The Use of Pre-Chemoradiotherapy Total Masseter Muscle Volume as a Novel Predictor of Radiation-Induced Trismus in Locally Advanced Nasopharyngeal Carcinoma Patients
Abstract
1. Introduction
2. Patients and Methods
2.1. Ethical Approval
2.2. Study Population
2.3. Treatment Protocol
2.4. Baseline Total Masseter Muscle Volume Measurements
2.5. Baseline and Follow-Up Oral Evaluation and the Determination of RIT
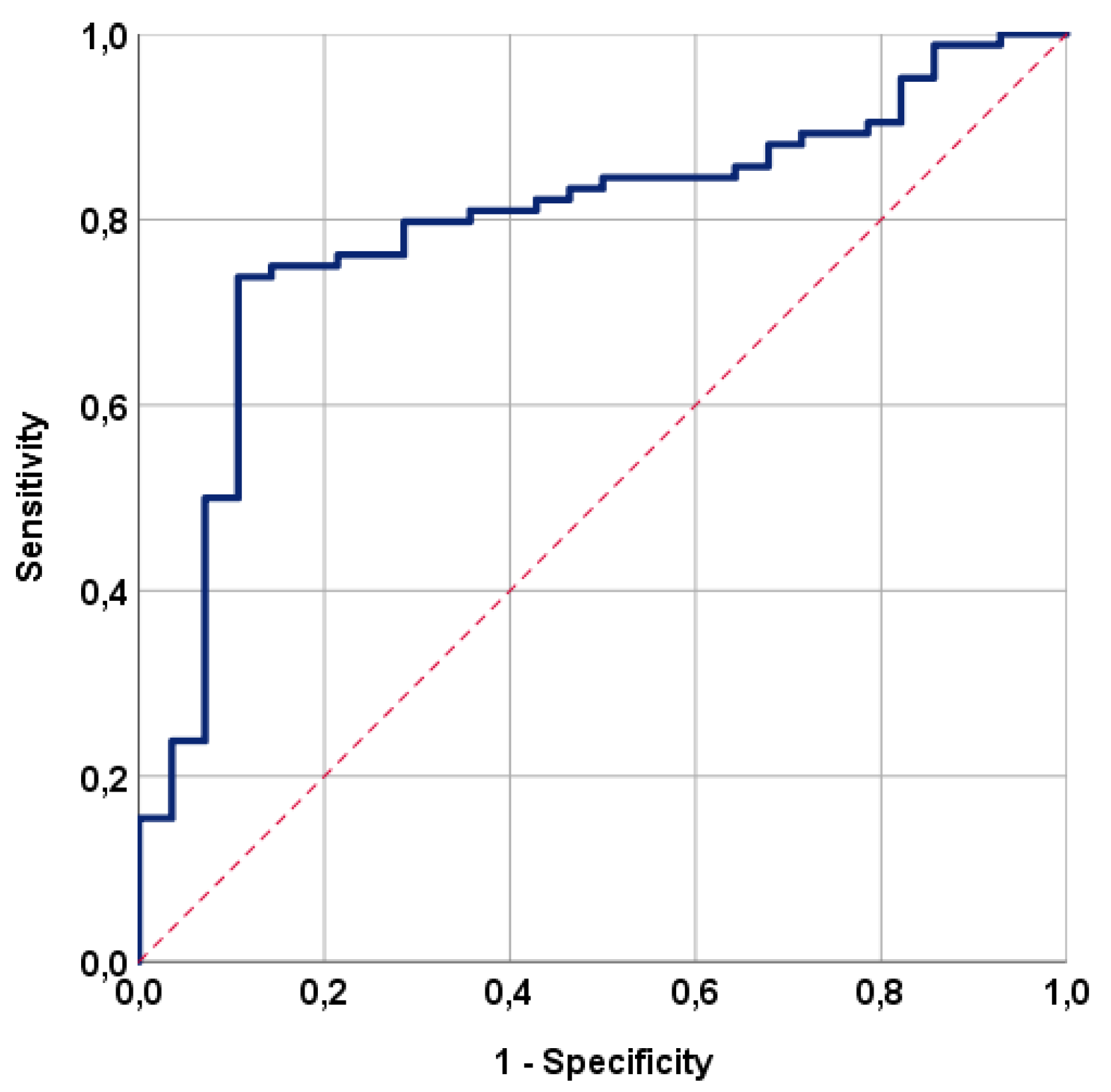
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adoga, A.A.; Kokong, D.D.; Ma’an, N.D.; Silas, O.A.; Dauda, A.M.; Yaro, J.P.; Mugu, J.G.; Mgbachi, C.J.; Yabak, C.J. The epidemiology, treatment, and determinants of outcome of primary head and neck cancers at the Jos University Teaching Hospital. South Asian J. Cancer 2018, 7, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.; Nguyen, F.; Moya-Plan, A.; Pignon, J.P.; Even, C.; Bidault, F.; Temam, S.; Ruffier, A.; Tao, Y. New developments in the management of nasopharyngeal carcinoma. Cancer Radiother. 2018, 22, 492–495. [Google Scholar] [CrossRef]
- Lee, N.; Harris, J.; Garden, A.S.; Straube, W.; Glisson, B.; Xia, P.; Bosch, W.; Morrison, W.H.; Quivey, J.; Thorstad, W.; et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: Radiation therapy oncology group phase II trial 0225. J. Clin. Oncol. 2009, 27, 3684–3690. [Google Scholar] [CrossRef] [PubMed]
- Skiba-Tatarska, M.; Kusa-Podkańska, M.; Surtel, A.; Wysokińska-Miszczuk, J. The side-effects of head and neck tumors radiotherapy. Pol. Merkur. Lekarski. 2016, 41, 47–49. [Google Scholar]
- Pauli, N.; Johnson, J.; Finizia, C.; Andréll, P. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncol. 2013, 52, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Garnett, M.J.; Nohl, F.S.; Barclay, S.C. Management of patients with reduced oral aperture and mandibular hypomobility (trismus) and implications for operative dentistry. Br. Dent. J. 2008, 204, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Musha, A.; Shimada, H.; Kubo, N.; Kawamura, H.; Okano, N.; Miyasaka, Y.; Sato, H.; Shirai, K.; Saitoh, J.I.; Yokoo, S.; et al. Evaluation of Carbon Ion Radiation-Induced Trismus in Head and Neck Tumors Using Dose-Volume Histograms. Cancers 2020, 12, 3116. [Google Scholar] [CrossRef]
- Chou, C.; Chen, C.C.; Lai, C.S.; Lin, S.D.; Kuo, Y.R. Simultaneous double free radial forearm flaps combined with coronoidectomy and myotomy to release bilateral severe trismus: A case report. Microsurgery 2017, 37, 831–835. [Google Scholar] [CrossRef]
- Somay, E.; Yilmaz, B.; Topkan, E.; Kucuk, A.; Haksoyler, V.; Pehlivan, B.; Selek, U.; Araz, K. Hemoglobin-to-platelet ratio in predicting the incidence of trismus after concurrent chemoradiotherapy. Oral Dis. 2022, 29, 2962–2970. [Google Scholar] [CrossRef]
- Somay, E.; Yilmaz, B.; Topkan, E.; Pehlivan, B.; Selek, U. Low hemoglobin levels predict increased radiation-induced trismus rates in nasopharyngeal cancer. Oral Dis. 2023, 8, 1–9. [Google Scholar] [CrossRef]
- Somay, E.; Yilmaz, B.; Topkan, E.; Kucuk, A.; Pehlivan, B.; Selek, U. Initial neutrophil-to-lymphocyte ratio predicts radiation-induced trismus in parotid gland cancer. Oral Dis. 2022, 29, 2772–2779. [Google Scholar] [CrossRef]
- Anjanappa, M.; Corden, M.; Green, A.; Roberts, D.; Hoskin, P.; McWilliam, A.; Choudhury, A. Sarcopenia in cancer: Risking more than muscle loss. Tech. Innov. Patient Support Radiat. Oncol. 2020, 16, 50–57. [Google Scholar] [CrossRef]
- Douglas, E.; McMillan, D.C. Towards a simple objective framework for the investigation and treatment of cancer cachexia: The Glasgow Prognostic Score. Cancer Treat. Rev. 2014, 40, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ma, J.; Li, L.; Zhu, X.D. Severe muscle loss during radical chemoradiotherapy for non-metastatic nasopharyngeal carcinoma predicts poor survival. Cancer Med. 2019, 8, 6604–6613. [Google Scholar] [CrossRef]
- Kamada, T.; Ohdaira, H.; Ito, E.; Takahashi, J.; Nakashima, K.; Nakaseko, Y.; Suzuki, N.; Yoshida, M.; Eto, K.; Suzuki, Y. Association between masseter muscle sarcopenia and postoperative pneumonia in patients with esophageal cancer. Sci. Rep. 2022, 12, 16374. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, U.A.; Somay, E.; Yilmaz, B.; Besen, A.A.; Mertsoylu, H.; Selek, U.; Topkan, E. Pretreatment Masseter Muscle Volume Predicts Survival in Locally Advanced Nasopharyngeal Carcinoma Patients Treated with Concurrent Chemoradiotherapy. J. Clin. Med. 2023, 12, 6863. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Mao, Y.P.; Tang, L.L.; Chen, L.; Sun, Y.; Ma, J. The evolution of nasopharyngeal carcinoma staging. Br. J. Radiol. 2019, 92, 20190244. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.B.; Possebon, A.P.D.R.; Schuster, A.J.; Marcello-Machado, R.M.; de Rezende Pinto, L.; Faot, F. Relationship Between Masticatory Function Impairment and Oral Health-Related Quality of Life of Edentulous Patients: An interventional Study. J. Prosthodont. 2019, 28, 634–642. [Google Scholar] [CrossRef]
- Yilmaz, B.; Somay, E.; Selek, U.; Topkan, E. Pretreatment Systemic Immune-Inflammation Index Predict Needs for Teeth Extractions for Locally Advanced Head and Neck Cancer Patients Undergoing Concurrent Chemoradiotherapy. Ther. Clin. Risk Manag. 2021, 17, 1113–1121. [Google Scholar] [CrossRef]
- Dijkstra, P.U.; Kalk, W.W.; Roodenburg, J.L. Trismus in head and neck oncology: A systematic review. Oral. Oncol. 2004, 40, 879–889. [Google Scholar] [CrossRef]
- Bhargava, D.; Jain, M.; Deshpande, A.; Singh, A.; Jaiswal, J. Temporomandibular joint arthrocentesis for internal derangement with disc displacement without reduction. J. Maxillofac. Oral Surg. 2015, 14, 454–459. [Google Scholar] [CrossRef]
- Owosho, A.A.; Pedreira Ramalho, L.M.; Rosenberg, H.I.; Yom, S.K.; Drill, E.; Riedel, E.; Tsai, C.J.; Lee, N.Y.; Huryn, J.M.; Estilo, C.L. Objective assessment of trismus in oral and oropharyngeal cancer patients treated with intensity-modulated radiation therapy (IMRT). J. Cranio-Maxillofac. Surg. 2016, 44, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Kraaijenga, S.A.; Hamming-Vrieze, O.; Verheijen, S.; Lamers, E.; van der Molen, L.; Hilgers, F.J.; van den Brekel, M.W.; Heemsbergen, W.D. Radiation dose to the masseter and medial pterygoid muscle in relation to trismus after chemoradiotherapy for advanced head and neck cancer. Head Neck 2019, 41, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Rajalalitha, P.; Vali, S. Molecular pathogenesis of oral submucous fibrosis–a collagen metabolic disorder. J. Oral Pathol. Med. 2005, 34, 321–328. [Google Scholar] [CrossRef]
- Wallace, J.D.; Calvo, R.Y.; Lewis, P.R.; Brill, J.B.; Shackford, S.R.; Sise, M.J.; Sise, C.B.; Bansal, V. Sarcopenia as a predictor of mortality in elderly blunt trauma patients: Comparing the masseter to the psoas using computed tomography. J. Trauma Acute Care Surg. 2017, 82, 65–72. [Google Scholar] [CrossRef]
- McGoldrick, D.M.; Yassin Alsabbagh, A.; Shaikh, M.; Pettit, L.; Bhatia, S.K. Masseter muscle defined sarcopenia and survival in head and neck cancer patients. Br. J. Oral Maxillofac. Surg. 2022, 60, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Huang, J.; Wu, H.; Wang, Y.; Du, Z.; Ling, Y.; Wang, W.; Wu, Q.; Gao, W. Molecular mechanisms of cancer cachexia-induced muscle atrophy (Review). Mol. Med. Rep. 2020, 22, 4967–4980. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa, E.; Silva, M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef]
- Gould, D.W.; Lahart, I.; Carmichael, A.R.; Koutedakis, Y.; Metsios, G.S. Cancer cachexia prevention via physical exercise: Molecular mechanisms. J. Cachexia Sarcopenia Muscle 2013, 4, 111–124. [Google Scholar] [CrossRef]
- Muthanandam, S.; Muthu, J. Understanding Cachexia in Head and Neck Cancer. Asia Pac. J. Oncol. Nurs. 2021, 8, 527–538. [Google Scholar] [CrossRef]
- Topkan, E.; Yavuz, A.A.; Ozyilkan, O. Cancer cachexia: Pathophysiologic aspects and treatment options. Asian Pac. J. Cancer Prev. 2007, 8, 445–451. [Google Scholar] [PubMed]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
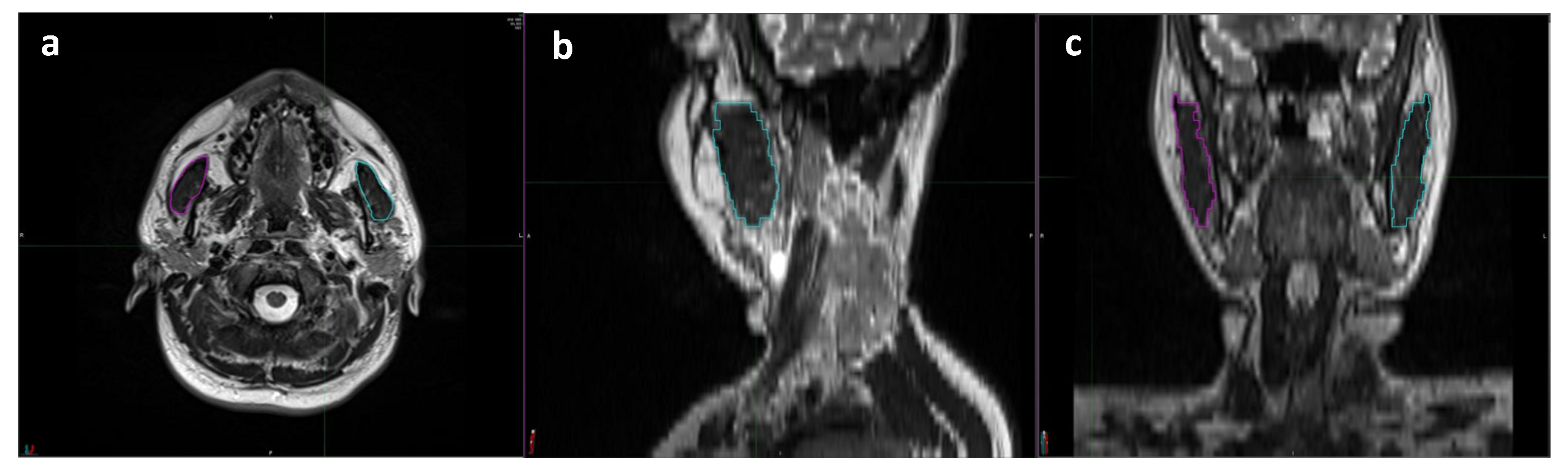
| Characteristics | All Patients (N = 112) | TMMV ≤ 35 cc (N = 43) | TMMV > 35 cc (N = 69) | p-Value |
|---|---|---|---|---|
| Median age, y (range) | 60 (18–79) | 59 (28–73) | 61 (18–79) | 0.82 |
| Age group, n (%) | 0.56 | |||
| ≥60 years | 57 (50.9) | 20 (46.5) | 37 (53.6) | |
| <60 years | 55 (49.1) | 23 (53.5) | 32 (46.4) | |
| Gender, N (%) | 0.29 | |||
| Female | 32 (28.6) | 28 (65.1) | 17 (24.6) | |
| Male | 80 (71.4) | 15 (34.9) | 52 (75.4) | |
| Smoking status, N (%) | 0.06 | |||
| No | 37 (33.0) | 19 (44.2) | 18 (26.1) | |
| Yes | 75 (67.0) | 24 (55.8) | 51 (73.9) | |
| Alcohol consumption, N (%) | 0.83 | |||
| No | 78 (69.6) | 29 (67.4) | 49 (71.0) | |
| Yes | 34 (30.4) | 14 (32.6) | 20 (29.0) | |
| Median pre-C-CRT MMO, mm (range) | 41.6 (37.0–46.8) | 41.0 (47.8–45) | 41.9 (38.9–46.8) | 0.027 * |
| Pre-C-CRT MMO group, N (%) | 0.05 * | |||
| ≤41.6 mm | 57 (50.9) | 27 (62.8) | 30 (43.5) | |
| >41.6 mm | 55 (49.1) | 16 (37.2) | 39 (56.5) | |
| T stage, N (%) | 0.70 | |||
| 1–2 | 47 (42.0) | 17 (39.5) | 30 (43.5) | |
| 3–4 | 65 (58.0) | 26 (60.5) | 39 (56.5) | |
| N stage, N (%) | 0.30 | |||
| 0–1 | 37 (33.0) | 17 (39.5) | 20 (29.0) | |
| 2–3 | 75 (67.0) | 26 (60.5) | 49 (71.0) |
| Characteristic | All Patients (N = 112) | TMMV ≤ 35 cc (N = 43) | TMMV > 35 cc (N = 69) | p-Value |
|---|---|---|---|---|
| Concurrent chemotherapy cycles, N (%) | 0.76 | |||
| 1 | 20 (17.6) | 8 (18.6) | 12 (17.4) | |
| 2–3 | 92 (82.4) | 35 (81.4) | 57 (82.6) | |
| Adjuvant chemotherapy cycles, N (%) | 0.32 | |||
| 0 | 29 (25.9) | 13 (30.2) | 16 (23.4) | |
| 1–2 | 83 (74.1) | 30 (69.8) | 53 (76.6) | |
| Mean MAD, Gy (range) | 35.2 (11.9–66.8) | 34.9 (11.9–65.7) | 35.6 (12.3–66.8) | 0.87 |
| MAD V56.5, N (%) | 0.54 | |||
| <34 Gy | 70 (62.5) | 28 (65.1) | 42 (60.7) | |
| ≥34 Gy | 42 (37.5) | 15 (34.9) | 27 (39.3) | |
| Mean C-CRT to RIT interval, mo. (range) | 9.5 (4–18) | 9.6 (4–18) | 13.8 (9–18) | 0.02 * |
| Median post-C-CRT MMO, mm (range) | 39.0 (25.0–42.0) | 34.0 (25.9–41.0) | 39.2 (30.7–42.0) | 0.05 |
| RIT, N (%) | ˂0.001 | |||
| Present | 28 (25.0) | 22 (51.2) | 6 (8.7) | |
| Absent | 84 (75.0) | 21 (48.8) | 63 (91.3) |
| Factors | RIT (%) | Univariate p-Value | Multivariate p-Value | HR (95% CI) |
|---|---|---|---|---|
| Age group (≥60 years vs. <60 years) | 19.3 vs. 30.9 | 0.19 | - | 1.54 (0.94–2.07) |
| Gender (male vs. female) | 22.5 vs. 31.3 | 0.34 | - | 1.28 (0.87–163) |
| Smoking status (no vs. yes) | 22.7 vs. 29.7 | 0.50 | - | 1.14 (0.96–1.33) |
| Alcohol consumption (no vs. yes) | 23.1 vs. 29.4 | 0.50 | - | 1.09 (0.78–1.76) |
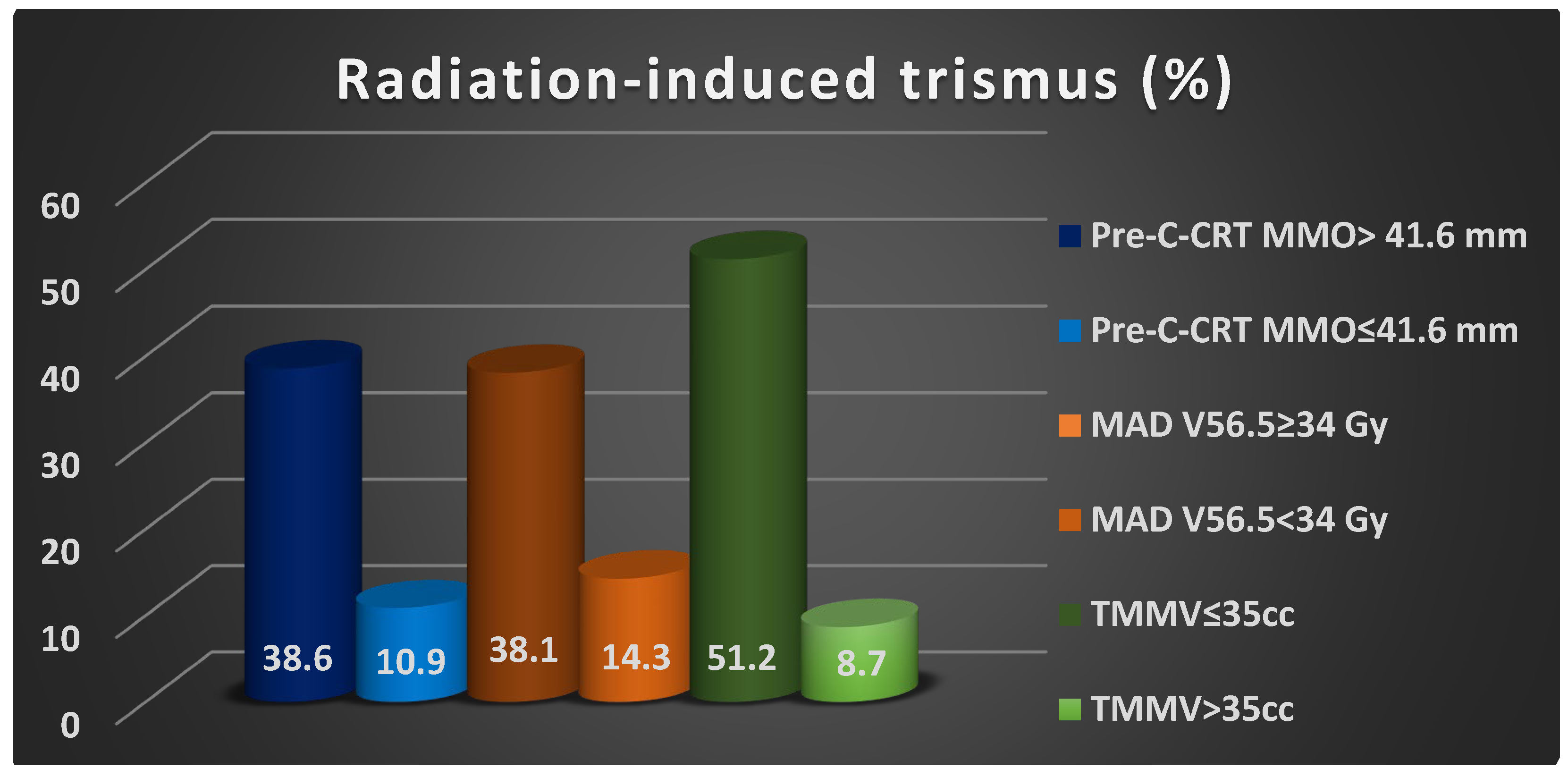
| Pre-C-CRT MMO group (≤41.6 mm vs. >41.6 mm) | 38.6 vs. 10.9 | 0.001 | 0.007 | 3.71 (2.68–5.19) |
| T-stage group (1–2 vs. 3–4) | 23.4 vs. 26.2 | 0.83 | - | 1.04 (0.88–1.23) |
| N-stage group (0–1 vs. 2–3) | 24.3 vs. 25.3 | 1.00 | - | 1.02 (0.95–1.07) |
| Concurrent chemotherapy cycles (1 vs. 2–3) | 23.3 vs. 26.1 | 0.87 | - | 1.12 (0.89–1.24) |
| Adjuvant chemotherapy cycles (0 vs. 1–2) | 24.1 vs. 25.3 | 0.61 | - | 1.07 (0.94–1.11) |
| MAD V56.5 group (<34 Gy vs. ≥34 Gy) | 14.3 vs. 38.1 | 0.002 | 0.004 | 2.58 (1.74–3.64) |
| TMMV group (≤35 cc vs. >35 cc) | 51.2 vs. 8.7 | ˂0.001 | <0.001 | 6.79 (4.87–9.16) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somay, E.; Topkan, E.; Pehlivan, U.A.; Yilmaz, B.; Besen, A.A.; Mertsoylu, H.; Pehlivan, B.; Selek, U. The Use of Pre-Chemoradiotherapy Total Masseter Muscle Volume as a Novel Predictor of Radiation-Induced Trismus in Locally Advanced Nasopharyngeal Carcinoma Patients. Tomography 2024, 10, 79-89. https://doi.org/10.3390/tomography10010007
Somay E, Topkan E, Pehlivan UA, Yilmaz B, Besen AA, Mertsoylu H, Pehlivan B, Selek U. The Use of Pre-Chemoradiotherapy Total Masseter Muscle Volume as a Novel Predictor of Radiation-Induced Trismus in Locally Advanced Nasopharyngeal Carcinoma Patients. Tomography. 2024; 10(1):79-89. https://doi.org/10.3390/tomography10010007
Chicago/Turabian StyleSomay, Efsun, Erkan Topkan, Umur Anil Pehlivan, Busra Yilmaz, Ali Ayberk Besen, Huseyin Mertsoylu, Berrin Pehlivan, and Ugur Selek. 2024. "The Use of Pre-Chemoradiotherapy Total Masseter Muscle Volume as a Novel Predictor of Radiation-Induced Trismus in Locally Advanced Nasopharyngeal Carcinoma Patients" Tomography 10, no. 1: 79-89. https://doi.org/10.3390/tomography10010007
APA StyleSomay, E., Topkan, E., Pehlivan, U. A., Yilmaz, B., Besen, A. A., Mertsoylu, H., Pehlivan, B., & Selek, U. (2024). The Use of Pre-Chemoradiotherapy Total Masseter Muscle Volume as a Novel Predictor of Radiation-Induced Trismus in Locally Advanced Nasopharyngeal Carcinoma Patients. Tomography, 10(1), 79-89. https://doi.org/10.3390/tomography10010007






