Metabolic Differences in Neuroimaging with [18F]FDG in Rats Under Isoflurane and Hypnorm–Dormicum
Abstract
:1. Introduction
2. Materials and Methods
2.1. License, Power and Design
2.2. Animals and Housing
2.3. Anesthesia and Monitoring
2.4. [18F]FDG PET/MR Imaging
2.5. PET Data Analyses
2.6. Voxel-Based Analysis
2.7. Statistics
3. Results
3.1. General Study Information
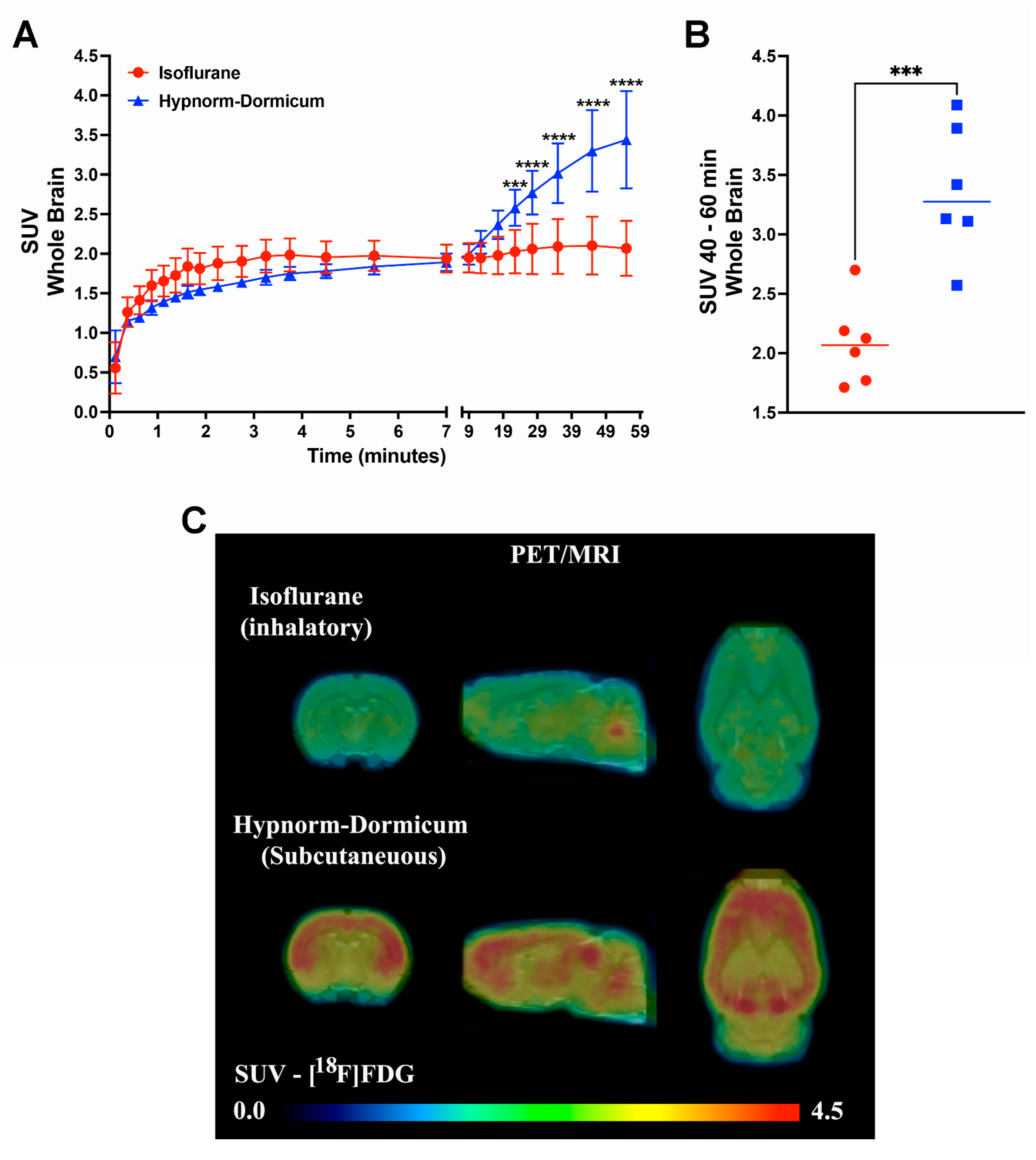
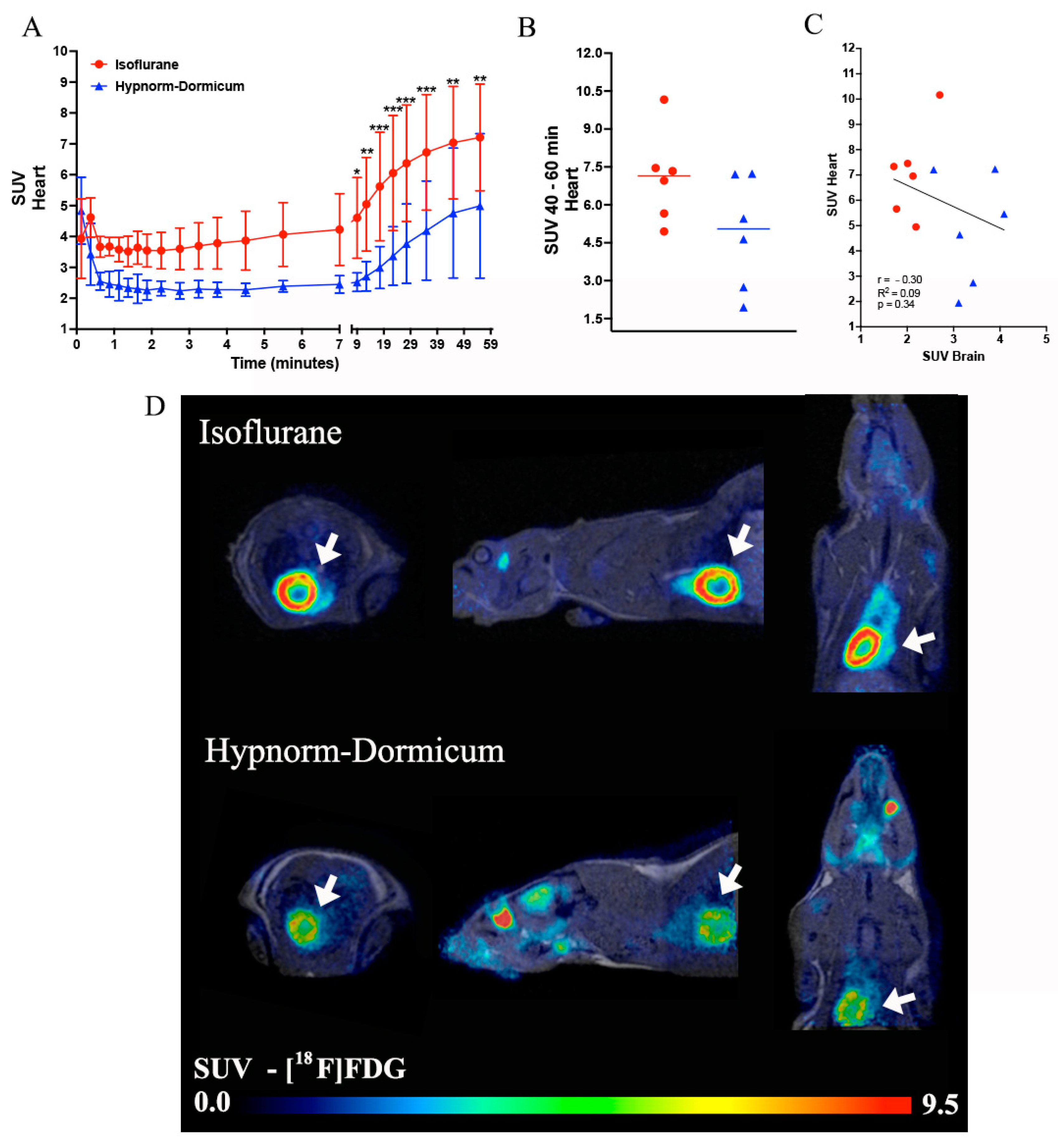
3.2. [18F]FDG PET Imaging—Standardized Uptake Value (SUV)
3.2.1. Brain Uptake
3.2.2. Heart Uptake
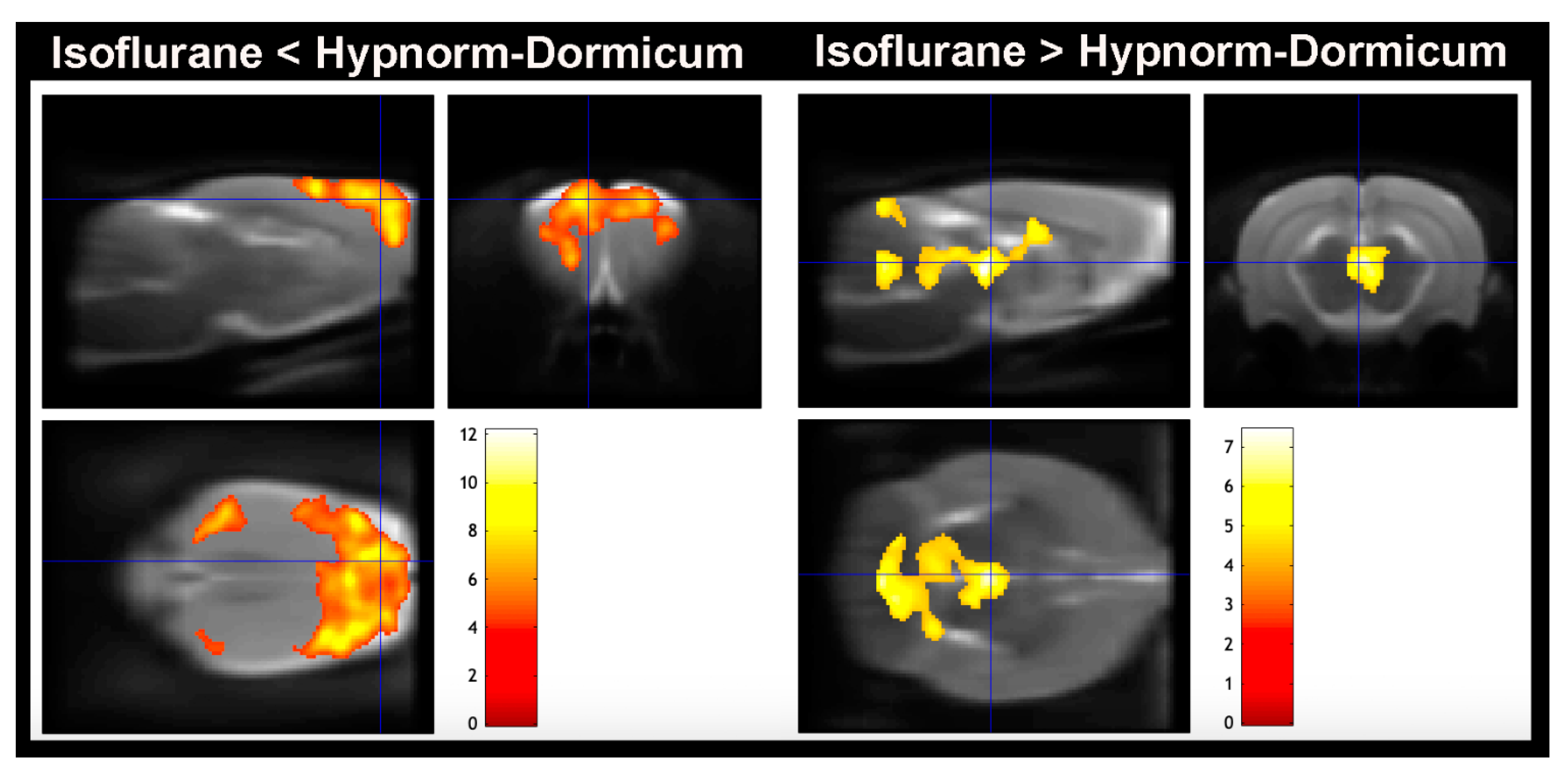
3.3. [18F]FDG PET Imaging—Voxel-Based Analysis—Brain
4. Discussion
4.1. Overview
4.2. Possible Causes of the Anesthetic Effects
4.3. Brain Regions Uptake Difference
4.4. Other Parameters
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tremoleda, J.L.; Kerton, A.; Gsell, W. Anaesthesia and physiological monitoring during in vivo imaging of laboratory rodents: Considerations on experimental outcomes and animal welfare. EJNMMI Res. 2012, 2, 44. [Google Scholar] [CrossRef] [PubMed]
- Alstrup, A.K.O.; Smith, D.F. Anaesthesia for positron emission tomography scanning of animal brains. Lab. Anim. 2013, 47, 12–18. [Google Scholar] [CrossRef]
- Schulz, D.; Southekal, S.; Junnarkar, S.S.; Pratte, J.F.; Purschke, M.L.; Stoll, S.P.; Ravindranath, B.; Maramraju, S.H.; Krishnamoorthy, S.; Henn, F.A.; et al. Simultaneous assessment of rodent behavior and neurochemistry using a miniature positron emission tomograph. Nat. Meth. 2011, 8, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A. PET brain imaging of awake small animals. Doctor Thesis, University of Antwerp, Antwerp, Belgium, 2018. [Google Scholar]
- Miranda, A.; Kroll, T.; Schweda, V.; Staelens, S.; Verhaeghe, J. Correction of motion tracking errors for PET head rigid motion correction. Phys. Med. Biol. 2023, 68, 175009. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Bertoglio, D.; Stroobants, S.; Staelens, S.; Verhaeghe, J. Translation of preclinical PET imaging findings: Challenges and motion correction to overcome the confounding effects of anesthesia. Front. Med. 2021, 8, 753977. [Google Scholar] [CrossRef]
- Momosaki, S.; Hatano, K.; Kawasumi, Y.; Kato, T.; Hosoi, R.; Kobayashi, K.; Inoue, O.; Ito, K. Rat-PET study without anesthesia: Anesthetics modify the dopamine D1 receptor binding in rat brain. Synapse 2004, 54, 207–213. [Google Scholar] [CrossRef]
- Lasbennes, F.; Lestage, P.; Bobillier, P.; Seylaz, J. Stress and local cerebral blood flow: Studies on restrained and unrestrained rats. Exp. Brain Res. 1986, 63, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.D.; Lee, D.E.; Alexoff, D.L.; Dewey, S.L.; Schiffer, W.K. Imaging dopamine release with positron emission tomography (PET) and 11C-raclopride in freely moving animals. Neuroimage 2008, 41, 1051–1066. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen & Co Ltd.: London, UK, 1959. [Google Scholar]
- Kepes, Z.; Arato, V.; Csikos, C.; Hegedus, E.; Esze, R.; Nagy, T.; Joszai, I.; Emri, M.; Kertesz, I.; Trencsenyi, G. Evaluation of brain [18F]F-FDG uptake pattern under different anaesthesia protocols. In Vivo 2024, 38, 587–597. [Google Scholar] [CrossRef]
- Severance, A.J.; Parsey, R.V.; Kumar, J.S.; Underwood, M.D.; Arango, V.; Majo, V.J.; Prabhakaran, J.; Simpson, N.R.; Van Heertum, R.L.; Mann, J.J. In vitro and in vivo evaluation of [11C]MPEPy as a potential PET ligand for mGlu5 receptors. Nucl. Med. Biol. 2006, 33, 1021–1027. [Google Scholar] [CrossRef]
- Todd, M.M.; Weeks, J. Comparative effects of propofol, pentobarbital, and isoflurane om cerebral blood flow and blood volume. J. Neurosurg. Anesthesiol. 1996, 8, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Lagerkranser, M.; Stånge, K.; Sollevi, A. Effects of propofol on cerebral blood flow, metabolim, and cerebral autoregulation in the anesthetized pig. J. Neurosurg. Anesthesiol. 1997, 9, 188–193. [Google Scholar] [CrossRef]
- Noda, A.; Takamatsu, H.; Minoshima, S.; Tsukada, H.; Nishimura, S. Determination of kinetic rate constants for 2-[18F]fluoro-2-deoxy-D-glucose and partition coefficient of water in conscious macaques and alterations in aging or anesthesia examined on parametric images with an anatomic standardization technique. J. Cereb. Blood Flow Metab. 2003, 23, 1441–1447. [Google Scholar] [CrossRef]
- Toyama, H.; Ichise, M.; Liow, J.S.; Modell, K.J.; Vines, D.C.; Esaki, T.; Cook, M.; Seidel, J.; Sokoloff, L.; Green, M.V.; et al. Absolute quantification of regional cerebral glucose utilization in mice by 18F-FDG small animal PET scanning and 2-14C-DG autoradiography. J. Nucl. Med. 2004, 45, 1398–1405. [Google Scholar] [PubMed]
- Mizuma, H.; Shukuri, M.; Hayashi, T.; Watanabe, Y.; Onoe, H. Establishment of in vivo brain imaging in conscious mice. J. Nucl. Med. 2010, 51, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Ko, J.; Lee, A.R.; Lee, I.H.; Jung, M.A.; Austin, B.; Chung, H.; Nahm, S.; Eom, K. Effects of anesthetic protocol on normal canine brain uptake of 18F-FDG assessed by PET/CT. Vet. Radiol. Ultrasound 2010, 51, 130–135. [Google Scholar] [PubMed]
- Lee, Y.A.; Kim, J.I.; Lee, J.W.; Cho, Y.J.; Lee, B.H.; Chung, H.W.; Park, K.K.; Han, J.S. Effects of various anesthetic protocols on 18F-fluorodeoxyglucose uptake into the brain and hearts of normal miniature pigs (Sus scrofa domestica). J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 246–252. [Google Scholar] [PubMed]
- Park, T.Y.; Nishida, K.S.; Wilson, C.M.; Jaiswal, S.; Scott, J.; Hoy, A.R.; Selwyn, R.G.; Dardzinski, B.J.; Choi, K.H. Effects of isoflurane anaesthesia and intravenous morphine selv-administration on regional glucose metabolism ([18F]FDG-PET) of male Sprague-Dawley rats. Eur. J. Neurosci. 2017, 45, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Alstrup, A.K.O.; Simonsen, M.; Landau, A.M. Type of anesthesia influences positron emission tomography measurements of dopamine D2/3 receptor binding in the rat brain. Scand. J. Lab. Anim. Sci. 2011, 38, 195–200. [Google Scholar]
- Lind, N.M.; Olsen, A.K.; Moustgaard, A.; Jensen, S.B.; Jakobsen, S.; Hansen, A.K.; Arnfred, S.M.; Hemmingsen, R.P.; Gjedde, A.; Cumming, P. Mapping the amphetamine-evoked dopamine release in the brain of the Göttingen minipig. Brain Res. Bull. 2005, 65, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.; Simonsen, M.; Østergaard, S.D.; Munk, O.L.; Rosa-Neto, P.; Olsen, A.K.; Jensen, S.B.; Møller, A.; Cumming, P. Mapping the amphetamine-evoked changes in [11C]raclopride binding in living rat using small animal PET: Modulation by MAO-inhibition. Neuroimage 2007, 35, 38–46. [Google Scholar] [CrossRef]
- Elfving, B.; Bjørnholm, B.; Knudsen, G.M. Interference of anaesthetics with radioligand binding in neuroreceptor studies. Eu. J. Nucl. Med. Mol. Imag. 2003, 30, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Spelta, L.E.W.; Real, C.C.; Buchpiguel, C.A.; de Paula, F.D.; Marcourakis, T.J. [18F]FDG brain uptake of C57/Bl/6 male mice is affected by locomotor activity after cocaine use: A small animal positron emission tomography study. Neurosci. Res. 2022, 100, 1876–1889. [Google Scholar] [CrossRef]
- Busk, M.; Munk, O.L.; Jakobsen, S.; Frøkiær, J.; Overgaard, J.; Horsman, M.R. FDG-PET reproducibility in tumor-bearing mice: Comparing a traditional SUV approach with a tumor-to-brain tissue ratio approach. Acta. Oncol. 2017, 56, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Jødal, L.; Afzelius, P.; Alstrup, A.K.O.; Jensen, S.B. Radiotracers for bone marrow infection imaging. Molecules 2021, 26, 3159. [Google Scholar] [CrossRef]
- Festing, M.F. On determining sample size in experiments involving laboratory animals. Lab. Anim. 2018, 52, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Guillen, J. FELASA Guidelines and Recommendations. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 311–321. [Google Scholar]
- de Paula, F.D.; Estessi de Souza, L.; Duran, F.L.S.; Buchpiguel, C.A.; Britto, L.R.; Crippa, J.A.S.; Filho, G.B.; Real, C.C. Cannabidiol treatment improves glucose metabolism and memory in Streptozotocin-induced Alzheimer’s disease rat model: A Proof-of-concept study. Int. J. Mol. Sci. 2022, 23, 1076. [Google Scholar] [CrossRef]
- Wei, Z.; Roh, S.-E.; Yang, X.; Wang, W.; Wang, J.; Chen, L.; Li, Y.; Bibic, A.; Lu, H. The impact of isoflurane anesthesia on brain metabolism in mice: An MRI and electroencephalography study. NMR Biomed. 2024, 37, e5260. [Google Scholar] [CrossRef] [PubMed]
- Flecknell, P.A.; Mitchell, M. Midazolam and fentanyl-fluanisone: Assessment of anaesthetic effects in laboratory rodents and rabbits. Lab. Anim. 1984, 18, 143–146. [Google Scholar] [CrossRef]
- Driesen, N.R.; Herman, P.; Rowland, M.A.; Thompson, G.; Qiu, M.; He, G.; Fineberg, S.; Barron, D.S.; Helgeson, L.; Lacadie, C.; et al. Ketamine effects on energy metabolism, functional connectivity and working memory in healthy humans. bioRxiv 2023. [Google Scholar] [CrossRef]
- Thiel, A.; Zickmann, B.; Zimmermann, R.; Hempelmann, G. Transcranial doppler sonography; effects of halothale, enflurane and isoflurane on blood flow velocity in the middle cerebral artery. Br. J. Anaesth. 1992, 68, 388–393. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.W.; Kirsch, J.R.; Tobin, J.R.; Ghaly, R.F.; Traystman, R.J. Cerebral blood flow in primates is increased by isoflurane over time and is descreased by nitric oxide synthase inhibition. Anesthesiology 1994, 80, 132–1327. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.; Juge, O.; Morel, D. Effects of midazolam on cerebral blood flow in human volunteers. Anesthesiology 1982, 56, 453–455. [Google Scholar] [CrossRef]
- Eftekhari, S.; Westgate, C.S.J.; Johansen, K.P.; Bruun, S.R.; Jensen, R.H. Long-term monitoring of intracranial pressure in freely-moving rats: Impact of different physiological states. Fluids Barriers CNS 2020, 17, 39. [Google Scholar] [CrossRef]
- Mannheim, J.G.; Kara, F.; Doorduin, J.; Fuchs, K.; Reischl, G.; Liang, S.; Verhoye, M.; Gremse, F.; Mezzanotte, L.; Huisman, M.C. Standardization of small animal imaging–current status and future prospects. Mol. Imaging Biol. 2017, 20, 716–731. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.K.; Challet, E.; Kalsbeek, A. Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol. Cell. Endocrinol. 2015, 418, 74–88. [Google Scholar]
- Bárez-López, S.; Gadd, G.J.; Pauža, A.G.; Murphy, D.; Greenwood, M.P. Isofluran rapidly modifies synaptic and cytoskeletal phoshoproteomes of supraoptic nucleus of the hypothalamus and the cortex. Neuroendocrinology 2023, 113, 1008–1023. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Fujino, Y.; Furue, H. Anesthesia and surgery induce a functional decrease in excitatory synaptic transmission in prefrontal cortex neurons, and intraoperative administration of dexmedotomidine does not elicit the synaptic dysfunction. Biochem. Biophys. Res. Commun. 2021, 572, 27–34. [Google Scholar] [CrossRef]
- Makaryus, R.; Lee, H.; Yu, M.; Zhang, S.; Smith, S.D.; Rebecchi, M.; Glass, P.S.; Benveniste, H. The metabolomic profile during isoflurane anesthesia differs from propofol anesthesia in the live rodent brain. J. Cereb. Blood Flow Metab. 2011, 31, 1432–1442. [Google Scholar] [CrossRef]
- Boritius, S.; Tammer, R.; Michaelis, T.; Brockmøller, J.; Frahm, J. Halogenated volatile anaesthetics alter brain metabolism as revealed by proton magnetic resonance spectroscopy of mice in vivo. Neuroimage 2013, 69, 244–255. [Google Scholar] [CrossRef]
- Litaudon, P.; Bouillot, C.; Zimmer, L.; Costes, N.; Ravel, N. Activity in the rat olfactory cortex is correlated with behavioral response to odor: A microPET study. Brain Struct. Funct. 2017, 222, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Jong, W.M.C.; Zuurbier, C.J.; Winter, R.J.D.; Heuvel, D.A.F.V.D.; Reitsma, P.H.; Cate, H.T.; Ince, C. Fentanyl-fluanisone-midazolam combination results in more stable hemodynamics than does urethane alpha-chloralose and 2,2,2-tribromoethanol in mice. Contemp. Top. Lab. Anim. Sci. 2002, 41, 28–32. [Google Scholar]
- Toyama, H.; Ichise, M.; Liow, J.S.; Vines, D.C.; Seneca, N.M.; Modell, K.J.; Seidel, J.; Green, M.V.; Innis, R.B. Evaluation of anesthesia effects on [18F]FDG uptake in mouse brain and heart using small animal PET. Nucl. Med. Biol. 2004, 31, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Pertiwi, K.R.; Hillman, R.M.; Scott, C.A.; Chilton, E.L. Ischemia Reperfusion Injury Produces, and Ischemic Preconditioning Prevents, Rat Cardiac Fibroblast Differentiation: Role of K(ATP) Channels. J. Cardiovasc. Dev. Dis. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.E.; Wang, X.; Jin, J.H. Mechanisms of cardioprotection by isoflurane against I/R injury. Front. Biosci. 2013, 18, 387–393. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Fidler, J.A.; Meng, Q.C.; Eckenhoff, R.G.; Garcia, P.S. Sex effects on behavioral markers of emergence from propofol and isoflurane anesthesia in rats. Behav. Brain Res. 2019, 367, 59–67. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Isoflurane | N | Hypnorm–Dormicum | N | p-Value |
|---|---|---|---|---|---|
| Weight [g] | 274 (20) | 6 | 273 (23) | 6 | 0.937 |
| [18F]FDG [MBq] | 29 (6) | 6 | 28 (4) | 6 | 0.822 |
| Pulse [min−1] | 381 (22) | 6 | 371 (17) | 6 | 0.376 |
| Respiration rate [min−1] | 48 (10) | 6 | 58 (12) | 6 | 0.125 |
| Temperature [°C] | 36.9 (0.2) | 6 | 36.5 (0.6) | 6 | 0.113 |
| Cluster Level | Voxel Level | Coordinates | Brain Area | |||||
|---|---|---|---|---|---|---|---|---|
| PFWE-Corr | kE | T | Puncorr | x | y | z | ||
| Isoflurane < Hypnorm–Dormicum | <0.0001 | 20,055 | 12.15 | <0.0001 | 5.4 | 0.8 | −4.2 | Right Primary somatosensorial cortex |
| 9.6 | <0.0001 | −3.4 | 2.4 | −1.6 | Left Primary motor cortex | |||
| 5.49 | <0.0001 | 3.4 | −6.6 | −1 | Right Primary motor cortex | |||
| <0.0001 | 1207 | 8.25 | <0.0001 | −3 | −6 | −1 | Left visual cortex | |
| 0.025 | 465 | 6.08 | <0.0001 | 7 | −3 | −3.6 | Right Primary motosensorial cortex | |
| Isoflurane > Hypnorm–Dormicum | <0.0001 | 6480 | 7.44 | <0.0001 | 0.2 | −5.6 | −5.8 | Dorsomedial periaqueductal gray |
| 0.088 | 313 | 5.86 | <0.0001 | −0.4 | −11.8 | −2 | Left molecular layer cerebellum | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alstrup, A.K.O.; Simonsen, M.; Hansen, K.V.; Real, C.C. Metabolic Differences in Neuroimaging with [18F]FDG in Rats Under Isoflurane and Hypnorm–Dormicum. Tomography 2025, 11, 4. https://doi.org/10.3390/tomography11010004
Alstrup AKO, Simonsen M, Hansen KV, Real CC. Metabolic Differences in Neuroimaging with [18F]FDG in Rats Under Isoflurane and Hypnorm–Dormicum. Tomography. 2025; 11(1):4. https://doi.org/10.3390/tomography11010004
Chicago/Turabian StyleAlstrup, Aage Kristian Olsen, Mette Simonsen, Kim Vang Hansen, and Caroline C. Real. 2025. "Metabolic Differences in Neuroimaging with [18F]FDG in Rats Under Isoflurane and Hypnorm–Dormicum" Tomography 11, no. 1: 4. https://doi.org/10.3390/tomography11010004
APA StyleAlstrup, A. K. O., Simonsen, M., Hansen, K. V., & Real, C. C. (2025). Metabolic Differences in Neuroimaging with [18F]FDG in Rats Under Isoflurane and Hypnorm–Dormicum. Tomography, 11(1), 4. https://doi.org/10.3390/tomography11010004






