Screening for Bioactive Metabolites in Leaves, Branches, and Roots of Mansoa hirsuta: Phytochemical, Toxicological and Antioxidant Aspects
Abstract
:1. Introduction
2. Experiment
2.1. Reagent Solvents and Equipment
2.2. Plant Material
2.3. Liquid–Liquid Partition Chromatography
2.4. General Experimental Methods
2.4.1. HPLC-PDA
2.4.2. LC–MS
2.5. Nuclear Magnetic Resonance
2.6. Toxicity against Artemia salina Leach
2.7. Oxidative/Antioxidant Action for Saccharomyces cerevisiae
2.8. Cytotoxicity Test for the MTT Method
2.9. Statistical Analysis
2.10. Molecular Docking
2.11. Pharmacophore Model Generation
3. Results and Discussion
3.1. Metabolite Profiling of M. hirsuta Fractions
3.1.1. HPLC-PDA
3.1.2. LC–MS
3.1.3. H-NMR
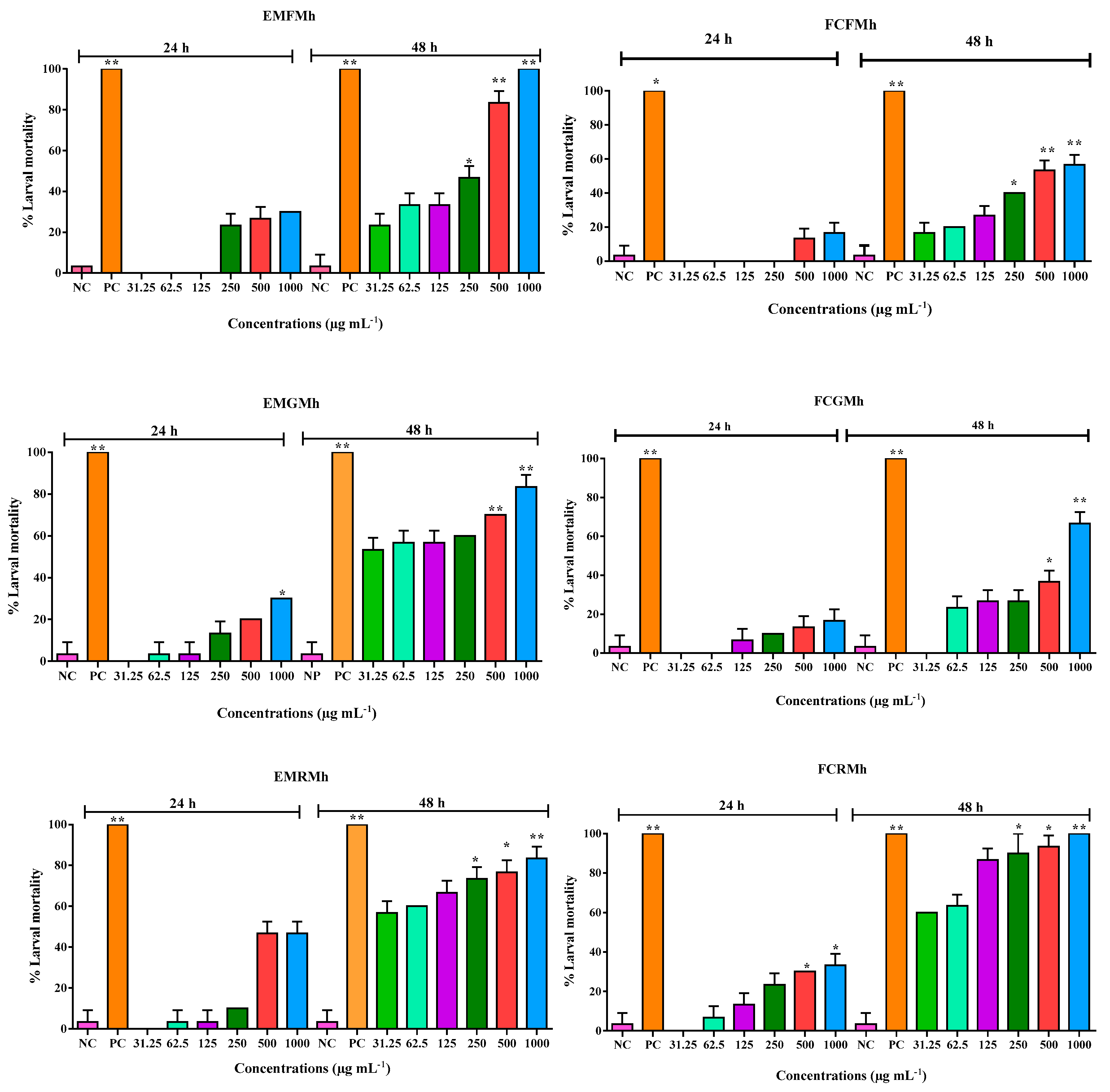
3.2. Bioassay of Toxicity in Brine Shrimp
3.3. Oxidant/Antioxidant Activity in Saccharomyces cerevisiae
3.4. Cell Viability for the MTT Method
3.5. Molecular Docking
3.6. Pharmacophore Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vieira, P.M.; Costa, P.M.; e Silva, C.R.; Chen-Chen, L. Assessment of the genotoxic, antigenotoxic, and cytotoxic activities of the ethanolic fruit extract of Solanum lycocarpum A. St. Hill. (Solanaceae) by micronucleus test in mice. J. Med. Food 2010, 13, 1409–1414. [Google Scholar] [CrossRef]
- Sharma, V.; Sarkar, I.N. Bioinformatics opportunities for identification and study of medicinal plants. Brief. Bioinform. 2013, 14, 238–250. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Ogbole, O.O.; Segun, P.A.; Adeniji, A.J. In vitro cytotoxic activity of medicinal plants from Nigeria ethnomedicine on Rhabdomyosarcoma cancer cell line and HPLC analysis of active extracts. BMC Complement. Altern. Med. 2017, 17, 494. [Google Scholar] [CrossRef]
- Maciel, M.; Pinto, A.C.; Veiga, J.R. Plantas medicinais: A necessidade de estudos multidisciplinares. Quím. Nova 2002, 25, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Shitan, N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293. [Google Scholar] [CrossRef] [Green Version]
- Rates, S.M.K. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Ke, B.; Tian, M.; Li, J.; Liu, B.; He, G. Targeting Programmed Cell Death Using Small-Molecule Compounds to Improve Potential Cancer Therapy. Med. Res. Rev. 2016, 36, 983–1035. [Google Scholar] [CrossRef] [PubMed]
- Oren, O.; Smith, B.D. Eliminating Cancer Stem Cells by Targeting Embryonic Signaling Pathways. Stem Cell Rev. Rep. 2017, 13, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer Molecular Mechanisms of Resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, S.; Sadhukhan, P.; Saha, S.; Sil, P.C. Regulation of oxidative stress by different naturally occurring polyphenolic compounds: An emerging anticancer therapeutic approach. ROS 2017, 3, 81–95. [Google Scholar] [CrossRef]
- Dutta, S.; Saha, S.; Mahalanobish, S.; Sadhukhan, P.; Sil, P.C. Melatonin attenuates arsenic induced nephropathy via the regulation of oxidative stress and inflammatory signaling cascades in mice. FCT 2018, 118, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Endringer, D.C.; Valadares, Y.M.; Campana, P.R.V.; Campos, J.J.; Guimarães, K.G.; Pezzuto, J.M.; Braga, F.C. Evaluation of Brazilian Plants on Cancer Chemoprevention Targets in vitro. Phytother. Res. 2010, 24, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Campana, P.R.; Coleman, C.M.; Sousa, L.P.; Teixeira, M.M.; Ferreira, D.; Braga, F.C. Mansoins C–F, Oligomeric Flavonoid Glucosides Isolated from Mansoa hirsuta Fruits with Potential Anti-inflammatory Activity. J. Nat. Prod. 2016, 79, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.R.; Queiroz, R.F.; Siqueira, E.A.; Brasileiro-Vidal, A.C.; Sant’ana, A.E.G.; Silva, D.M. Evaluation of cytogenotoxicity, antioxidant and hypoglycemiant activities of isolate compounds from Mansoa hirsuta D.C. (Bignoniaceae). An. Acad. Bras. Cienc. 2017, 89, 317–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, P.S.; Preet, G.; Dias, L.; Oliveira, M.; Silva, R.; Castro, I.; Silva, G.; Joaquim, J.; Lima, N.; Silva, D.H. The Free Radical Scavenging Property of the Leaves, Branches, and Roots of Mansoa hirsuta DC: In vitro assessment, 3d pharmacophore, and molecular docking study. Molecules 2022, 27, 6016. [Google Scholar] [CrossRef]
- Zoghbi, M.G.; Oliveira, J.; Skelding, G.M.; Guilhon, P. The genus Mansoa (Bignoniaceae): A source of organosulphur compounds. Rev. Bras. Farmacogn. 2009, 19, 795–804. [Google Scholar] [CrossRef] [Green Version]
- Lohmann, L.G. Untangling the phylogeny of neotropical lianas (Bignonieae, Bignoniaceae). Am. J. Bot. 2006, 93, 304–318. [Google Scholar] [CrossRef]
- Lohmann, L.G.; Taylor, C.M. A new generic classification for tribe Bignonieae (Bignoniaceae). Ann. Mo. Bot. Gard. 2014, 99, 348–489. [Google Scholar] [CrossRef]
- Padhy, G.K. Spathodea campanulata P. Beauv. A review of its ethnomedicinal, phytochemical, and pharmacological profile. J. Appl. Pharm. Sci. 2021, 11, 017–044. [Google Scholar] [CrossRef]
- Queiroz, M.M.F. Identificação dos Inibidores de Acetilcolinesterase em Tetrapterys mucronata Cav. (Malpighiaceae) e Comparação Quali e Quantitativa dos Derivados Triptamícos Presentes na Espécie em Estudo e Ayahuasca. Ph.D. Thesis, Universidade Estadual Paulista, São Paulo, Brazil, 2013. [Google Scholar]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.M.; Melecchi, M.I.; Halmenschlager, R.; Abad, F.C.; Simoni, C.R.; Caramão, E.B.; Henriques, J.A.; Saffi, J.; de Paula Ramos, A.L. Antioxidant and antimutagenic properties of Hibiscus tiliaceus L. methanolic extract. J. Agric. Food Chem. 2006, 54, 7324–7330. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolber, G.; Thierry, L. LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J. Chem. Inf. Model. 2005, 45, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Sendovski, M.; Kanteev, M.; Ben-Yosef, V.S.; Adir, N.; Fishman, A. First structures of an active bacterial tyrosinase reveal copper plasticity. J. Mol. Biol. 2011, 405, 227–237. [Google Scholar] [CrossRef]
- Declercq, J.P.; Evrard, C.; Clippe, A.; Stricht, D.V.; Bernard, A.; Knoops, B. Crystal structure of human peroxiredoxin 5, a novel type of mammalian peroxiredoxin at 1.5 A resolution. J. Mol. Biol. 2001, 311, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Strange, R.W.; Antonyuk, S.V.; Hough, M.A.; Doucette, P.A.; Valentine, J.S.; Hasnain, S.S. Variable metallation of human superoxide dismutase: Atomic resolution crystal structures of Cu-Zn, Zn-Zn and as-isolated wild-type enzymes. J. Mol. Biol. 2006, 356, 1152–1162. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera- a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- SAMSON: Software for Adaptive Modeling and Simulation Of Nanosystems. Available online: https://www.samson-connect.net (accessed on 22 January 2023).
- Simões, C.M.O.; Schenkel, E.P.; De Mello, J.C.P.; Mentz, L.A.; Petrovick, P.S. Farmacognosia: Do Produto Natural ao Medicamento; Artmed Editora: Porto Alegre, Brazil, 2016. [Google Scholar]
- Shao, S.Y.; Ting, Y.; Wang, J.; Sun, J.; Guo, X.F. Characterization and identification of the major flavonoids in Phyllostachys edulis leaf extract by UPLC–QTOF–MS/MS. Acta Chromatogr. 2020, 32, 228–237. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Zhang, W.; Chen, Z. Identification and quantification of oleanolic acid and ursolic acid in Chinese herbs by liquid chromatography–ion trap mass spectrometry. Biomed. Chromatogr. 2011, 25, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Dapic, I.; Jakasa, I.; Kobetic, R.; Brkljacic, L. Characterization of Ceramides with Phytosphingosine Backbone by Hydrogen-deuterium Exchange Mass Spectrometry. Croat. Chem. Acta 2019, 92, 411–417. [Google Scholar] [CrossRef]
- Munikishore, R.; Padmaja, A.; Gunasekar, D.; Blond, A.; Bodo, B. Two new flavonoids from Ageratum conyzoides. Indian J. Chem. Sect. B 2013, 52, 1479–1482. [Google Scholar] [CrossRef]
- Ramamurthy, V.; Rajeswari, D.M.; Gowri, R.; Vadivazhagi, M.; Jayanthi, G.; Raveendran, S. Study of the phytochemical analysis and antimicrobial activity of Dodonaea viscosa. J. Pure Appl. Zool. 2013, 1, 178–184. [Google Scholar]
- Rand, G.M. Fundamentals of Aquatic Toxicology: Effects, Environmental Fate and Risk Assessment, 2nd ed.; Taylor & Francis: Washington, DC, USA, 1995; cap. 1. [Google Scholar]
- Fonseca, A.L. A Biologia das Espécies Daphnia laevis, Ceriodaphnia dubi Silvestris (Crustacea, Cladocera) e Poecilia reticulata (Piscis, Poeciledae) e o Comportamento Destes em Testes de Toxicidade Aquática com Efluente Industriais. Dissertação de Mestrado em Hidráulica e Saneamento, USP São Carlos, São Carlos, Brazil, 1991. [Google Scholar]
- Queiroz, S. Tratado de Toxicologia Ocupacional, 2nd ed.; Biblioteca 24 Horas: Rio de Janeiro, Brazil, 2015; p. 554. [Google Scholar]
- Olowa, L.F.; Nuñeza, O.M. Brine shrimp lethality assay of the ethanolic extracts of three selected species of medicinal plants from Iligan City Philippines. Int. J. Biol. Sci. 2013, 2, 74–77. [Google Scholar]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of medicinal plants native to ornaturalized in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef]
- Chakraborty, A.K.; Rambhade, S.; Patil, U.K. Chromolaena odorata (L.): An overview. J. Pharm. Res. 2011, 4, 573–576. [Google Scholar]
- Moshi, M.J.; Cosam, J.C.; Mbwambo, Z.H.; Kapingu, M.; Nkunya, M.H.H. Testing Beyond Ethnomedical Claims: Brine Shrimp Lethality of Some Tanzanian Plants. Pharm. Biol. 2004, 42, 547–551. [Google Scholar] [CrossRef]
- Moshi, M.J.; Mbwambo, Z.H.; Nondo, R.S.O.; Masimba, P.J.; Kamuhabwa, A.; Kapingu, M.C.; Thomas, P.; Richard, M. Evaluation of ethnomedical claims and brine shrimp toxicity of some plants used in Tanzania as traditional medicines. AJTCAM 2006, 3, 48–58. [Google Scholar]
- Mascarenhas, C.; Edwards-Ingram, L.C.; Zeef, L.; Shenton, D.; Ashe, M.P.; Grant, C.M. Gcn4 is required for the response to peroxide stress in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 2008, 19, 2995–3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Spent brewer’s yeast (Saccharomyces cerevisiae) as a potential source of bioactive peptides: An overview. Int. J. Biol. Macromol. 2022, 208, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Nemudzivhadi, V.; Masoko, P. In vitro assessment of cytotoxicity, antioxidant, and anti-inflammatory activities of Ricinus communis (Euphorbiaceae) leaf extracts. Evid.-Based Complement. Altern. Med. 2014, 2014, 625961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campana, P.R.; Braga, F.C.; Cortes, S.F. Endothelium-dependent vasorelaxation in rat thoracic aorta by Mansoa hirsuta D.C. Phytomedicine 2009, 16, 456–461. [Google Scholar] [CrossRef]
- Stoclet, J.C.; Chataigneau, T.; Ndiaye, M.; Oak, M.; Bedoui, J.; Chataigneau, M.; Schini-Kerth, V.B. Vascular protection by dietary polyphenols. Eur. J. Pharmacol. 2004, 500, 299–313. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Yanez, J.; Vicente, V.; Alcaraz, M.; Castillo, J.; Benavente-García, O.; Canteras, M.; Teruel, J.A. Cytotoxicity and antiproliferative activities of several phenolic compounds against three melanocytes cell lines: Relationship between structure and activity. Nutr. Cancer 2004, 49, 191–199. [Google Scholar] [CrossRef]
- Onyancha, J.M.; Gikonyo, N.K.; Wachira, S.W.; Mwitari, P.G.; Gicheru, M.M. Anticancer activities and safety evaluation of selected Kenyan plant extracts against breast cancer cell lines. J. Pharmacogn. Phytother. 2018, 10, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar]
- Seo, S.Y.; Kim, Y.J.; Kim, J.; Nam, M.H.; Park, K.Y. Phytosphingosine induces systemic acquired resistance through activation of sphingosine kinase. Plant Direct 2021, 5, e351. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Bang, S.; Chung, B.Y.; Jung, H.; Oh, E.S.; Chang, S.E. Inhibitory effects of N,N,N-trimethyl phytosphingosine-iodide on melanogenesis via ERK activation-mediated MITF degradation. Biosci. Biotechnol. Biochem. 2016, 80, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Ho, J.N.; Yoon, S.H.; Yang, G.Y.; Hwang, S.G.; Um, H.D. Peroxiredoxin 6 promotes lung cancer cell invasion by inducing urokinase-type plasminogen activator via p38 kinase, phosphoinositide 3-kinase, and Akt. Mol. Cells 2009, 28, 583–588. [Google Scholar] [CrossRef]
- Miyamoto, N.; Izumi, H.; Miyamoto, R.; Kondo, H.; Tawara, A.; Sasaguri, Y.; Kohno, K. Quercetin induces the expression of peroxiredoxins 3 and 5 via the Nrf2/NRF1 transcription pathway. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1055–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Description | Genotype | Deficiency in Enzymatic Defenses | Origin |
|---|---|---|---|
| EG103 (SODWT) | MATa leu2-3.112 trp1-289 ura3-52 | None | Edith Gralla, Los Angeles |
| EG118 (Sod1∆) | Sod 1:: URA3 all other markers such as EG103 | Cu-Zn superoxide dismutase (cytoplasmic) | Edith Gralla, Los Angeles |
| EG110 (Sod2∆) | Sod 2:: TRP1 all other markers such as EG103 | Mn superoxide dismutase (mitochondrial) | Edith Gralla, Los Angeles |
| EG133 (Sod1∆Sod2∆) | Sod 1:: URA3 Sod2::TRP1 double mutant all other markers as EG103 | Cytoplasmic and mitochondrial superoxide dismutase | Edith Gralla, Los Angeles |
| EG223 (Cat1∆) | EG103, except Cat 1:: TRP1 | cytoplasmic catalase | Edith Gralla, Los Angeles |
| EG (Sod1∆Cat1∆) | EG103, except Sod1::URA3 and Cat1::TRP1 | Cu-Zn superoxide dismutase and cytoplasmic catalase | Edith Gralla, Los Angeles |
| Fractions | Rt (Min) | Ionization Mode | Fragments (Relative Abundance (R %) | Compound | References |
|---|---|---|---|---|---|
| FCFMh | 2.0 | Positive | m/z 285 (1.32%), m/z 309 (1.10%), m/z 339 (4.73%) | Isoorientin-2″-O -arabinoside | [35] |
| FCGMh | 2.0 | Positive | m/z 429 (0.18%), m/z 447 (2.95%), m/z 459 (0.77%) | Isoorientin-2″-O -arabinoside | [35] |
| FCRMh | 2.0 | Positive | m/z 429 (5.5%), m/z 447 (11.64%), m/z 459 (1.49%) | Isoorientin-2″-O -arabinoside | [35] |
| FCFMh | 2.2 | Positive | m/z 393 (3.84%) and m/z 411 (5.83%) | Oleanolic acid and ursolic acid | [36] |
| FCGMh | 2.2 | Positive | m/z 393 (2.95%), m/z 411 (3.35%) and m/z 457 (4.21%) | Oleanolic acid and ursolic acid | [36] |
| FCRMh | 2.3 | Positive | m/z 393 (0.22%) and m/z 411 (0.36%) | Oleanolic acid and ursolic acid | [36] |
| FCFMh | 5.1 | Positive | m/z 300 (5.55%) and m/z 318 (100%) | Phytosphingosine | [37] |
| FCGMh | 5.1 | Positive | m/z 300 (19.93%) and m/z 318 (100%) | Phytosphingosine | [37] |
| FCRMh | 5.1 | Positive | m/z 300 (5.43%) and m/z 318 (100%) | Phytosphingosine | [37] |
| Samples | LC50 (µg·mL−1) | LC50 (µg·mL−1) |
|---|---|---|
| 24 h | 48 h | |
| EMFMh | 355.7 | 339.9 |
| EMGMh | 316.5 | 89.26 |
| EMRMh | 287.3 | 83.92 |
| FCFMh | 479.0 | 421.9 |
| FCGMh | 421.4 | 64.21 |
| FCRMh | 142.9 | 87.61 |
| Groups | SodWT | Sod1∆ | Sod2∆ | Sod1∆Sod2∆ | Cat1∆ | Sod1∆Cat1∆ |
|---|---|---|---|---|---|---|
| NC | 0.50 ± 0.71 | 0.50 ± 0.61 | 0.37 ± 0.39 | 0.41 ± 0.45 | 0.52 ± 0.49 | 0.50 ± 0.55 |
| PC | 18.25 ± 0.70 a | 20.25 ± 2.16 a | 19.25 ± 0.55 a | 23.00 ± 0.39 a | 21.75 ± 0.55 a | 24.25 ± 0.34 a |
| FCFMh µg mL−1 | ||||||
| 50 | 0.75 ± 0.30 b | 1.66 ± 0.58 b | 1.00 ± 0.60 b | 2.33 ± 0.61 b | 0.71 ± 0.59 b | 1.33 ± 0.34 b |
| 250 | 10.50 ± 0.4 ab | 6.66 ± 0.62 ab | 7.33 ± 0.55 ab | 9.00 ± 0.42 ab | 7.50 ± 0.42 ab | 7.00 ± 0.37 ab |
| 500 | 12.33 ± 0.60 ab | 12.00 ± 0.63 ab | 12.00 ± 0.60 ab | 12.00 ± 0.40 ab | 11.50 ± 0.40 ab | 11.50 ± 0.30 ab |
| FCGMh µg mL−1 | ||||||
| 50 | 0.50 ± 0.52 b | 2.66 ± 0.9 b | 0.75 ± 0.25 b | 2.75 ± 0.63 b | 0.75 ± 0.55 b | 1.25 ± 0.33 b |
| 250 | 7.00 ± 0.95 ab | 7.33 ± 0.92 ab | 7.33 ± 0.73 ab | 7.66 ± 0.68 ab | 6.66 ± 0.58 ab | 7.00 ± 0.36 ab |
| 500 | 9.75 ± 0.88 ab | 9.75 ± 0.85 ab | 13.00 ± 0.70 ab | 11.25 ± 0.55 ab | 9.25 ± 0.51 ab | 11.33 ± 0.35 ab |
| FCRMh µg mL−1 | ||||||
| 50 | 1.50 ± 0.83 b | 2.25 ± 0.75 b | 1.50 ± 0.54 b | 2.00 ± 0.48 b | 1.66 ± 0.67 b | 1.25 ± 0.34 b |
| 250 | 7.00 ± 0.95 ab | 5.33 ± 0.82 ab | 7.33 ± 0.59 ab | 7.66 ± 0.52 ab | 7.25 ± 0.62 ab | 7.00 ± 0.36 ab |
| 500 | 9.75 ± 0.88 ab | 9.00 ± 0.76 ab | 10.00 ± 0.54 ab | 10.50 ± 0.44 ab | 8.00 ± 0.52 ab | 10.50 ± 0.32 ab |
| Groups | SodWT | Sod1∆ | Sod2∆ | Sod1∆Sod2∆ | Cat1∆ | Sod1∆Cat1∆ |
|---|---|---|---|---|---|---|
| NC | 0.50 ± 0.57 | 0.50 ± 0.57 | 0.50 ± 0.57 | 0.50 ± 0.57 | 0.50 ± 0.57 | 0.50 ± 0.57 |
| PC | 18.75 ± 0.65 b | 21.25 ± 0.63 b | 19.25 ± 0.58 b | 23.00 ± 0.71 b | 21.75 ± 0.64 b | 24.25 ± 0.34 b |
| FCFMh µg mL−1 | ||||||
| 50 | 2.00 ± 0.71 b | 4.00 ± 0.60 ab | 1.33 ± 0.62 b | 4.00 ± 0.77 ab | 3.00 ± 0.64 ab | 4.33 ± 0.37 ab |
| 250 | 4.33 ± 0.71 ab | 4.66 ± 0.68 ab | 4.00 ± 0.63 ab | 4.33 ± 0.77 ab | 4.00 ± 0.64 ab | 5.00 ± 0.37 ab |
| 500 | 12.00 ± 0.70 ab | 14.50 ± 0.63 ab | 14.33 ± 0.60 ab | 14.33 ± 0.70 ab | 11.50 ± 0.64 ab | 12.50 ± 0.34 ab |
| FCGMh µg mL−1 | ||||||
| 50 | 0.66 ± 0.16 b | 4.00 ± 0.51 ab | 2.67 ± 0.98 b | 2.00 ± 0.83 b | 2.33 ± 0.59 b | 2.33 ± 0.41 b |
| 250 | 3.00 ± 0.66 ab | 4.00 ± 0.56 ab | 4.66 ± 0.93 ab | 4.75 ± 0.77 ab | 4.33 ± 0.58 ab | 5.33 ± 0.40 ab |
| 500 | 10.00 ± 0.60 ab | 9.50 ± 0.54 ab | 10.00 ± 0.80 ab - | 8.00 ± 0.84 ab | 8.50 ± 0.54 ab | 8.50 ± 0.38 ab |
| FCRMh µg mL−1 | ||||||
| 50 | 1.50 ± 0.77 b | 4.00 ± 0.57 ab | 5.66 ± 1.10 ab | 3.33 ± 0.82 b | 1.25 ± 0.68 b | 2.75 ± 0.48 b |
| 250 | 3.00 ± 0.83 b | 5.33 ± 0.62 ab | 6.00 ± 1.02 ab | 3.50 ± 0.76 b | 3.00 ± 0.73 b | 4.66 ± 0.53 ab |
| 500 | 13.00 ± 0.78 ab | 9.00 ± 0.58 ab | 10.00 ± 0.90 ab | 9.66 ± 0.81 ab | 10.00 ± 0.68 ab | 11.00 ± 0.49 ab |
| Groups | SodWT | Sod1∆ | Sod2∆ | Sod1∆Sod2∆ | Cat1∆ | Sod1∆Cat1∆ |
|---|---|---|---|---|---|---|
| NC | 0.50 ± 0.58 | 0.50 ± 0.58 | 0.50 ± 0.58 | 0.50 ± 0.58 | 0.50 ± 0.58 | 0.50 ± 0.58 |
| PC | 18.75 ± 0.63 b | 21.25 ± 0.48 b | 19.25 ± 0.50 b | 23.00 ± 0.45 b | 21.00 ± 0.50 b | 23.00 ± 0.37 b |
| FCFMh µg mL−1 | ||||||
| 50 | 0.50 ± 0.68 b | 1.00 ± 0.52 b | 1.00 ± 0.55 b | 1.66 ± 0.49 b | 0.50 ± 0.49 b | 1.33 ± 0.40 b |
| 250 | 6.00 ± 0.68 ab | 4.75 ± 0.48 ab | 6.00 ± 0.50 ab | 4.33 ± 0.45 ab | 6.00 ± 0.54 ab | 3.33 ± 0.37 ab |
| 500 | 11.75 ± 0.63 ab | 12.33 ± 0.50 ab | 12.00 ± 0.55 ab | 11.67 ± 0.36 ab | 12.25 ± 0.50 ab | 12.25 ± 0.34 ab |
| FCGMh µg mL−1 | ||||||
| 50 | 0.50 ± 0.69 b | 1.33 ± 0.76 b | 0.75 ± 0.25 b | 1.50 ± 0.68 b | 0.75 ± 0.62 b | 1.25 ± 0.00 b |
| 250 | 0.50 ± 0.62 b | 2.00 ± 0.79 b | 2.00 ± 0.65 b | 4.00 ± 0.73 ab | 0.75 ± 0.64 b | 1.66 ± 0.40 b |
| 500 | 11.75 ± 0.74 ab | 13.75 ± 0.73 ab | 13.67 ± 0.71 ab | 12.50 ± 0.66 ab | 12.75 ± 0.63 ab | 11.25 ± 0.37 ab |
| FCRMh µg mL−1 | ||||||
| 50 | 1.66 ± 0.68 b | 1.50 ± 0.51 b | 1.75 ± 0.50 b | 2.50 ± 0.51 b | 1.50 ± 0.56 b | 2.75 ± 0.47 b |
| 250 | 4.00 ± 0.63 ab | 6.66 ± 0.55 ab | 6.00 ± 0.48 ab | 6.66 ± 0.55 ab | 5.00 ± 0.56 ab | 4.66 ± 0.53 ab |
| 500 | 10.50 ± 0.60 ab | 11.50 ± 0.53 ab | 11.00 ± 0.52 ab | 11.75 ± 0.59 ab | 10.75 ± 0.58 ab | 11.00 ± 0.49 ab |
| Groups | SodWT | Sod1∆ | Sod2∆ | Sod1∆Sod2∆ | Cat1∆ | Sod1∆Cat1∆ |
|---|---|---|---|---|---|---|
| NC | 0.50 ± 0.58 | 0.50 ± 0.58 | 0.50 ± 0.58 | 0.50 ± 0.58 | 0.50 ± 0.58 | 0.50 ± 0.58 |
| PC | 18.75 ± 0.72 a | 21.25 ± 0.71 a | 19.25 ± 0.58 a | 23.00 ± 0.44 a | 21.75 ± 0.41 a | 24.25 ± 0.44 a |
| FCFMh µg mL−1 | ||||||
| 50 | 0.50 ± 0.72 | 1.25 ± 0.73 b | 4.00 ± 0.62 ab | 1.50 ± 0.44 b | 1.66 ± 0.44 b | 2.50 ± 0.44 b |
| 250 | 1.00 ± 0.78 b | 4.33 ± 0.80 ab | 4.66 ± 0.63 ab | 1.50 ± 0.40 b | 2.00 ± 0.45 b | 5.00 ± 0.47 ab |
| 500 | 9.50 ± 0.70 ab | 8.00 ± 0.79 ab | 9.66 ± 0.62 ab | 8.50 ± 0.45 ab | 9.00 ± 0.45 ab | 9.50 ± 0.44 ab |
| FCGMh µg mL−1 | ||||||
| 50 | 0.50 ± 0.96 b | 1.25 ± 1.26 b | 1.50 ± 1.15 b | 2.66 ± 0.85 b | 2.75 ± 0.64 b | 3.00 ± 0.89 b |
| 250 | 1.25 ± 0.96 b | 3.75 ± 1.06 ab | 5.66 ± 1.24 ab | 3.66 ± 0.85 ab | 3.33 ± 0.69 ab | 4.00 ± 0.96 ab |
| 500 | 15.67 ± 1.04 ab | 11.00 ± 1.10 ab | 10.00 ± 1.16 ab | 9.66 ± 0.84 ab | 11.75 ± 0.63 ab | 13.75 ± 0.89 ab |
| FCRMh µg mL−1 | ||||||
| 50 | 1.25 ± 0.66 b | 1.75 ± 0.56 b | 2.25 ± 0.54 b | 0.33 ± 0.44 b | 0.50 ± 0.49 b | 1.75 ± 0.38 b |
| 250 | 2.25 ± 0.63 b | 4.00 ± 0.52 ab | 6.33 ± 0.58 ab | 3.50 ± 0.40 ab | 3.75 ± 0.43 ab | 4.00 ± 0.44 ab |
| 500 | 12.00 ± 0.80 ab | 15.00 ± 0.50 ab | 12.00 ± 0.54 ab | 10.00 ± 0.39 ab | 10.00 ± 0.48 ab | 12.00 ± 0.45 ab |
| Samples | IC50 (μg mL−1) | Range IC50 (μg mL−1) |
|---|---|---|
| 4T1 a | L929 b | |
| FCFMh | 33.13 6.003–82.9 | 45.05 31.40–64.64 |
| FCGMh | 47.13 2.329–953.8 | 47.82 34.45–68.06 |
| FCRMh | 16.48 10.64–25.52 | 33.74 23.65–48.15 |
| Compounds | Docking Score kcalmol−1 | Docking Score kcalmol−1 | Docking Score kcalmol−1 |
|---|---|---|---|
| PDB ID: 1HD2 (Human Peroxiredoxin 5) | PDB ID: 2C9V (Human Superoxide Dismutase) | PDB ID: 3NM8 (Bacterial Tyrosinase) | |
| H2O2 (Standard) | −2.8 | −2.6 | −2.4 |
| Isoorientin-2″-O-arabinoside | −5.0 | −8.2 | −8.2 |
| Phytosphingosine | −4.0 | −4.7 | −5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, P.e.S.; Preet, G.; Oliveira, M.; Dias, L.; Silva, G.; Nascimento, M.L.; Reis, A.; Sousa, J.M.; Júnior, J.; Lima, N.M.; et al. Screening for Bioactive Metabolites in Leaves, Branches, and Roots of Mansoa hirsuta: Phytochemical, Toxicological and Antioxidant Aspects. Biophysica 2023, 3, 425-445. https://doi.org/10.3390/biophysica3030028
Alves PeS, Preet G, Oliveira M, Dias L, Silva G, Nascimento ML, Reis A, Sousa JM, Júnior J, Lima NM, et al. Screening for Bioactive Metabolites in Leaves, Branches, and Roots of Mansoa hirsuta: Phytochemical, Toxicological and Antioxidant Aspects. Biophysica. 2023; 3(3):425-445. https://doi.org/10.3390/biophysica3030028
Chicago/Turabian StyleAlves, Patrícia e Silva, Gagan Preet, Maria Oliveira, Leandro Dias, Giovanna Silva, Maria Luísa Nascimento, Antonielly Reis, João Marcelo Sousa, Joaquim Júnior, Nerilson Marques Lima, and et al. 2023. "Screening for Bioactive Metabolites in Leaves, Branches, and Roots of Mansoa hirsuta: Phytochemical, Toxicological and Antioxidant Aspects" Biophysica 3, no. 3: 425-445. https://doi.org/10.3390/biophysica3030028
APA StyleAlves, P. e. S., Preet, G., Oliveira, M., Dias, L., Silva, G., Nascimento, M. L., Reis, A., Sousa, J. M., Júnior, J., Lima, N. M., Andrade, T., & Feitosa, C. (2023). Screening for Bioactive Metabolites in Leaves, Branches, and Roots of Mansoa hirsuta: Phytochemical, Toxicological and Antioxidant Aspects. Biophysica, 3(3), 425-445. https://doi.org/10.3390/biophysica3030028








