Prediction of the Benign or Malignant Nature of Pulmonary Pure Ground-Glass Nodules Based on Radiomics Analysis of High-Resolution Computed Tomography Images
Abstract
:1. Introduction
2. Materials and Methods
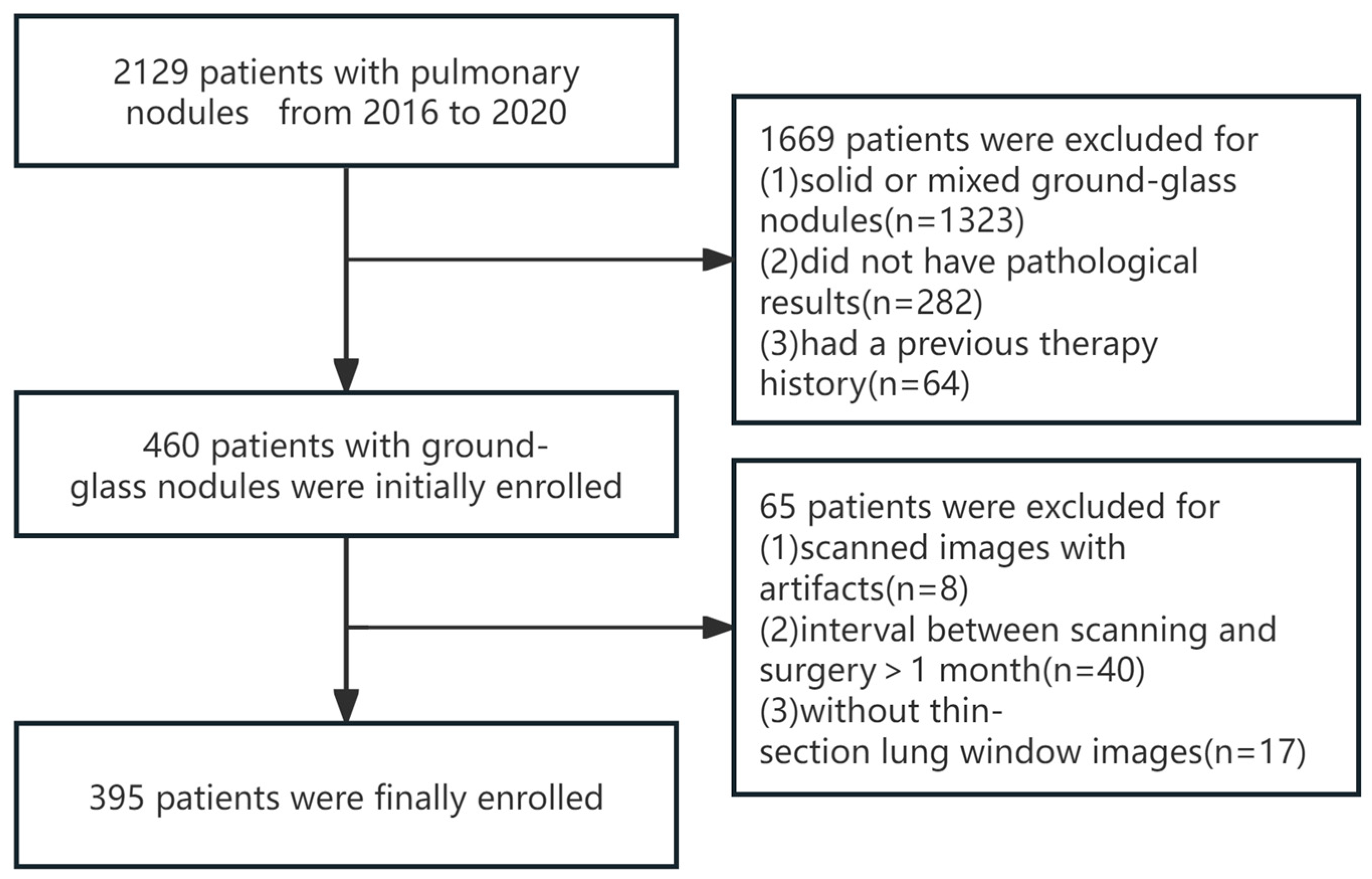
2.1. Study Population
2.2. Image Acquisition
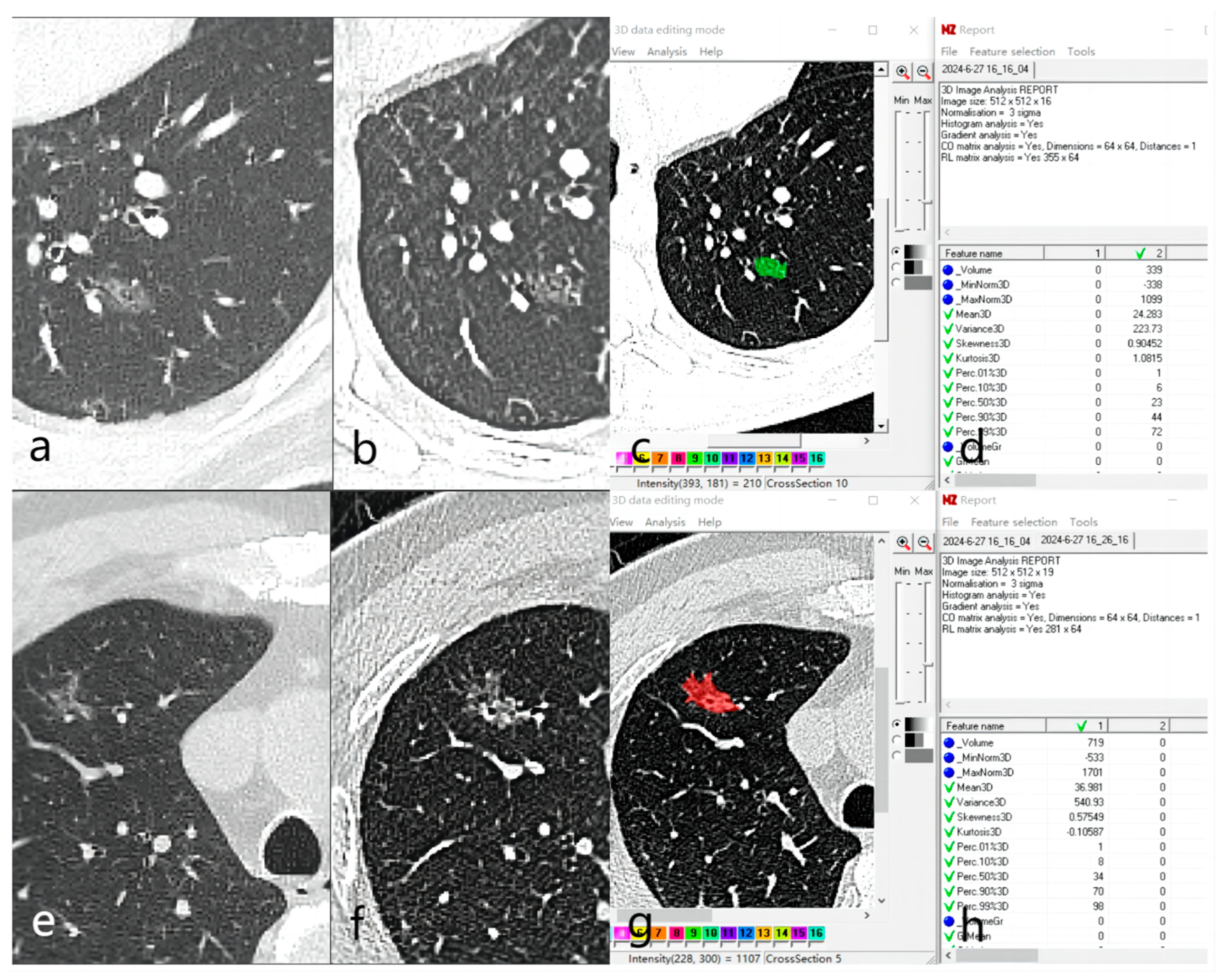
2.3. Image Analysis and Feature Extraction
2.4. Statistical Analysis
3. Results
3.1. General Information and CT Imaging Features
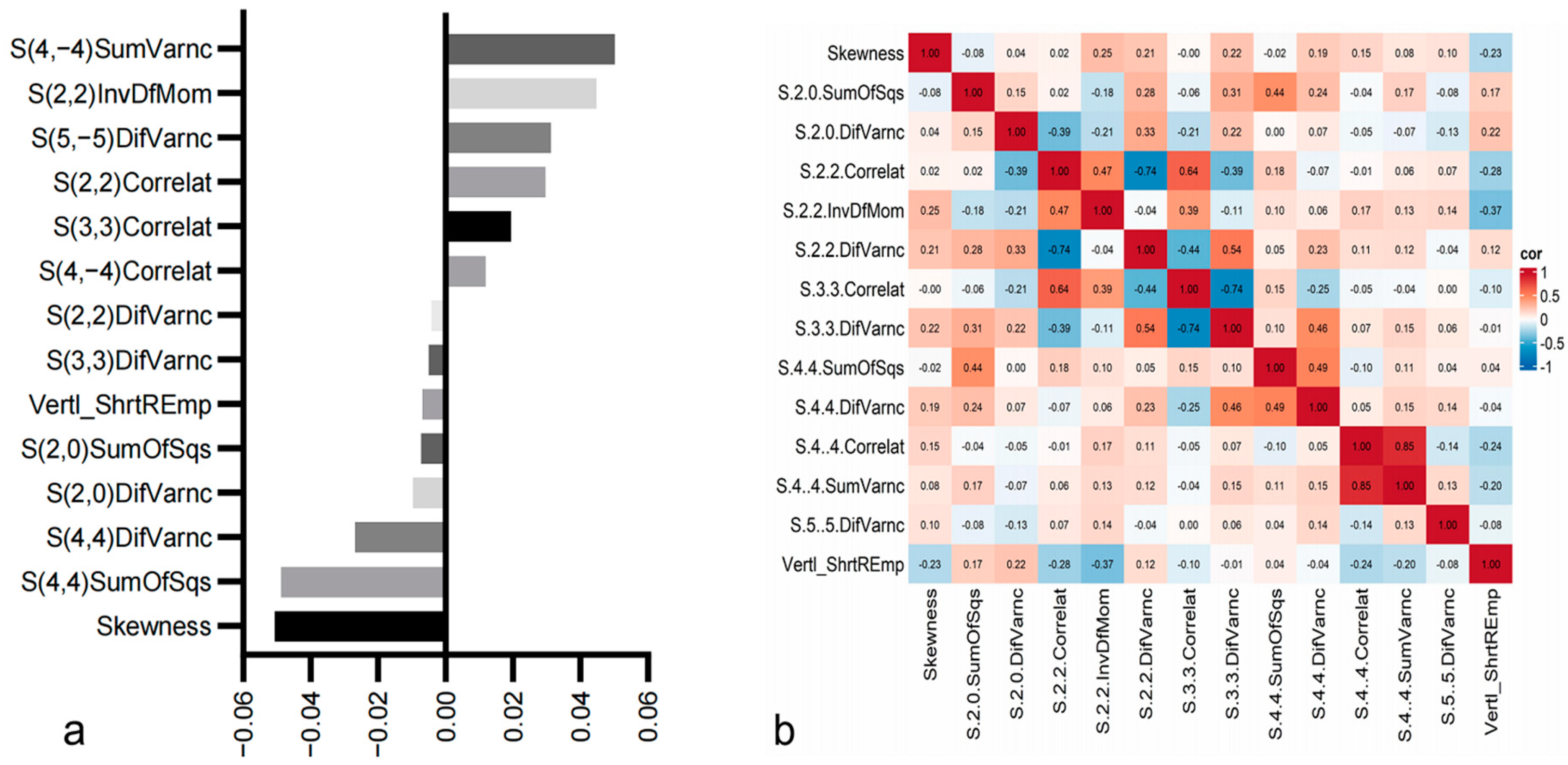
3.2. Radiomics Feature Analysis
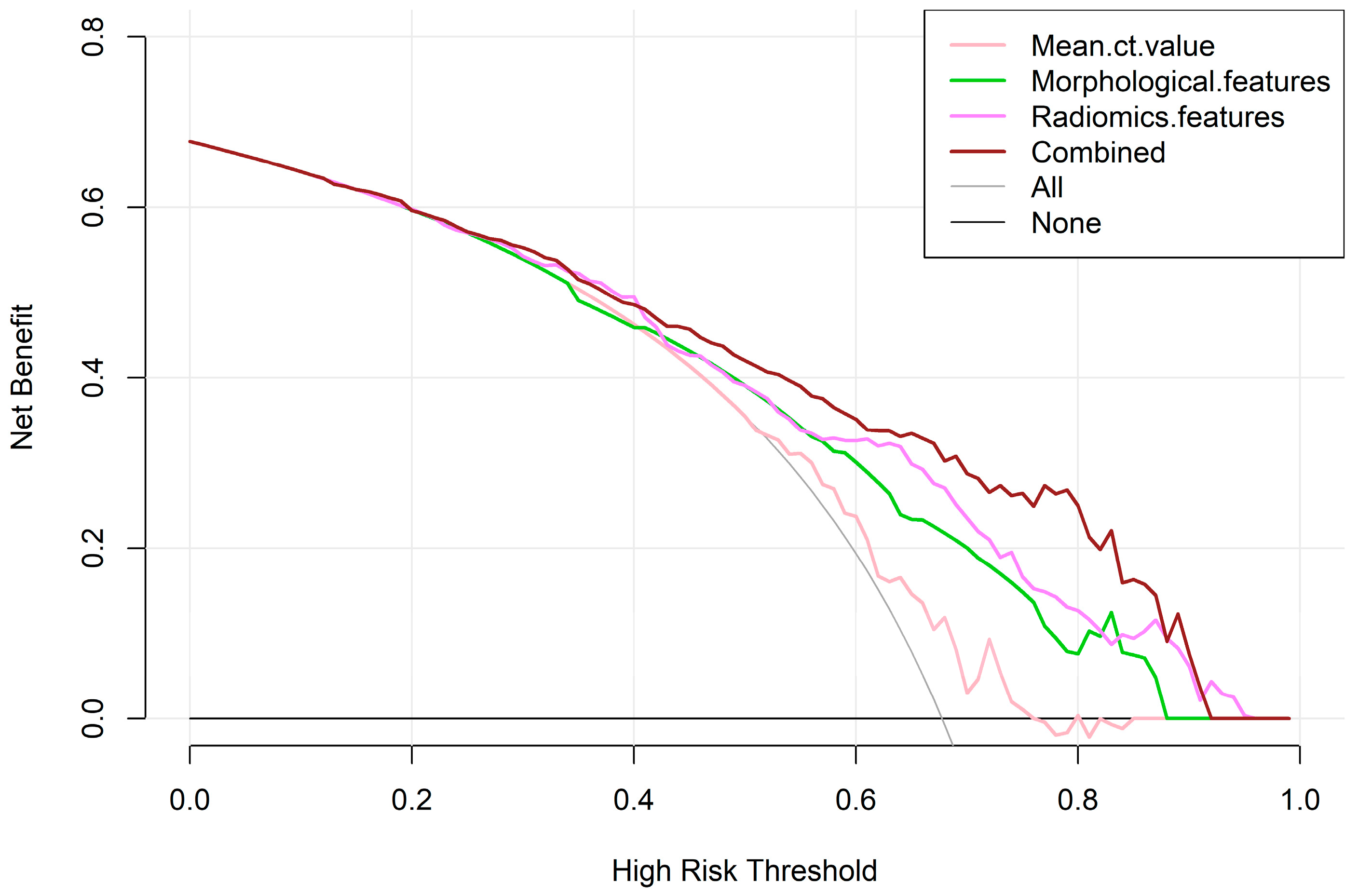
3.3. Construction and Diagnostic Performance of Predictive Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Austin, J.H.; Müller, N.L.; Friedman, P.J.; Hansell, D.M.; Naidich, D.P.; Remy-Jardin, M.; Webb, W.R.; Zerhouni, E.A. Glossary of Terms for CT of the Lungs: Recommendations of the Nomenclature Committee of the Fleischner Society. Radiology 1996, 200, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Choi, Y.-L.; Lee, K.S.; Han, J.; Zo, J.I.; Shim, Y.M.; Moon, J.W. Pure Ground-Glass Opacity Neoplastic Lung Nodules: Histopathology, Imaging, and Management. Am. J. Roentgenol. 2014, 202, W224–W233. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sun, Y.; Fang, W.; Qian, F.; Ye, J.; Chen, Q.; Jiang, Y.; Yu, K.; Han, B. High-Resolution Computed Tomography Features Distinguishing Benign and Malignant Lesions Manifesting as Persistent Solitary Subsolid Nodules. Clin. Lung Cancer 2018, 19, e75–e83. [Google Scholar] [CrossRef] [PubMed]
- Succony, L.; Rassl, D.M.; Barker, A.P.; McCaughan, F.M.; Rintoul, R.C. Adenocarcinoma Spectrum Lesions of the Lung: Detection, Pathology and Treatment Strategies. Cancer Treat. Rev. 2021, 99, 102237. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, A.; Tammemagi, M.C.; Mayo, J.R.; Roberts, H.; Liu, G.; Soghrati, K.; Yasufuku, K.; Martel, S.; Laberge, F.; Gingras, M.; et al. Probability of Cancer in Pulmonary Nodules Detected on First Screening CT. N. Engl. J. Med. 2013, 369, 910–919. [Google Scholar] [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef]
- Wood, D.E.; Kazerooni, E.A.; Baum, S.L.; Eapen, G.A.; Ettinger, D.S.; Hou, L.; Jackman, D.M.; Klippenstein, D.; Kumar, R.; Lackner, R.P.; et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 412–441. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Jin, K.-N.; Lee, J.-K.; Kim, D.K.; Chung, H.S.; Heo, E.Y.; Choi, S.H. Long-Term Follow-Up of Ground-Glass Nodules After 5 Years of Stability. J. Thorac. Oncol. 2019, 14, 1370–1377. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Fukui, T.; Ito, S.; Usami, N.; Hatooka, S.; Yatabe, Y.; Mitsudomi, T. How Long Should Small Lung Lesions of Ground-Glass Opacity Be Followed? J. Thorac. Oncol. 2013, 8, 309–314. [Google Scholar] [CrossRef]
- Liang, Z.-R.; Ye, M.; Lv, F.-J.; Fu, B.-J.; Lin, R.-Y.; Li, W.-J.; Chu, Z.-G. Differential Diagnosis of Benign and Malignant Patchy Ground-Glass Opacity by Thin-Section Computed Tomography. BMC Cancer 2022, 22, 1206. [Google Scholar] [CrossRef]
- Liu, A.; Wang, Z.; Yang, Y.; Wang, J.; Dai, X.; Wang, L.; Lu, Y.; Xue, F. Preoperative Diagnosis of Malignant Pulmonary Nodules in Lung Cancer Screening with a Radiomics Nomogram. Cancer Commun. 2020, 40, 16–24. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting More Information from Medical Images Using Advanced Feature Analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Zhang, T.; Yuan, M.; Zhong, Y.; Zhang, Y.-D.; Li, H.; Wu, J.-F.; Yu, T.-F. Differentiation of Focal Organising Pneumonia and Peripheral Adenocarcinoma in Solid Lung Lesions Using Thin-Section CT-Based Radiomics. Clin. Radiol. 2019, 74, 78.e23–78.e30. [Google Scholar] [CrossRef]
- Elia, S.; Pompeo, E.; Santone, A.; Rigoli, R.; Chiocchi, M.; Patirelis, A.; Mercaldo, F.; Mancuso, L.; Brunese, L. Radiomics and Artificial Intelligence Can Predict Malignancy of Solitary Pulmonary Nodules in the Elderly. Diagnostics 2023, 13, 384. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, M.; Ma, W.; Duan, S.; Huang, X.; Jin, L.; Tang, L.; Li, M. Preoperative Prediction of the Degree of Differentiation of Lung Adenocarcinoma Presenting as Sub-Solid or Solid Nodules with a Radiomics Nomogram. Clin. Radiol. 2022, 77, e680–e688. [Google Scholar] [CrossRef]
- Zhang, C.-R.; Wang, Q.; Feng, H.; Cui, Y.-Z.; Yu, X.-B.; Shi, G.-F. Computed-Tomography-Based Radiomic Nomogram for Predicting the Risk of Indeterminate Small (5–20 Mm) Solid Pulmonary Nodules. Diagn. Interv. Radiol. 2023, 29, 283–290. [Google Scholar] [CrossRef]
- He, R.; Yang, X.; Li, T.; He, Y.; Xie, X.; Chen, Q.; Zhang, Z.; Cheng, T. A Machine Learning-Based Predictive Model of Epidermal Growth Factor Mutations in Lung Adenocarcinomas. Cancers 2022, 14, 4664. [Google Scholar] [CrossRef]
- Sacconi, B.; Anzidei, M.; Leonardi, A.; Boni, F.; Saba, L.; Scipione, R.; Anile, M.; Rengo, M.; Longo, F.; Bezzi, M.; et al. Analysis of CT Features and Quantitative Texture Analysis in Patients with Lung Adenocarcinoma: A Correlation with EGFR Mutations and Survival Rates. Clin. Radiol. 2017, 72, 443–450. [Google Scholar] [CrossRef]
- Zhang, R.; Wei, Y.; Shi, F.; Ren, J.; Zhou, Q.; Li, W.; Chen, B. The Diagnostic and Prognostic Value of Radiomics and Deep Learning Technologies for Patients with Solid Pulmonary Nodules in Chest CT Images. BMC Cancer 2022, 22, 1118. [Google Scholar] [CrossRef]
- Sun, Q.; Huang, Y.; Wang, J.; Zhao, S.; Zhang, L.; Tang, W.; Wu, N. Applying CT Texture Analysis to Determine the Prognostic Value of Subsolid Nodules Detected during Low-Dose CT Screening. Clin. Radiol. 2019, 74, 59–66. [Google Scholar] [CrossRef]
- Kao, T.-N.; Hsieh, M.-S.; Chen, L.-W.; Yang, C.-F.J.; Chuang, C.-C.; Chiang, X.-H.; Chen, Y.-C.; Lee, Y.-H.; Hsu, H.-H.; Chen, C.-M.; et al. CT-Based Radiomic Analysis for Preoperative Prediction of Tumor Invasiveness in Lung Adenocarcinoma Presenting as Pure Ground-Glass Nodule. Cancers 2022, 14, 5888. [Google Scholar] [CrossRef] [PubMed]
- Strzelecki, M.; Szczypinski, P.; Materka, A.; Klepaczko, A. A Software Tool for Automatic Classification and Segmentation of 2D/3D Medical Images. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2013, 702, 137–140. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Cao, S.; Xu, R. Cancer Incidence, Mortality, and Burden in China: A Time-Trend Analysis and Comparison with the United States and United Kingdom Based on the Global Epidemiological Data Released in 2020. Cancer Commun. 2021, 41, 1037–1048. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global Surveillance of Trends in Cancer Survival 2000-14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Kuroda, H. Narrative Review of Optimal Prognostic Radiological Tools Using Computed Tomography for T1N0-Staged Non-Small Cell Lung Cancer. J. Thorac. Dis. 2021, 13, 3171–3181. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Weng, Q.; Hui, J.; Fang, S.; Wu, X.; Mao, W.; Chen, M.; Zheng, L.; Wang, Z.; Zhao, Z.; et al. Value of TSCT Features for Differentiating Preinvasive and Minimally Invasive Adenocarcinoma From Invasive Adenocarcinoma Presenting as Subsolid Nodules Smaller Than 3 Cm. Acad Radiol. 2020, 27, 395–403. [Google Scholar] [CrossRef]
- Li, Q.; Fan, L.; Cao, E.-T.; Li, Q.-C.; Gu, Y.-F.; Liu, S.Y. Quantitative CT Analysis of Pulmonary Pure Ground-Glass Nodule Predicts Histological Invasiveness. Eur. J. Radiol. 2017, 89, 67–71. [Google Scholar] [CrossRef]
- Bianconi, F.; Palumbo, I.; Fravolini, M.L.; Rondini, M.; Minestrini, M.; Pascoletti, G.; Nuvoli, S.; Spanu, A.; Scialpi, M.; Aristei, C.; et al. Form Factors as Potential Imaging Biomarkers to Differentiate Benign vs. Malignant Lung Lesions on CT Scans. Sensors 2022, 22, 5044. [Google Scholar] [CrossRef]
- Yang, Y.; Li, K.; Sun, D.; Yu, J.; Cai, Z.; Cao, Y.; Wu, J. Invasive Pulmonary Adenocarcinomas Versus Preinvasive Lesions Appearing as Pure Ground-Glass Nodules: Differentiation Using Enhanced Dual-Source Dual-Energy CT. AJR Am. J. Roentgenol. 2019, 213, W114–W122. [Google Scholar] [CrossRef]
- Gao, F.; Li, M.; Zhang, Z.; Xiao, L.; Zhang, G.; Zheng, X.; Hua, Y.; Li, J. Morphological Classification of Pre-Invasive Lesions and Early-Stage Lung Adenocarcinoma Based on CT Images. Eur. Radiol. 2019, 29, 5423–5430. [Google Scholar] [CrossRef]
- Liang, G.; Yu, W.; Liu, S.-Q.; Xie, M.-G.; Liu, M. The Value of Radiomics Based on Dual-Energy CT for Differentiating Benign from Malignant Solitary Pulmonary Nodules. BMC Med. Imaging 2022, 22, 95. [Google Scholar] [CrossRef]
- Gong, J.; Liu, J.; Hao, W.; Nie, S.; Wang, S.; Peng, W. Computer-Aided Diagnosis of Ground-Glass Opacity Pulmonary Nodules Using Radiomic Features Analysis. Phys. Med. Biol. 2019, 64, 135015. [Google Scholar] [CrossRef]
| Variables | Total (n = 276) | Benign (n = 89) | Malignant (n = 187) | Statistic | p |
|---|---|---|---|---|---|
| Age, mean ± SD | 52.42 ± 11.88 | 51.54 ± 12.30 | 52.83 ± 11.68 | t = −0.85 | 0.398 |
| Mean diameter, M (Q1, Q3) | 10.03 (7.68, 13.34) | 9.31 (7.67, 12.59) | 10.36 (7.72, 14.07) | Z = −1.53 | 0.127 |
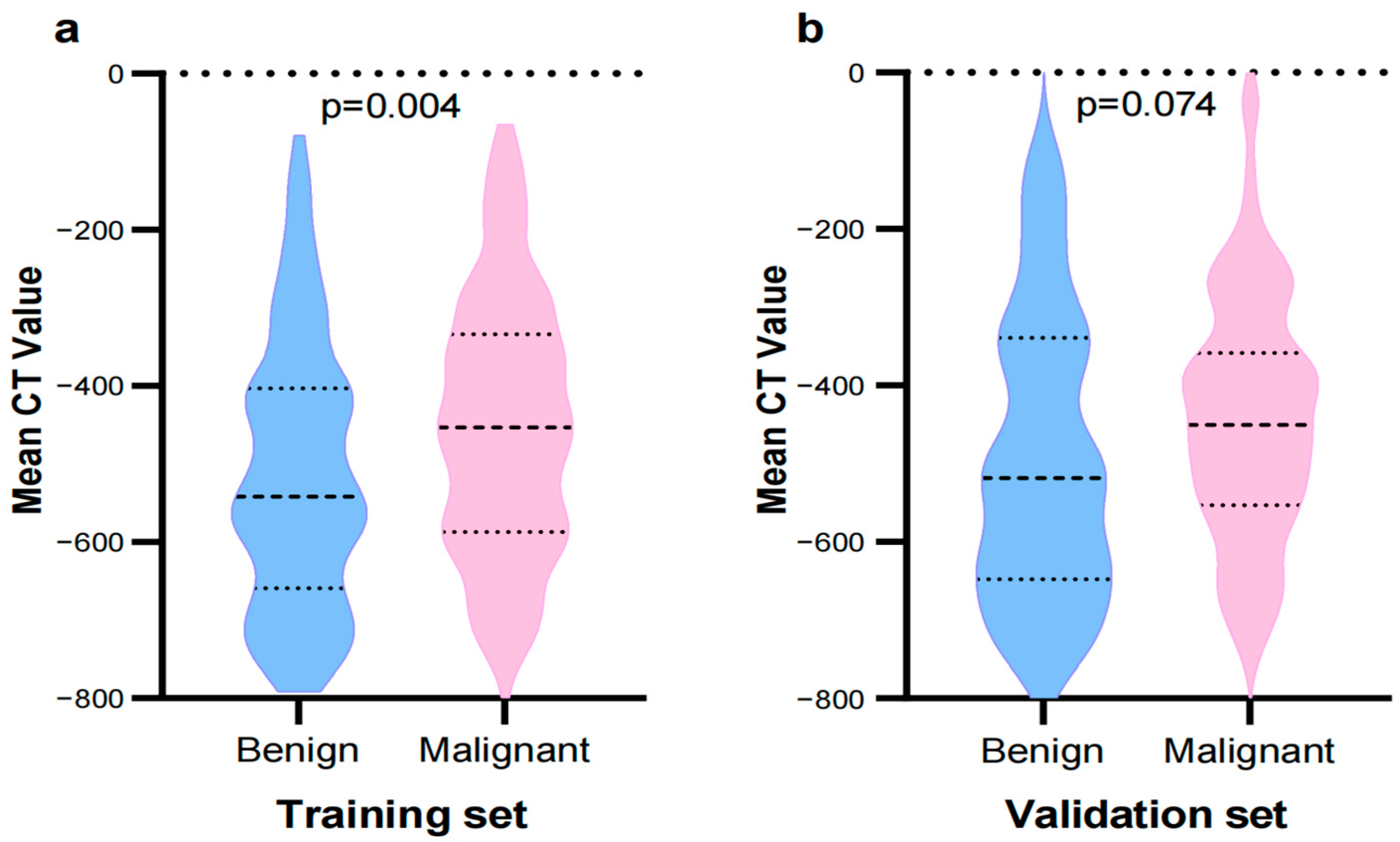
| Mean CT value, M (Q1, Q3) | −477.30 (−599.59, −356.35) | −541.56 (−647.60, −403.13) | −452.90 (−585.46, −337.68) | Z = −2.85 | 0.004 |
| Sex, n (%) | χ2 = 15.62 | <0.001 | |||
| female | 176 (63.77) | 42 (47.19) | 134 (71.66) | ||
| male | 100 (36.23) | 47 (52.81) | 53 (28.34) | ||
| Location, n (%) | χ2 = 5.50 | 0.24 | |||
| RUL | 96 (34.78) | 34 (38.20) | 62 (33.16) | ||
| RML | 23 (8.33) | 3 (3.37) | 20 (10.70) | ||
| RLL | 43 (15.58) | 17 (19.10) | 26 (13.90) | ||
| LUL | 71 (25.72) | 22 (24.72) | 49 (26.20) | ||
| LLL | 43 (15.58) | 13 (14.61) | 30 (16.04) | ||
| Shape, n (%) | χ2 = 0.50 | 0.481 | |||
| round or oval | 197 (71.38) | 66 (74.16) | 131 (70.05) | ||
| irregular | 79 (28.62) | 23 (25.84) | 56 (29.95) | ||
| Margin, n (%) | χ2 = 4.63 | 0.031 | |||
| not spiculated | 180 (65.22) | 66 (74.16) | 114 (60.96) | ||
| spiculated | 96 (34.78) | 23 (25.84) | 73 (39.04) | ||
| Tumor−lung interface, n (%) | χ2 = 14.82 | <0.001 | |||
| clear smooth | 130 (47.10) | 48 (53.93) | 82 (43.85) | ||
| clear rough | 78 (28.26) | 12 (13.48) | 66 (35.29) | ||
| blurry | 68 (24.64) | 29 (32.58) | 39 (20.86) | ||
| Pleural, n (%) | χ2 = 11.54 | <0.001 | |||
| no retraction | 199 (72.10) | 76 (85.39) | 123 (65.78) | ||
| retraction | 77 (27.90) | 13 (14.61) | 64 (34.22) | ||
| External vascular change, n (%) | χ2 = 2.66 | 0.103 | |||
| absence | 102 (36.96) | 39 (43.82) | 63 (33.69) | ||
| presence | 174 (63.04) | 50 (56.18) | 124 (66.31) | ||
| Internal vascular change, n (%) | χ2 = 14.10 | <0.001 | |||
| absence | 144 (52.17) | 61 (68.54) | 83 (44.39) | ||
| presence | 132 (47.83) | 28 (31.46) | 104 (55.61) | ||
| Vacuole sign, n (%) | χ2 = 1.90 | 0.168 | |||
| absence | 226 (81.88) | 77 (86.52) | 149 (79.68) | ||
| presence | 50 (18.12) | 12 (13.48) | 38 (20.32) |
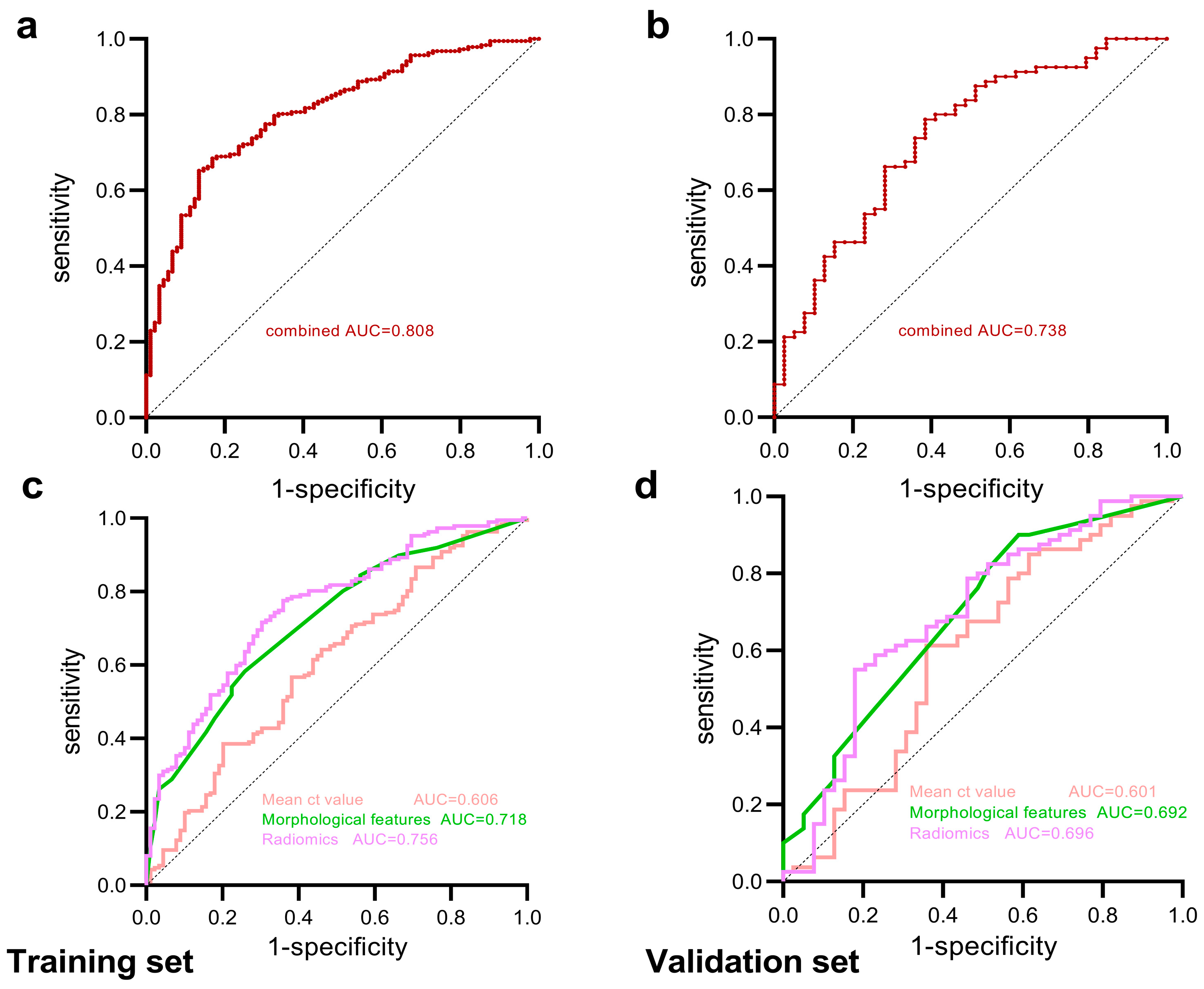
| Models | Area | 95% CI | Specificity | Sensitivity | NPV | PPV | FDR | FPR | |
|---|---|---|---|---|---|---|---|---|---|
| Training set | Mean CT value | 0.606 | 0.534–0.678 | 0.618 | 0.567 | 0.404 | 0.757 | 0.243 | 0.382 |
| Morphological features | 0.718 | 0.656–0.781 | 0.742 | 0.583 | 0.458 | 0.826 | 0.174 | 0.258 | |
| Radiomics features | 0.756 | 0.696–0.815 | 0.640 | 0.775 | 0.576 | 0.819 | 0.181 | 0.360 | |
| Combined | 0.808 | 0.755–0.861 | 0.865 | 0.652 | 0.542 | 0.910 | 0.090 | 0.135 | |
| Validation set | Mean CT value | 0.601 | 0.486–0.717 | 0.641 | 0.613 | 0.446 | 0.778 | 0.222 | 0.359 |
| Morphological features | 0.692 | 0.589–0.795 | 0.410 | 0.900 | 0.667 | 0.758 | 0.242 | 0.590 | |
| Radiomics features | 0.696 | 0.590–0.802 | 0.821 | 0.550 | 0.471 | 0.863 | 0.137 | 0.179 | |
| Combined | 0.738 | 0.641–0.835 | 0.615 | 0.788 | 0.585 | 0.808 | 0.192 | 0.385 |
| Models | Statistic | p | 95% Confidence Interval |
|---|---|---|---|
| Combined vs. Radiomics | 2.509 | 0.012 | 0.011 to 0.093 |
| Combined vs. Morphological | 3.500 | <0.001 | 0.039 to 0.139 |
| Combined vs. Mean CT value | 5.016 | <0.001 | 0.123 to 0.281 |
| Radiomics vs. Morphological | 0.881 | 0.378 | −0.046 to 0.119 |
| Radiomics vs. Mean CT value | 3.561 | <0.001 | 0.067 to 0.232 |
| Morphological vs. Mean CT value | 2.548 | 0.011 | 0.026 to 0.199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ping, X.; Jiang, N.; Meng, Q.; Hu, C. Prediction of the Benign or Malignant Nature of Pulmonary Pure Ground-Glass Nodules Based on Radiomics Analysis of High-Resolution Computed Tomography Images. Tomography 2024, 10, 1042-1053. https://doi.org/10.3390/tomography10070078
Ping X, Jiang N, Meng Q, Hu C. Prediction of the Benign or Malignant Nature of Pulmonary Pure Ground-Glass Nodules Based on Radiomics Analysis of High-Resolution Computed Tomography Images. Tomography. 2024; 10(7):1042-1053. https://doi.org/10.3390/tomography10070078
Chicago/Turabian StylePing, Xiaoxia, Nan Jiang, Qian Meng, and Chunhong Hu. 2024. "Prediction of the Benign or Malignant Nature of Pulmonary Pure Ground-Glass Nodules Based on Radiomics Analysis of High-Resolution Computed Tomography Images" Tomography 10, no. 7: 1042-1053. https://doi.org/10.3390/tomography10070078
APA StylePing, X., Jiang, N., Meng, Q., & Hu, C. (2024). Prediction of the Benign or Malignant Nature of Pulmonary Pure Ground-Glass Nodules Based on Radiomics Analysis of High-Resolution Computed Tomography Images. Tomography, 10(7), 1042-1053. https://doi.org/10.3390/tomography10070078







