; Ignacio Gutierrez-Ortiz, J.; Ramon Gonzalez-Velasco, J.; Lopez-Fonseca, R. Synthesis, Characterization and Kinetic Behavior of Supported Cobalt Catalysts for Oxidative after-Treatment of Methane Lean Mixtures. Materials 2019, 12, 3174. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhao, Y.; Chen, X.; Guo, Y.; Wang, H.; Jin, Y.; He, P.; Wei, Q.; Wang, B. Preparation of polymeric carbon nitride/TiO2 heterostructure with NH4Cl as template: Structural and photocatalytic studies. J. Phys. Chem. Solids 2022, 164, 110629. [Google Scholar] [CrossRef]
- Zasada, F.; Gryboś, J.; Hudy, C.; Janas, J.; Sojka, Z. Total oxidation of lean methane over cobalt spinel nanocubes—Mechanistic vistas gained from DFT modeling and catalytic isotopic investigations. Catal. Today 2020, 354, 183–195. [Google Scholar] [CrossRef]
- Wang, K.; Cao, Y.; Hu, J.; Li, Y.; Xie, J.; Jia, D. Solvent-Free Chemical Approach to Synthesize Various Morphological Co3O4 for CO Oxidation. ACS Appl. Mater. Interfaces 2017, 9, 16128–16137. [Google Scholar] [CrossRef]
- Liotta, L.F.; Wu, H.J.; Pantaleo, G.; Venezia, A.M. Co3O4 nanocrystals and Co3O4-MOx binary oxides for CO, CH4 and VOC oxidation at low temperatures: A review. Catal. Sci. Technol. 2013, 3, 3085–3102. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, X.; Zhang, D.; Cheng, Y.; Zhao, H.; Chang, L.; Yu, Z.; Yang, S. Preparation and capacitive performance of three-dimensional bulk-phase core-shell structured activated carbon @ Ni(OH)2 composites. Ionics 2023, 29, 1777–1787. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Yang, L.; Dong, L.; Wang, D.; Xu, X.; Wang, D.; Huang, J.; Lv, M.; Wang, H. A Review of Cobalt-Containing Nanomaterials, Carbon Nanomaterials and Their Composites in Preparation Methods and Application. Nanomaterials 2022, 12, 2042. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, Q.; Yang, K.; Xu, X.; Huang, J.; Chen, H.; Wang, H. A Review on the Application of Cobalt-Based Nanomaterials in Supercapacitors. Nanomaterials 2022, 12, 4065. [Google Scholar] [CrossRef]
- Singh, S.A.; Madras, G.; Sreedhar, I. Transition Metal (Ni, Cu and Fe) Substituted Co3O4–ZrO2 Catalysts for Lean Methane Combustion. Top. Catal. 2021, 64, 243–255. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, J.; Xue, F.; Deng, J.; Dai, Y.; Li, L.; Cui, M.; Qiao, X.; Fei, Z. Plasma-engraved Co3O4 nanostructure toward improved formaldehyde oxidation performance: Insight into the structure–activity relationship. Appl. Surf. Sci. 2022, 600, 154183. [Google Scholar] [CrossRef]
- Feng, C.; Wang, D.; Deng, R.; Tang, J.; Song, S.; Lei, Y.; Song, W.; Su, S.; Yang, X.; Zhang, H. Porous Co3O4 microcubes: Hydrothermal synthesis, catalytic and magnetic properties. Crystengcomm 2011, 13, 2123–2129. [Google Scholar]
- Xiong, S.; Yuan, C.; Zhang, X.; Xi, B.; Qian, Y. Controllable Synthesis of Mesoporous Co3O4Nanostructures with Tunable Morphology for Application in Supercapacitors. Chem. A Eur. J. 2009, 15, 5320–5326. [Google Scholar]
- Kumar, M.; Bhatt, V.; Yun, J.H. Hierarchical 3D micro flower-like Co3O4 structures for NO2 detection at room temperature. Phys. Lett. A 2020, 384, 126477. [Google Scholar] [CrossRef]
- Fei, Z.; He, S.; Li, L.; Ji, W.; Au, C.T. Morphology-directed synthesis of Co3O4 nanotubes based on modified Kirkendall effect and its application in CH4 combustion. Chem. Commun. 2011, 48, 853–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.A.; Li, S.; An, L. Hierarchically porous Co3O4 hollow spheres with tunable pore structure and enhanced catalytic activity. Chem. Commun. 2013, 49, 7427–7429. [Google Scholar]
- Xie, X.; Yong, L.; Liu, Z.Q.; Haruta, M.; Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746. [Google Scholar]
- Wang, Z.; Hou, X.; Shen, J.; Li, T. Supported cobalt oxide nanocrystals: Morphology control and catalytic performance for styrene oxidation. RSC Adv. 2016, 6, 89503–89509. [Google Scholar] [CrossRef]
- Zhang, F.; Liang, Y.; Wang, J. Impacts of Nano-Sized Co3O4 on Ignition and Oxidation Performance of N-Decane and N-Decane/1,2,4-Trimethylbenzene Mixtures. Combust. Sci. Technol. 2022. [Google Scholar]
- Wang, S.B.; Zhao, C.C.; Li, S.G.; Sun, Y.H. First principles prediction of CH4 reactivities with Co3O4 nanocatalysts of different morphologies. Phys. Chem. Chem. Phys. 2017, 19, 30874–30882. [Google Scholar] [CrossRef]
- Teng, F.; Chen, M.D.; Li, G.Q.; Teng, Y.; Xu, T.G.; Hang, Y.C.; Yao, W.Q.; Santhanagopalan, S.; Meng, D.D.; Zhu, Y.F. High combustion activity of CH4 and catalluminescence properties of CO oxidation over porous Co3O4 nanorods. Appl. Catal. B Environ. 2011, 110, 133–140. [Google Scholar] [CrossRef]
- Tang, X.F.; Hao, J.M.; Li, J.H. Complete oxidation of methane on Co3O4-SnO2 catalysts. Front. Environ. Sci. Eng. China 2009, 3, 265–270. [Google Scholar] [CrossRef]
- Trivedi, S.; Prasad, R. Reactive calcination route for synthesis of active Mn-Co3O4 spinel catalysts for abatement of CO-CH4 emissions from CNG vehicles. J. Environ. Chem. Eng. 2016, 4, 1017–1028. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Bulk Co3O4 for Methane Oxidation: Effect of the Synthesis Route on Physico-Chemical Properties and Catalytic Performance. Catalysts 2022, 12, 87. [Google Scholar]
- Zhao, S.; Hu, F.; Li, J. Hierarchical Core-Shell Al2O3@Pd-CoAlO Microspheres for Low-Temperature Toluene Combustion. ACS Catal. 2016, 6, 3433–3441. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Deng, J.; Xie, S.; Yang, H.; Tan, W.; Han, W.; Jiang, Y.; Guo, G.-S. Mesoporous Co3O4-supported gold nanocatalysts: Highly active for the oxidation of carbon monoxide, benzene, toluene, and o-xylene. J. Catal. 2014, 309, 408–418. [Google Scholar]
- Mo, S.; Zhang, Q.; Li, J.; Sun, Y.; Ren, Q.; Zou, S.; Zhang, Q.; Lu, J.; Fu, M.; Mo, D.; et al. Highly efficient mesoporous MnO2 catalysts for the total toluene oxidation: Oxygen-Vacancy defect engineering and involved intermediates using in situ DRIFTS. Appl. Catal. B Environ. 2020, 264, 118464. [Google Scholar] [CrossRef]
- Zengzan, Z.; Lu, G.; Zhang, Z.; Guo, Y.; Guo, Y.; Wang, Y. Highly Active and Stable Co3O4/ZSM-5 Catalyst for Propane Oxidation: Effect of the Preparation Method. ACS Catal. 2013, 3, 1154–1164. [Google Scholar]
- Ahmad, W.; Noor, T.; Zeeshan, M. Effect of synthesis route on catalytic properties and performance of Co3O4/TiO2 for carbon monoxide and hydrocarbon oxidation under real engine operating conditions. Catal. Commun. 2017, 89, 19–24. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Sun, Y.; Wu, X. High-efficiency NiCo layered double hydroxide electrocatalyst. New J. Chem. 2022, 46, 18535–18542. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Longo, A.; Pantaleo, G.; Venezia, A.M. Support effect on the catalytic performance of Au/Co3O4-CeO2 catalysts for CO and CH4 oxidation. Catal. Today 2008, 139, 174–179. [Google Scholar] [CrossRef]
- Zavyalova, U.; Scholz, P.; Ondruschka, B. Influence of cobalt precursor and fuels on the performance of combustion synthesized Co3O4/gamma-Al2O3 catalysts for total oxidation of methane. Appl. Catal. A Gen. 2007, 323, 226–233. [Google Scholar] [CrossRef]
- Wang, X.; Chang, L.; Zhao, H.; Yu, Z.; Xia, Y.; Huang, C.; Yang, S.; Pan, G.; Xia, S.; Liu, Y.; et al. Theoretical Study on the Swelling Mechanism and Structural Stability of Ni3Al-LDH Based on Molecular Dynamics. ACS Omega 2023, 8, 3286–3297. [Google Scholar] [CrossRef]
- Wang, X.; Wu, F.; Fan, J.; Tian, A.; Cheng, Y.; Yang, S. High specific surface area NiTiAl layered double hydroxide derived via alkali etching for high performance supercapacitor electrode. J. Alloys Compd. 2021, 888, 161502. [Google Scholar] [CrossRef]
- Deraz, N.A.M. Surface and catalytic properties of Co3O4-doped CuO-Al2O3 catalysts. Colloids Surf. A Physicochem. Eng. Asp. 2002, 207, 197–206. [Google Scholar] [CrossRef]
- Li, D.; Xu, R.; Tian, M.; Jia, Y.; Gu, Z.; Zhu, X.; Li, K. Encapsulated Co3O4/(SiAl@Al2O3) thermal storage functional catalysts for catalytic combustion of lean methane. Appl. Therm. Eng. 2020, 181, 116012. [Google Scholar] [CrossRef]
- Wang, H.Y.; Ruckenstein, E. Conversions of methane to synthesis gas over Co/gamma-Al2O3 by CO2 and/or O-2. Catal. Lett. 2001, 75, 13–18. [Google Scholar] [CrossRef]
- Pu, Z.; Liu, Y.; Zhou, H.; Huang, W.; Zheng, Y.; Li, X. Catalytic combustion of lean methane at low temperature over ZrO2-modified Co3O4 catalysts. Appl. Surf. Sci. 2017, 422, 85–93. [Google Scholar]
- Rubio-Marcos, F.; Calvino-Casilda, V.; Baares, M.A.; Fernandez, J.F. Control of the Interphases Formation Degree in Co3O4/ZnO Catalysts. ChemCatChem 2013, 5, 1431–1440. [Google Scholar]
- Hu, S.; Cheng, T.; Zhang, Y.; Fang, Z.; Han, K.; Gao, M. Low-temperature CO oxidation over CUO/Co3O4 catalysts. Asian J. Chem. 2008, 20, 4719–4730. [Google Scholar]
- Comanescu, C. Synthesis and characterization of novel mesocomposites Co3O4 and CuO@OMS (ordered mesoporous silica) as active catalysts for hydrocarbon oxidation. J. Nanoparticle Res. 2014, 16, 2323. [Google Scholar] [CrossRef]
- Gao, H.; Lv, X.; Zhang, M.; Li, Q.; Chen, J.; Hu, Z.; Jia, H. Copper-cobalt strong interaction to improve photothermocatalytic performance of cobalt-copper oxides supported on copper foam for toluene oxidation. Chem. Eng. J. 2022, 434, 134618. [Google Scholar] [CrossRef]
- Moncada, C.; Ercolino, G.; Poozhikunnath, A.; Maric, R.; Specchia, S. Analysis of heat and mass transfer limitations for the combustion of methane emissions on PdO/Co3O4 coated on ceramic open cell foams. Chem. Eng. J. 2020, 405, 126970. [Google Scholar]
- Wang, X.; Cheng, Y.; Qiao, X.; Zhang, D.; Xia, Y.; Fan, J.; Huang, C.; Yang, S. High-loading and high-performance NiMn layered double hydroxide nanosheets supported on nickel foam for supercapacitor via sodium dodecyl sulfonate intercalation. J. Energy Storage 2022, 52, 104834. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Chang, L.; Yu, Z.; Xiao, Z.; Tang, S.; Huang, C.H.; Fan, J.-l.; Yang, S. First-Principles Study on Interlayer Spacing and Structure Stability of NiAl-Layered Double Hydroxides. ACS Omega 2022, 7, 39169–39180. [Google Scholar] [PubMed]
- Wu, Z.; Zhao, Y.; Mi, L.; Guo, Y.; Wang, H.; Liu, K.; Zhang, K.; Wang, B. Preparation of g-C3N4/TiO2 by template method and its photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126756. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, C.; Li, J.; Zhong, F.; Xiao, Y.; Jiang, L. Enhanced Methane Oxidation over Co3O4-In2O3-x Composite Oxide Nanoparticles via Controllable Substitution of Co3+/Co2+ by In3+ Ions. Acs Appl. Nano Mater. 2020, 3, 9470–9479. [Google Scholar] [CrossRef]
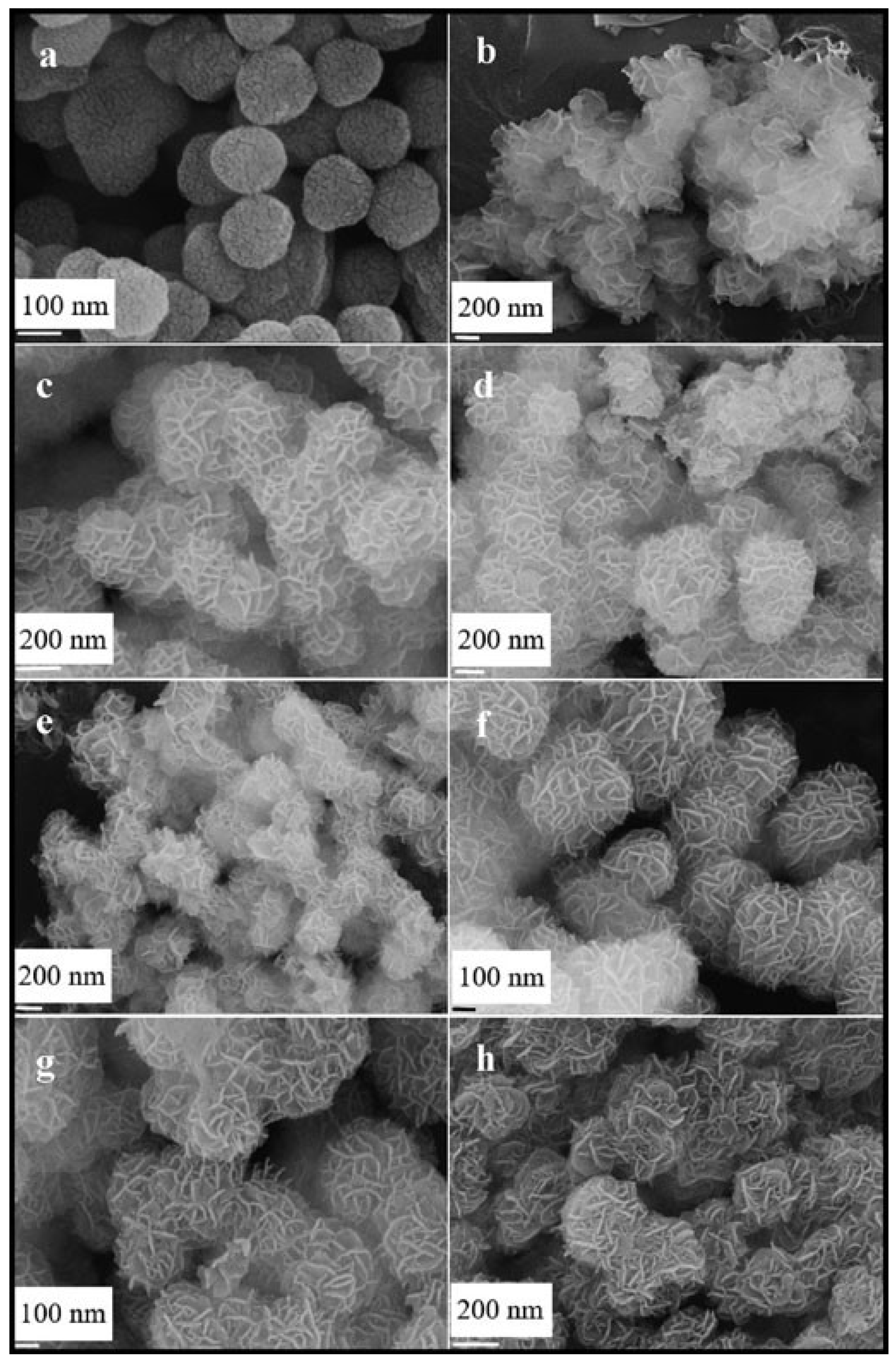
- Chen, Z.; Wang, S.; Liu, W.; Gao, X.; Gao, D.; Wang, M.; Wang, S. Morphology-dependent performance of Co3O4 via facile and controllable synthesis for methane combustion. Appl. Catal. A Gen. 2016, 525, 94–102. [Google Scholar] [CrossRef]
- Garcia, T.; Agouram, S.; Sanchez-Royo, J.F.; Murillo, R.; Maria Mastral, A.; Aranda, A.; Vazquez, I.; Dejoz, A.; Solsona, B. Deep oxidation of volatile organic compounds using ordered cobalt oxides prepared by a nanocasting route. Appl. Catal. A Gen. 2010, 386, 16–27. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Gui, P.; Zheng, L.; Liang, J.; Xue, G. Hydrothermal synthesis of sandwich interspersed LaCO3OH/Co3O4/graphene oxide composite and the enhanced catalytic performance for methane combustion. Catal. Today 2019, 327, 134–142. [Google Scholar] [CrossRef]
- Li, P.; Zhang, R.; Wang, X.; Liu, S.; Liu, N.; Chen, B. New evidence on the correlation between lattice fringe with catalytic performance for suprafacial CO and intrafacial CH4 oxidations over Co3O4 by isotopic O-18(2) exchange. Mol. Catal. 2017, 437, 26–36. [Google Scholar] [CrossRef]
- Wen, W.; Che, J.-W.; Wu, J.-M.; Kobayashi, H.; Pan, Y.; Wen, W.; Dai, Y.-H.; Huang, W.; Fu, C.; Zhou, Q.; et al. Co3+-O Bond Elongation Unlocks Co3O4 for Methane Activation under Ambient Conditions. ACS Catal. 2022, 12, 7037–7045. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Zhang, D.; Qiao, X.; Zhao, H.; Chang, L.; Yu, Z.; Xia, Y.; Fan, J.; Huang, C.; et al. High-capacity binderless supercapacitor electrode obtained from sulfidation large interlayer spacing of NiMn-LDH. Electrochim. Acta 2022, 429, 141039. [Google Scholar] [CrossRef]
- Wang, H.; Chao, L.; Wei, X.; Li, J.; Ji, C.; Wang, B.; Qi, X.; Hu, P.; Ying, Y.; Tian, M. Design of SiO2-TiO2-PAM composite flocculant with self-degrading characteristics and optimization of the flocculation process using a combination of central composite design and response surface methodology. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123982. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, K.; Yang, J.; Liu, P.; Song, L.; Zhang, J.; Fu, M.; Chen, L.; Huang, H.; Wu, J.; et al. The effect of existence states of PdOx supported by Co3O4 nanoplatelets on catalytic oxidation of methane. Appl. Surf. Sci. 2021, 539, 148211. [Google Scholar] [CrossRef]
- Yin, J.; Kim, E.-S.; Yang, J.; Deng, B. Fabrication of a novel thin-film nanocomposite (TFN) membrane containing MCM-41 silica nanoparticles (NPs) for water purification. J. Membr. Sci. 2012, 423, 238–246. [Google Scholar] [CrossRef]
- Fu, W.; Zhao, Y.; Wang, H.; Chen, X.; Liu, K.; Zhang, K.; Wei, Q.; Wang, B. Study on preparation, photocatalytic performance and degradation mechanism of polymeric carbon nitride/Pt/nano-spherical MoS2 composite. J. Phys. Chem. Solids 2022, 166, 110700. [Google Scholar] [CrossRef]
- Li, Z.H.; Hoflund, G.B. Catalytic oxidation of methane over Pd/Co3O4. React. Kinet. Catal. Lett. 1999, 66, 367–374. [Google Scholar] [CrossRef]
- Li, Z.H.; Xu, G.H.; Hoflund, G.B. In situ IR studies on the mechanism of methane oxidation over Pd/Al2O3 and Pd/Co3O4 catalysts. Fuel Process. Technol. 2003, 84, 1–11. [Google Scholar] [CrossRef]
- Castellazzi, P.; Groppi, G.; Forzatti, P.; Baylet, A.; Marécot, P.; Duprez, D. Role of Pd loading and dispersion on redox behaviour and CH4 combustion activity of Al2O3 supported catalysts. Catal. Today 2010, 155, 18–26. [Google Scholar]
- Ying, L.; Sheng, W.; Sun, T.; Gao, D.; Zhang, C.; Wang, S. Enhanced hydrothermal stability of high performance lean fuel combustion alumina-supported palladium catalyst modified by nickel. Appl. Catal. B Environ. 2012, 119–120, 321–328. [Google Scholar]
- Chen, L.; Zhu, Y. Tailoring Catalytic Properties of Pd/Co3O4 Catalysts via Structure Engineering for Methane Oxidation. J. Nanosci. Nanotechnol. 2018, 18, 2673–2679. [Google Scholar] [CrossRef]
- Ercolino, G.; Grodzka, A.; Grzybek, G.; Stelmachowski, P.; Specchia, S.; Kotarba, A. The Effect of the Preparation Method of Pd-Doped Cobalt Spinel on the Catalytic Activity in Methane Oxidation Under Lean Fuel Conditions. Top. Catal. 2017, 60, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Ercolino, G.; Grzybek, G.; Stelmachowski, P.; Specchia, S.; Kotarba, A.; Specchia, V. Pd/Co3O4-based catalysts prepared by solution combustion synthesis for residual methane oxidation in lean conditions. Catal. Today 2015, 257, 66–71. [Google Scholar] [CrossRef]
- Ercolino, G.; Karimi, S.; Stelmachowski, P.; Specchia, S. Catalytic combustion of residual methane on alumina monoliths and open cell foams coated with Pd/Co3O4. Chem. Eng. J. 2017, 326, 339–349. [Google Scholar] [CrossRef]
- Ercolino, G.; Stelmachowski, P.; Specchia, S. Catalytic Performance of Pd/Co3O4 on SiC and ZrO2 Open Cell Foams for Process Intensification of Methane Combustion in Lean Conditions. Ind. Eng. Chem. Res. 2017, 56, 6625–6636. [Google Scholar] [CrossRef]
- Hu, L.; Peng, Q.; Li, Y. Low-temperature CH4 Catalytic Combustion over Pd Catalyst Supported on Co3O4 Nanocrystals with Well-Defined Crystal Planes. Chemcatchem 2011, 3, 868–874. [Google Scholar] [CrossRef]
- Li, W.; Liu, D.; Feng, X.; Zhang, Z.; Jin, X.; Zhang, Y. High-Performance Ultrathin Co3O4 Nanosheet Supported PdO/CeO2 Catalysts for Methane Combustion. Adv. Energy Mater. 2019, 9, 1803583. [Google Scholar] [CrossRef]
- Liu, J.-W.; Yang, N.-T.; Zhu, Y. Pd/Co3O4 Nanoparticles Inlaid in Alkaline Al2O3 Nanosheets as an Efficient Catalyst for Catalytic Oxidation of Methane. Acta Phys.-Chim. Sin. 2017, 33, 1453–1461. [Google Scholar] [CrossRef]
- Zagoraios, D.; Athanasiadi, A.; Kalaitzidou, I.; Ntais, S.; Katsaounis, A.; Caravaca, A.; Vernoux, P.; Vayenas, C.G. Electrochemical promotion of methane oxidation over nanodispersed Pd/Co3O4 catalysts. Catal. Today 2020, 355, 910–920. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, S.; Ding, Y.; Zhang, L.; Lv, L.; Wang, M.; Wang, S. Pd catalysts supported on Co3O4 with the specified morphologies in CO and CH4 oxidation. Appl. Catal. A Gen. 2017, 532, 95–104. [Google Scholar]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M.; Deganello, G.; Borla, E.M.; Pidria, M.F. Pd/Co3O4 catalyst for CH4 emissions abatement: Study of SO2 poisoning effect. Top. Catal. 2007, 42–43, 425–428. [Google Scholar] [CrossRef]
- Miao, S.J.; Deng, Y.Q. Au-Pt/Co3O4 catalyst for methane combustion. Appl. Catal. B Environ. 2001, 31, L1–L4. [Google Scholar] [CrossRef]
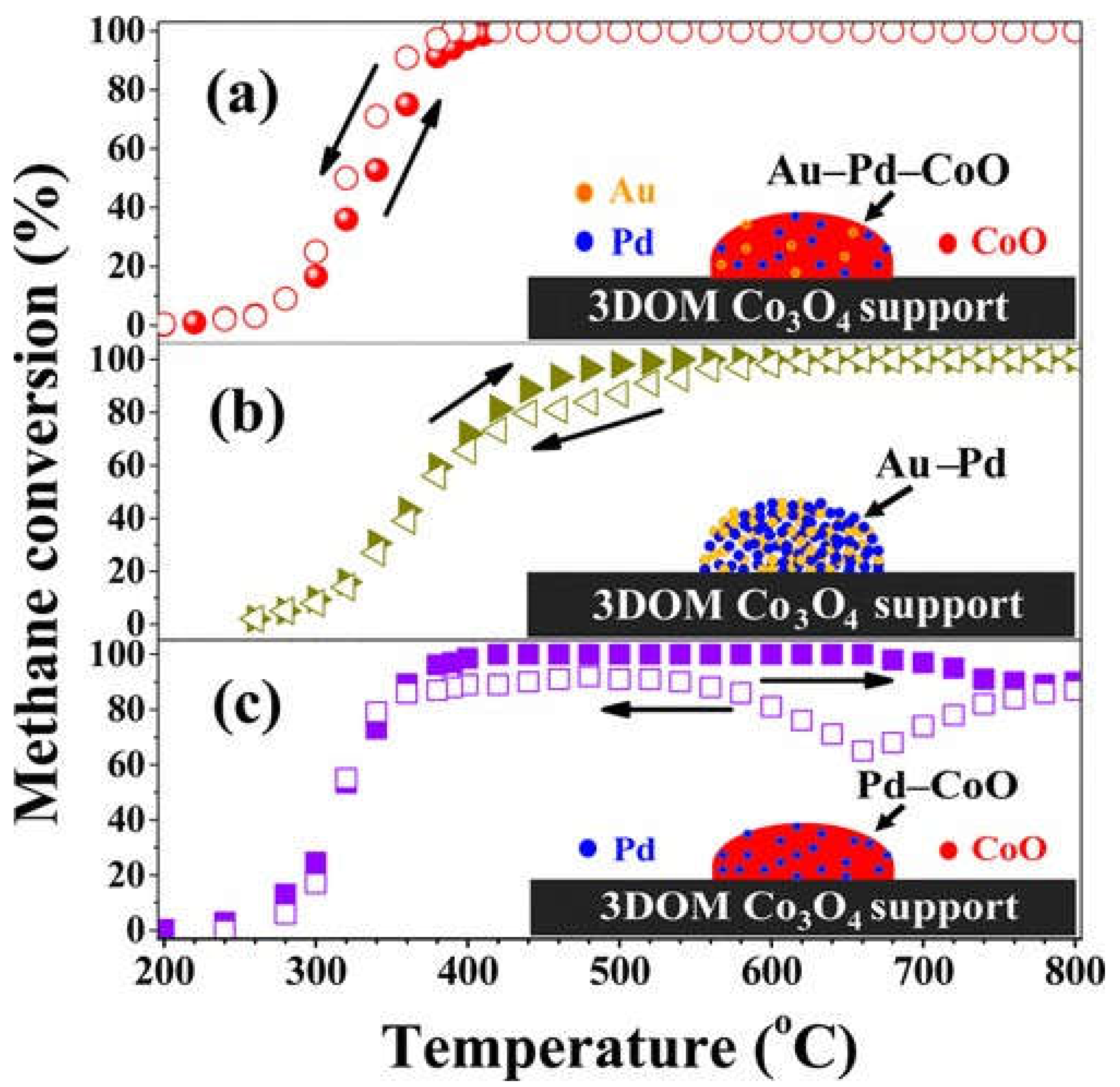
- Xie, S.; Liu, Y.; Deng, J.; Zang, S.; Zhang, Z.; Arandiyan, H.; Dai, H. Efficient Removal of Methane over Cobalt-Monoxide-Doped AuPd Nanocatalysts. Environ. Sci. Technol. 2017, 51, 2271–2279. [Google Scholar] [CrossRef]
- Hu, C.; Dai, P.; Chen, Z.; Zhang, H. Property and Reactivity Relationships of Co3O4 with Diverse Nanostructures for Soot Oxidation. ACS Omega 2022, 7, 44116–44123. [Google Scholar] [CrossRef]
- Deng, W.; Jia, Z.; Gao, B.; Zhu, S.; Liu, D.; Guo, L. Effect of preparation method on the performance of porous RuOx/Co3O4 catalysts for 1, 2-dichloroethane oxidation. Appl. Catal. A Gen. 2021, 624, 118300. [Google Scholar] [CrossRef]
- Jiang, B.; Huang, M.; Cai, D.; Tan, K.B.; Zhan, G. Fabrication of Pt/Co3O4 nanocatalysts based on pollen template for low-temperature CO oxidation. Catal. Commun. 2023, 174, 106597. [Google Scholar] [CrossRef]
- Xue, X.; Liao, W.; Liu, D.; Zhang, X.; Huang, Y. MgO/Co3O4 composite activated peroxymonosulfate for levofloxacin degradation: Role of surface hydroxyl and oxygen vacancies. Sep. Purif. Technol. 2023, 306, 122560. [Google Scholar] [CrossRef]
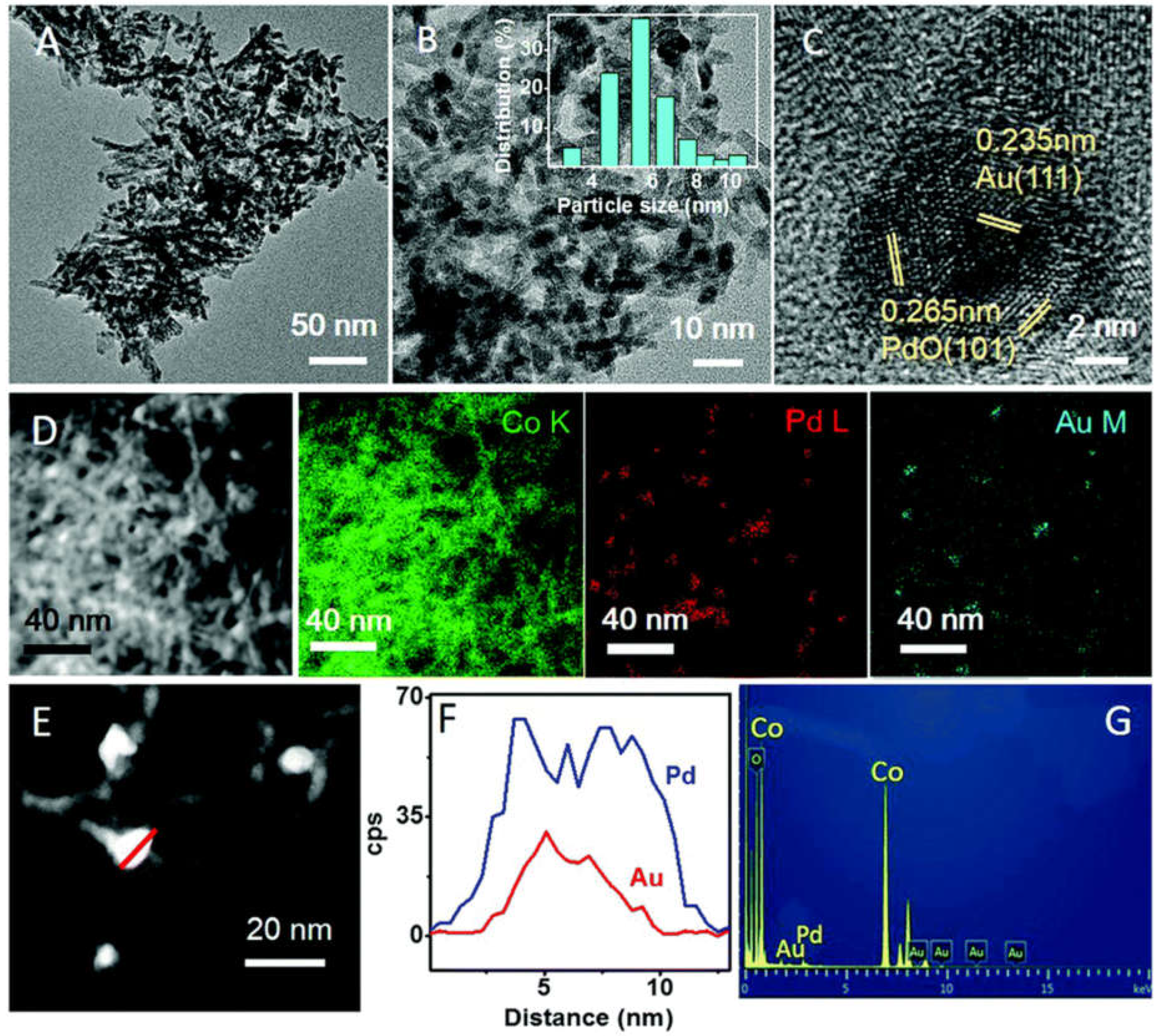
- Yang, N.; Liu, J.; Sun, Y.; Zhu, Y. Au@PdOx with a PdOx-rich shell and Au-rich core embedded in Co3O4 nanorods for catalytic combustion of methane. Nanoscale 2017, 9, 2123–2128. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, Y.; Fu, J.; Kyzas, G.Z.; Billah, S.; An, S. Synthesis, characterization, and catalytic evaluation of Co3O4/γ-Al2O3 as methane combustion catalysts: Significance of Co species and the redox cycle. Appl. Catal. B Environ. 2015, 168–169, 42–50. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; Ramon Gonzalez-Velasco, J.; Ignacio Gutierrez-Ortiz, J.; Lopez-Fonseca, R. Oxidation of residual methane from VNG vehicles over Co3O4-based catalysts: Comparison among bulk, Al2O3-supported and Ce-doped catalysts. Appl. Catal. B Environ. 2018, 237, 844–854. [Google Scholar] [CrossRef]
- Kirchnerova, J.; Alifanti, M.; Delmon, B. Evidence of phase cooperation in the LaCoO3-CeO2-Co3O4 catalytic system in relation to activity in methane combustion. Appl. Catal. A Gen. 2002, 231, 65–80. [Google Scholar] [CrossRef]
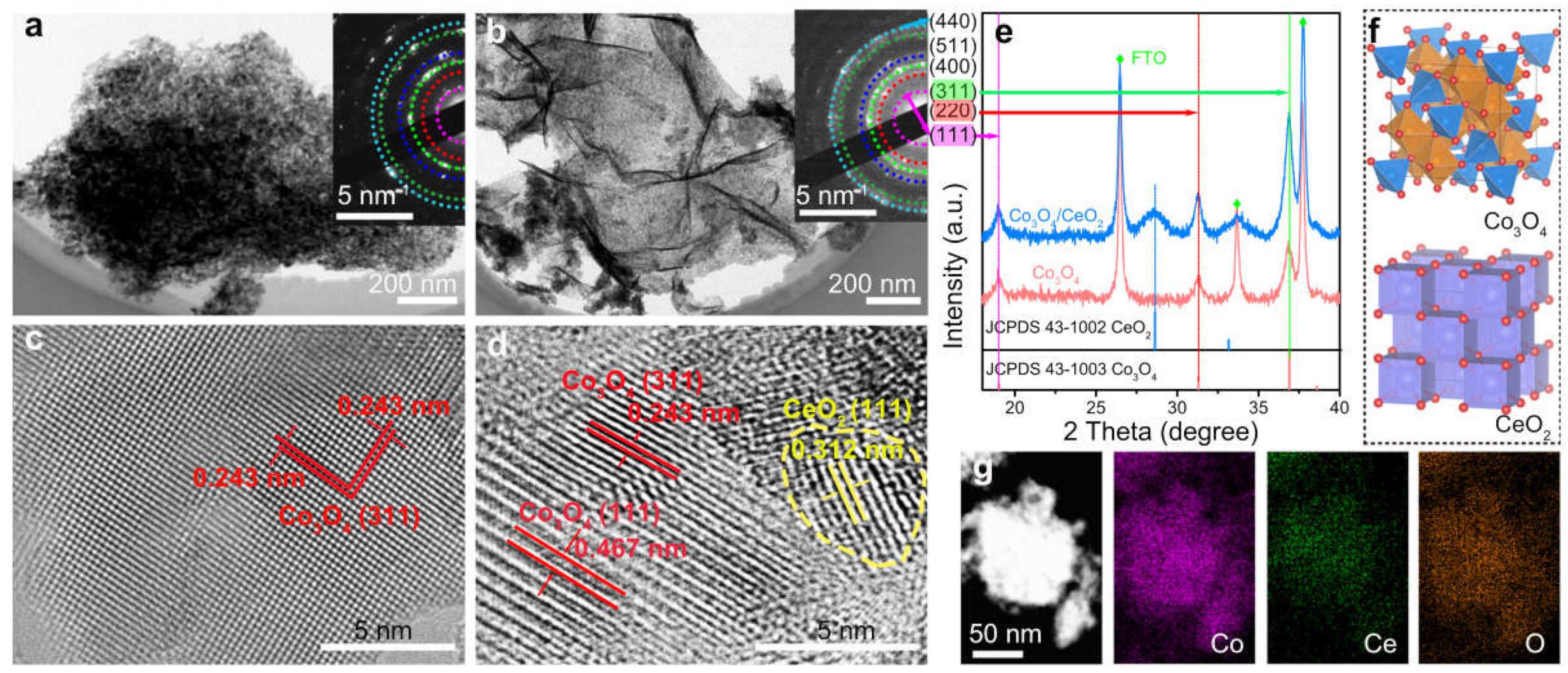
- Li, H.; Lu, G.; Qiao, D.; Wang, Y.; Guo, Y.; Guo, Y. Catalytic Methane Combustion over Co3O4/CeO2 Composite Oxides Prepared by Modified Citrate Sol-Gel Method. Catal. Lett. 2011, 141, 452–458. [Google Scholar] [CrossRef]
- Li, K.; Liu, K.; Xu, D.; Ni, H.; Shen, F.; Chen, T.; Guan, B.; Zhan, R.; Huang, Z.; Lin, H. Lean methane oxidation over Co3O4/Ce0.75Zr0.25 catalysts at low-temperature: Synergetic effect of catalysis and electric field. Chem. Eng. J. 2019, 369, 660–671. [Google Scholar] [CrossRef]
- Liotta, L.F.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Boreave, A.; Giroir-Fendler, A. Catalytic Removal of Toluene over Co3O4-CeO2 Mixed Oxide Catalysts: Comparison with Pt/Al2O3. Catal. Lett. 2009, 127, 270–276. [Google Scholar] [CrossRef]
- Cao, Y.; Peng, X.; Tan, Z.; Liu, Y.; Wang, X.; Zhao, W.; Jiang, L. Structural Evolution of Active Entities on Co3O4/Ceo(2) Catalyst during Water Gas Shift Reaction. Ind. Eng. Chem. Res. 2019, 58, 17692–17698. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M.; Deganello, G.; Borla, E.M.; Pidria, M. Supported Co3O4-CeO2 monoliths: Effect of preparation method and Pd-Pt promotion on the CO/CH4 oxidation activity. In Proceedings of the 9th International Symposium on the Scientific Bases for the Preparation of Heterogeneous Catalysts, Univ Cathol Louvain (UCL), Louvain-la-Neuve, Belgium, 10–14 September 2006; pp. 657–664. [Google Scholar]
- Wang, X.; Yan, H.; Zhang, J.; Hong, X.; Yang, S.; Wang, C.; Li, Z. Stamen-petal-like CeO2/NiMn layered double hydroxides composite for high-rate-performance supercapacitor. J. Alloys Compd. 2019, 810, 151911. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Deganello, G. Co3O4/CeO2 andCo(3)O(4)/CeO2-ZrO2 composite catalysts for methane combustion: Correlation between morphology reduction properties and catalytic activity. Catal. Commun. 2005, 6, 329–336. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M.; Deganello, G. Co3O4/CeO2 composite oxides for methane emissions abatement: Relationship between Co3O4-CeO2 interaction and catalytic activity. Appl. Catal. B Environ. 2006, 66, 217–227. [Google Scholar] [CrossRef]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M.; Deganello, G. Insights into SO2 Interaction with Pd/Co3O4-CeO2 Catalysts for Methane Oxidation. Top. Catal. 2009, 52, 1989–1994. [Google Scholar] [CrossRef]
- Lu, H.; Jiang, C.; Ding, Z.; Wang, W.; Chu, W.; Feng, Y. Effects of ultrasonic impregnation combined with calcination in N-2 atmosphere on the property of Co3O4/CeO2 composites for catalytic methane combustion. J. Energy Chem. 2016, 25, 387–392. [Google Scholar] [CrossRef]
- Zeng, S.; Fu, X.; Wang, X.; Su, H. Effect of Precursor Concentration on CeO2/Co3O4 Catalysts for CH4/CO2 Reforming. Catal. Lett. 2014, 144, 561–566. [Google Scholar] [CrossRef]
- Liotta, L.F.; Carlo, G.D.; Pantaleo, G.; Deganello, G.; Borla, E.M.; Pidria, M. Honeycomb supported Co3O4/CeO2 catalyst for CO/CH4 emissions abatement: Effect of low Pd–Pt content on the catalytic activity. Catal. Commun. 2007, 8, 299–304. [Google Scholar]
- Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Deganello, G. Catalytic performance of Co3O4/CeO2 and Co3O4/CeO2-ZrO2 composite oxides for methane combustion: Influence of catalyst pretreatment temperature and oxygen concentration in the reaction mixture. Appl. Catal. B Environ. 2007, 70, 314–322. [Google Scholar] [CrossRef]
- Dou, J.; Tang, Y.; Nie, L.; Andolina, C.M.; Zhang, X.; House, S.; Li, Y.; Yang, J.; Tao, F.F. Complete Oxidation of Methane on Co3O4/CeO2 Nanocomposite: A Synergic Effect. Catal. Today 2018, 311, 48–55. [Google Scholar]
- Wu, H.; Pantaleo, G.; Di Carlo, G.; Guo, S.; Marci, G.; Concepcion, P.; Venezia, A.M.; Liotta, L.F. Co3O4 particles grown over nanocrystalline CeO2: Influence of precipitation agents and calcination temperature on the catalytic activity for methane oxidation. Catal. Sci. Technol. 2015, 5, 1888–1901. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Fu, X.; Zhou, T.; Wang, X.; Su, H. Influence of pore distribution on catalytic performance over inverse CeO2/Co3O4 catalysts for CH4/CO2 reforming. Fuel Process. Technol. 2013, 114, 69–74. [Google Scholar]
- Ercolino, G.; Stelmachowski, P.; Kotarba, A.; Specchia, S. Reactivity of Mixed Iron–Cobalt Spinels in the Lean Methane Combustion. Top. Catal. 2017, 60, 1370–1379. [Google Scholar]
- Darda, S.; Pachatouridou, E.; Lappas, A.; Iliopoulou, E. Effect of Preparation Method of Co-Ce Catalysts on CH4 Combustion. Catalysts 2019, 9, 219. [Google Scholar] [CrossRef] [Green Version]
- Choya, A.; de Rivas, B.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. On the Effect of the Synthesis Route of the Support in Co3O4/CeO2 Catalysts for the Complete Oxidation of Methane. Ind. Eng. Chem. Res. 2022, 61, 17854–17865. [Google Scholar] [CrossRef]
- Huang, J.; Sheng, H.; Ross, R.D.; Han, J.; Wang, X.; Song, B.; Jin, S. Modifying redox properties and local bonding of Co3O4 by CeO2 enhances oxygen evolution catalysis in acid. Nat. Commun. 2021, 12, 3036. [Google Scholar] [CrossRef]
- Oh, C.; Kim, J.; Hwang, Y.J.; Ma, M.; Park, J.H. Electrocatalytic methane oxidation on Co3O4- incorporated ZrO2 nanotube powder. Appl. Catal. B Environ. 2021, 283, 119653. [Google Scholar] [CrossRef]
- Feng, Z.; Du, C.; Chen, Y.; Lang, Y.; Zhao, Y.; Cho, K.; Chen, R.; Shan, B. Improved durability of Co3O4 particles supported on SmMn2O5 for methane combustion. Catal. Sci. Technol. 2018, 8, 3785–3794. [Google Scholar] [CrossRef]
- Chen, J.; Huang, M.; Chen, W.; Tang, H.; Jiao, Y.; Zhang, J.; Wang, G.; Wang, R. Defect Engineering and Synergistic Effect in Co3O4 Catalysts for Efficient Removal of Formaldehyde at Room Temperature. Ind. Eng. Chem. Res. 2020, 59, 18781–18789. [Google Scholar] [CrossRef]
- Laghari, A.J.; Aftab, U.; Tahira, A.; Shah, A.A.; Gradone, A.; Solangi, M.Y.; Samo, A.H.; Kumar, M.; Abro, M.I.; Akhtar, M.w.; et al. MgO as promoter for electrocatalytic activities of Co3O4–MgO composite via abundant oxygen vacancies and Co2+ ions towards oxygen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 12672–12682. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, Y.; Wang, L.; Cao, Z.; Sun, J. Catalytic oxidation of toluene over Co-Cu bimetallic oxides derived from CoyCu3−y-MOF-74. J. Alloys Compd. 2022, 928, 167105. [Google Scholar] [CrossRef]
- Lei, J.; Wang, P.; Wang, S.; Li, J.; Xu, Y.; Li, S. Enhancement effect of Mn doping on Co3O4 derived from Co-MOF for toluene catalytic oxidation. Chin. J. Chem. Eng. 2022, 52, 1–9. [Google Scholar] [CrossRef]
- Assaouka, H.T.; Nsangou, I.N.; Daawe, D.M.; Mevoa, D.O.; Zigla, A.A.; Ndouka, P.N.; Kouotou, P.M. Copper and iron co-doping effects on the structure, optical energy band gap, and catalytic behaviour of Co3O4 nanocrystals towards low-temperature total oxidation of toluene. Energy Adv. 2023, 2, 829–842. [Google Scholar] [CrossRef]
- Zhao, S.; Jin, X.; Wu, P.; Zhao, Y.; Chen, G.; Li, Y.; Li, A.; Ye, D.; Qiu, Y. Cu2+-Decorated Porous Co3O4 Nanosheets for Photothermocatalytic Oxidation of Toluene. ACS Appl. Nano Mater. 2020, 3, 10454–10461. [Google Scholar] [CrossRef]
- Song, L.; Liu, Y.; Zhang, S.; Zhou, C.; Ma, K.; Yue, H. Tuning Oxygen Vacancies of the Co3O4 Catalyst through an Ethanol-Assisted Hydrothermal Method for Low-Temperature CO Oxidation. Ind. Eng. Chem. Res. 2022, 61, 14783–14792. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Han, N.; Orooji, Y. Hydrogen production through methane reforming processes using promoted-Ni/mesoporous silica: A review. J. Ind. Eng. Chem. 2022, 107, 20–30. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Guo, Q. Development of hybrid amine-functionalized MCM-41 sorbents for CO2 capture. Chem. Eng. J. 2015, 260, 573–581. [Google Scholar] [CrossRef]
- Xie, W.; Han, Y.; Wang, H. Magnetic Fe3O4/MCM-41 composite-supported sodium silicate as heterogeneous catalysts for biodiesel production. Renew. Energy 2018, 125, 675–681. [Google Scholar] [CrossRef]
- Xie, W.; Zang, X. Immobilized lipase on core-shell structured Fe3O4-MCM-41 nanocomposites as a magnetically recyclable biocatalyst for interesterification of soybean oil and lard. Food Chem. 2016, 194, 1283–1292. [Google Scholar] [CrossRef]
- Zheng, J.; Chu, W.; Zhang, H.; Jiang, C.; Dai, X. CO oxidation over Co3O4/SiO2 catalysts: Effects of porous structure of silica and catalyst calcination temperature. J. Nat. Gas Chem. 2010, 19, 583–588. [Google Scholar] [CrossRef]
- McCullen, S.; Vartuli, J.C.; Kresge, C.T.; Roth, W.J.; Beck, J.S.; Schmitt, K.D.; Leonowicz, M.E.; Schlenker, J.L.; Shih, S.S.; Lutner, J.D. A New Family of Mesoporous Molecular Sieves. In Access in Nanoporous Materials; Springer: Berlin/Heidelberg, Germany, 2002; pp. 1–11. [Google Scholar]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Mukti, R.R.; Taufiq-Yap, Y.H.; Sazegar, M.R. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl. Catal. B Environ. 2014, 147, 359–368. [Google Scholar] [CrossRef]
- de Clippel, F.; Dusselier, M.; Van Rompaey, R.; Vanelderen, P.; Dijkmans, J.; Makshina, E.; Giebeler, L.; Oswald, S.; Baron, G.V.; Denayer, J.F.M.; et al. Fast and Selective Sugar Conversion to Alkyl Lactate and Lactic Acid with Bifunctional Carbon-Silica Catalysts. J. Am. Chem. Soc. 2012, 134, 10089–10101. [Google Scholar] [CrossRef]
- Hoang, V.-T.; Kaliaguine, S. Predictive Models for Mixed-Matrix Membrane Performance: A Review. Chem. Rev. 2013, 113, 4980–5028. [Google Scholar] [CrossRef]
- Huo, C.; Ouyang, J.; Yang, H. CuO nanoparticles encapsulated inside Al-MCM-41 mesoporous materials via direct synthetic route. Sci. Rep. 2014, 4, 3682. [Google Scholar] [CrossRef] [Green Version]
- Petala, E.; Dimos, K.; Douvalis, A.; Bakas, T.; Tucek, J.; Zboril, R.; Karakassides, M.A. Nanoscale zero-valent iron supported on mesoporous silica: Characterization and reactivity for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2013, 261, 295–306. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Verboekend, D.; Bonilla, A.; Abelló, S. Zeolite Catalysts with Tunable Hierarchy Factor by Pore-Growth Moderators. Adv. Funct. Mater. 2010, 19, 3972–3979. [Google Scholar]
- Anilkumar, M.; Hoelderich, W.F. A one step synthesis ofcaprolactam out of cyclohexanone by combinded ammoximation and Beckmann rearrangement over Nb-MCM-41 catalysts. Appl. Catal. B Environ. 2015, 165, 87–93. [Google Scholar]
- Parvulescu, V.; Anastasescu, C.; Constantin, C.; Su, B.L. Mono (V, Nb) or bimetallic (V–Ti, Nb–Ti) ions modified MCM-41 catalysts: Synthesis, characterization and catalysis in oxidation of hydrocarbons (aromatics and alcohols). Catal. Today 2003, 78, 477–485. [Google Scholar]
- Prvulescu, V.; Anastasescu, C.; Su, B.L. Vanadium incorporated mesoporous silicates as catalysts for oxidation of alcohols and aromatics. J. Mol. Catal. A Chem. 2003, 198, 249–261. [Google Scholar]
- Lim, S.; Ciuparu, D.; Pak, C.; Dobek, F.; Chen, Y.; Harding, D.; Pfefferle, L.; Haller, G. Synthesis and characterization of highly ordered Co-MCM-41 for production of aligned single walled carbon nanotubes (SWNT). J. Phys. Chem. B 2003, 107, 11048–11056. [Google Scholar] [CrossRef]
- Somanathan, T.; Pandurangan, A.; Sathiyamoorthy, D. Catalytic influence of mesoporous Co-MCM-41 molecular sieves for the synthesis of SWNTs via CVD method. J. Mol. Catal. A Chem. 2006, 256, 193–199. [Google Scholar]
- Panpranot, J.; Goodwin Jr, J.G.; Sayari, A. Synthesis and characteristics of MCM-41 supported CoRu catalysts. Catal. Today 2002, 77, 269–284. [Google Scholar] [CrossRef]
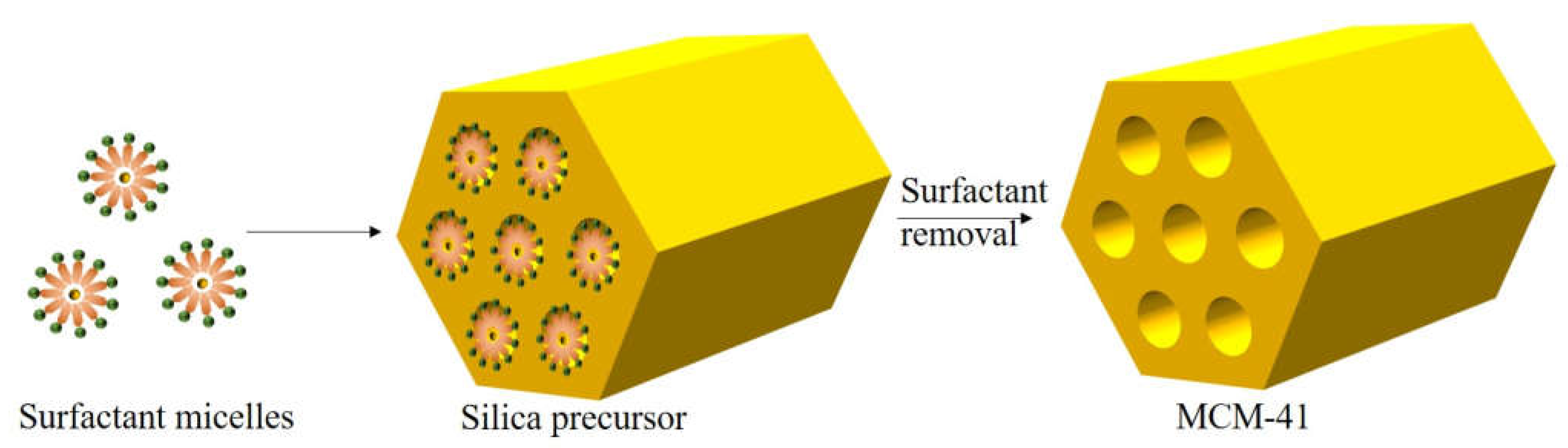
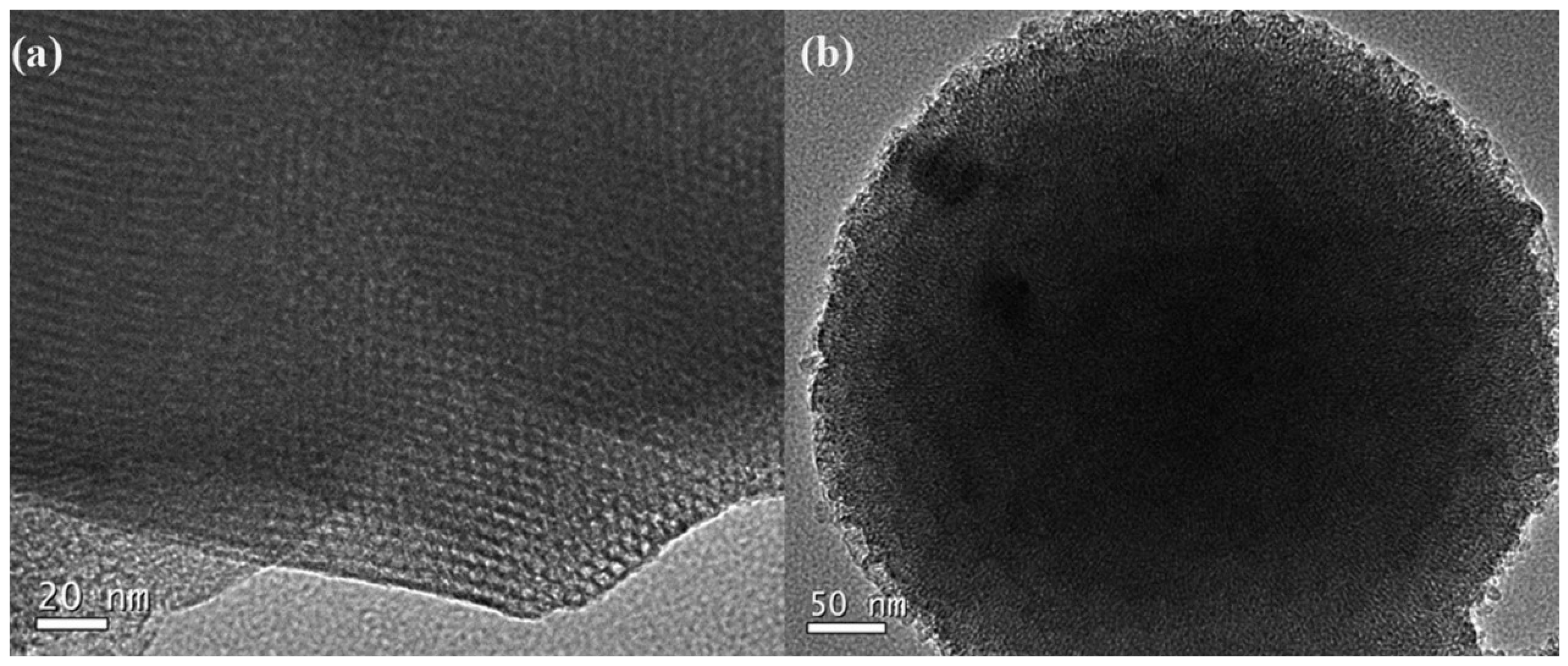
- Xu, X.; Wu, J.; Xu, W.; He, M.; Fu, M.; Chen, L.; Zhu, A.; Ye, D. High-efficiency non-thermal plasma-catalysis of cobalt incorporated mesoporous MCM-41 for toluene removal. Catal. Today 2016, 281, 527–533. [Google Scholar] [CrossRef]
- Todorova, S.; Parvulescu, V.; Kadinov, G.; Tenchev, K.; Somacescu, S.; Su, B.L. Metal states in cobalt- and cobalt–vanadium-modified MCM-41 mesoporous silica catalysts and their activity in selective hydrocarbons oxidation. Microporous Mesoporous Mater. 2008, 113, 22–30. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Liu, W.; Xu, X.; Wei, X.; Chao, L.; Zhao, R.; Qi, X.; Che, L. Enhancing catalytic CH4 oxidation over Co3O4/SiO2 core-shell catalyst by substituting Co2+ with Mn2+. J. Dispers. Sci. Technol. 2021, 42, 82–92. [Google Scholar] [CrossRef]



| Catalyst | Temperature (°C) | BET (m2 g−1) | Reference |
|---|---|---|---|
| Nanoplate Co3O4 | 325 | 45.5 | [47] |
| Nanoparticle Co3O4 | 150 | 15 | [45] |
| 325 | 112.6 | [47] | |
| 550 | 46 | [45] | |
| 650 | 42 | [45] | |
| Nanorob Co3O4 | 325 | 111.4 | [47] |
| 90 | 170.2 | [48] | |
| Nanotube Co3O4 | 350 | 36–48 | [48] |
| Bulk Co3O4 | 700 | 20.9 | [48] |
| Mn promoted Co3O4 spinel (Cat-R) | 340 | 127.94 | [50] |
| Mn promoted Co3O4 spinel (Cat-S) | 420 | 57.43 | [50] |
| Mn promoted Co3O4 spinel (Cat-F) | 380 | 94.5 | [50] |
| Measures | Results | Specific Mechanism |
|---|---|---|
| pure Co3O4 | The catalytic performance of pure Co3O4 is poor due to the lack of support and synergy of these vectors. | |
| doping with noble metal elements | The specific surface area of Co3O4 is increased, and the physical and chemical properties of the surface are changed, to improve the catalytic performance. | By providing additional protons and electrons in the catalytic reaction, the reducing power and catalytic activity of Co3O4 are increased, while also facilitating the contact between the cobalt tetroxide and the reactants, thus improving the efficiency of the catalyst. |
| compounding with metal oxides | By helping Co3O4 to form more active sites on the surface, the rate of catalytic reaction is increased. | |
| compounding with non-metal oxides | By helping Co3O4 to disperse evenly on the surface of the carrier, the contact area of the catalytic reaction is increased and the catalytic efficiency is improved. |
| Catalyst | SBET (m2 g−1) a | Co3O4 Particle Size (nm) b | T10 (°C) d | T50 (°C) d | T90 (°C) d | Ea (kJ mol−1) e |
|---|---|---|---|---|---|---|
| Co3O4 | 25.6 | 13.4 | 298 | 357 | 402 | 138.0 |
| Pd-Co3O4 | 28.7/26.9 c | 13.2 | 247 | 291 | 337 | 66.9 |
| Pd/Co3O4 | 21.6 | 13.8 | 265 | 315 | 361 | 89.7 |
| Pd@Co3O4 | 23.6 | 14.5 | 280 | 326 | 372 | 90.5 |
| Sample | Co 2p3/2 (eV) | S 2p (eV) | Co (at%) | Pd (at%) | S (at%) |
|---|---|---|---|---|---|
| Pd/Co3O4 | 779.6 | 38.8 | 4.0 | ||
| 781.4 | |||||
| Pd/Co3O4 after 4 runs without SO2 | 779.5 | 36.3 | 0.4 | ||
| 780.8 | |||||
| Pd/Co3O4 after 4 runs with SO2 (1, 10 ppm) | 779.9 | 169.5 | 34.8 | 0.2 | 1.9 |
| 781.3 | |||||
| Pd/Co3O4 after 15 h at 350 °C with 10 ppm SO2 and 1 run without SO2 | 780.0 | 169.5 | 33.5 | 0.4 | 2.4 |
| 781.4 | |||||
| Co3O4 | 779.5 | 38 | |||
| 780.0 | |||||
| Co3O4 after 15 h at 350 °C with 10 ppm SO2 and 1 run without SO2 | 779.9 | 169.6 | 36.6 | 5.0 | |
| 781.1 | |||||
| Co3O4 after 15 h at 350 °C with 10 ppm SO2 and 3 runs without SO2 | 779.7 | 169.6 | 37.5 | 1.1 | |
| 781.1 |
| Catalysts | Pd Content (wt.%) | Au Content (wt.%) | CO CAA (μmol g−1) | Pdx+/Pd0 Molar Ratio | Oads/Olatt Molar Ratio | r (μmol g−1 s−1) | Ea (kJ mol−1) | T10 (°C) | T90 (°C) |
|---|---|---|---|---|---|---|---|---|---|
| Au@PdOx (1:5)/Co3O4 | 2.44 | 0.49 | 92.8 | 29.3 | 0.51 | 194 | 50.9 | 210 | 344 |
| AuPd (1:5)/Co3O4 | 2.44 | 0.49 | 65.1 | 21.2 | 0.45 | 101 | 72.2 | 237 | 350 |
| Pd/Co3O4 | 2.45 | 0 | 123.6 | 26.7 | 0.49 | 70 | 63.0 | 251 | 372 |
| Au/Co3O4 | 0 | 2.70 | 57.2 | - | 0.44 | 75 | 53.5 | 250 | >450 |
| Co3O4 | 0 | 0 | 114.8 | - | 0.48 | 25 | 74.0 | 281 | 500 |
| Catalyst | Total Reduction Degree of Co3O4 | Reaction Temp and Time | Coke Deposition (wt.%) |
|---|---|---|---|
| CeO2/Co3O4 (1:1) | 0.8938 | 750 °C, 5 h | 40.84 |
| CeO2/Co3O4 (1:2) | 0.9073 | 750 °C, 5 h | 53.11 |
| CeO2/Co3O4 (1:3) | 0.9662 | 750 °C, 5 h | 61.21 |
| CeO2/Co3O4 (1:4) | 0.9743 | 750 °C, 5 h | 61.04 |
| CeO2/Co3O4 (1:5) | 0.9350 | 750 °C, 5 h | 68.62 |
| CeO2/Co3O4 (1:6) | 0.9159 | 750 °C, 5 h | 61.52 |
| CeO2/Co3O4 (1:8) | 0.9526 | 750 °C, 5 h | 47.33 |
| Catalysts | Content | Reaction Conditions | Temperature | Reference | |||
|---|---|---|---|---|---|---|---|
| T10 (°C) | T50 (°C) | T90 (°C) | T100 (°C) | ||||
| Co3O4 | 1 vol.% CH4, 4 vol.% O2, WHSV 78,000 mL g−1 h−1 | 597 | [37] | ||||
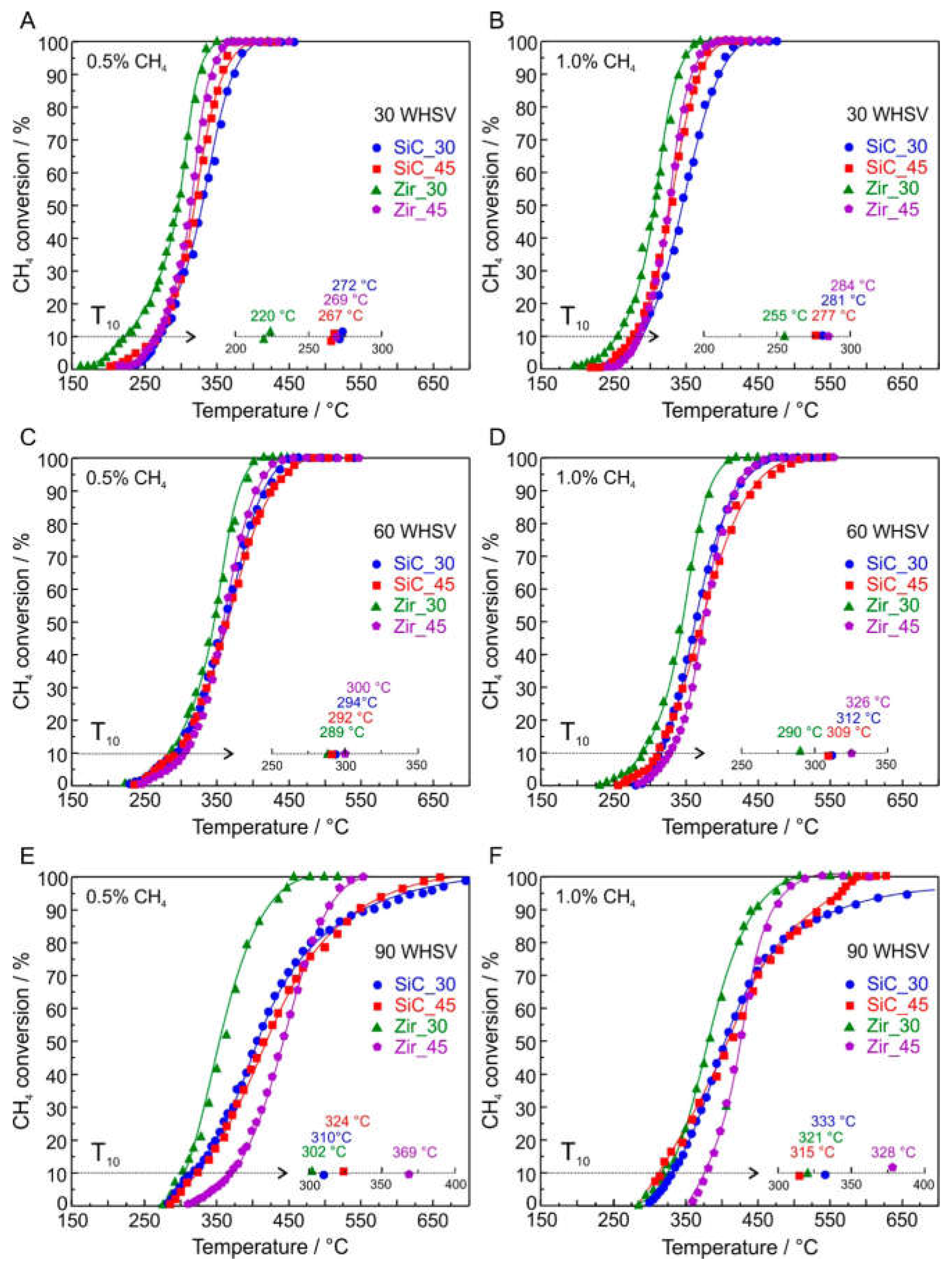
| Pd/Co3O4 | 3 wt.% Pd coated with SiC OCF | 0.5 vol.% CH4, 30 WHSV NL s−1 g−1cat | 272 | 305 | 350 | [93] | |
| Pd/Co3O4 | 3 wt.% Pd coated with Zir OCF | 0.5 vol.% CH4, 30 WHSV NL s−1 g−1cat | 220 | 250 | 275 | [93] | |
| Pd/Co3O4 | 0.7 wt.% Pd | 0.3 vol.% CH4, 0.6 vol.% O2/He | 300–350 | 383 | 500–550 | [99] | |
| Au/Co3O4 | 0.18 wt.% Au | 1 vol.% CH4, 5 vol.% O2, and N2 balance | 241 | 317 | 370 | [100] | |
| Pt/Co3O4 | 0.21 wt.% Pt | 2 vol.% CH4, 5 vol.% O2, and N2 balance | 238 | 312 | 358 | [100] | |
| Au-Pt/Co3O4 | 1.92 wt.% Au and 1.63 wt.% Pt | 3 vol.% CH4, 5 vol.% O2, and N2 balance | 218 | 295 | 332 | [100] | |
| Au-Pd/Co3O4 | 1.90 wt.% Au and 1.48 wt.% Pd | 4 vol.% CH4, 5 vol.% O2, and N2 balance | 241 | 317 | 363 | [100] | |
| Co3O4/SnO2 | Co/(Co + Sn) = 0.75 | 1.0 vol.% CH4, 10.0 vol.% O2, and N2 balance; GHSV 18,000 mL gcat−1 h−1 | 753 | [49] | |||
| Co3O4/γ-Al2O3 | 30 wt.% Co3O4 | 0.2 vol.% CH4, 10 vol.% O2, and N2 balance; GHSV 36,000 mL h−1 g−1 | 300 | 550 | [107] | ||
| Co3O4/γ-Al2O3 | 10.0 wt.% Co3O4 | 1.0 vol.% CH4, space velocity 15,000 h−1 | 320–340 | 400 | [59] | ||
| Co3O4/CeO2 | 20 mL min−1 of 10% CH4/Ar and 10 mL min−1 of O2 WHSV of 9000 mL·g−1·h−1 | 450 | [123] | ||||
| Co3O4/CeO2 | 30 wt.% Co3O4 | 0.3 vol.% CH4, 0.6 vol.% O2, and He balance WHSV 60,000 mL g−1 h−1 | 451 | 549 | [124] | ||
| Pd/Co3−xFexO4 | 3 wt.% Pd, x = 1.1 | 0.5 vol.% CH4 | 481 | [126] | |||
| Co3O4/CeO2 | 15 wt.% Co | 0.5% vol. CH4 and 10% vol. O2, He balance | 440 | [127] | |||
| SmMn2O5/Co3O4 | Co/SMO-50% | 1 vol.% CH4, 10 vol.% O2, N2 balance, and WHSV 60,000 mL g−1 h−1 | 334 | 390 | 437 | [131] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Kang, J.; Gan, L.; Wang, W.; Yang, L.; Wang, D.; Zhong, R.; Qi, J. Recent Advances in Co3O4-Based Composites: Synthesis and Application in Combustion of Methane. Nanomaterials 2023, 13, 1917. https://doi.org/10.3390/nano13131917
Wei X, Kang J, Gan L, Wang W, Yang L, Wang D, Zhong R, Qi J. Recent Advances in Co3O4-Based Composites: Synthesis and Application in Combustion of Methane. Nanomaterials. 2023; 13(13):1917. https://doi.org/10.3390/nano13131917
Chicago/Turabian StyleWei, Xinfang, Jiawei Kang, Lin Gan, Wei Wang, Lin Yang, Dijia Wang, Ruixia Zhong, and Jian Qi. 2023. "Recent Advances in Co3O4-Based Composites: Synthesis and Application in Combustion of Methane" Nanomaterials 13, no. 13: 1917. https://doi.org/10.3390/nano13131917
APA StyleWei, X., Kang, J., Gan, L., Wang, W., Yang, L., Wang, D., Zhong, R., & Qi, J. (2023). Recent Advances in Co3O4-Based Composites: Synthesis and Application in Combustion of Methane. Nanomaterials, 13(13), 1917. https://doi.org/10.3390/nano13131917








