Variability Between Radiation-Induced Cancer Risk Models in Estimating Oncogenic Risk in Intensive Care Unit Patients
Abstract
:1. Introduction
2. Materials and Methods
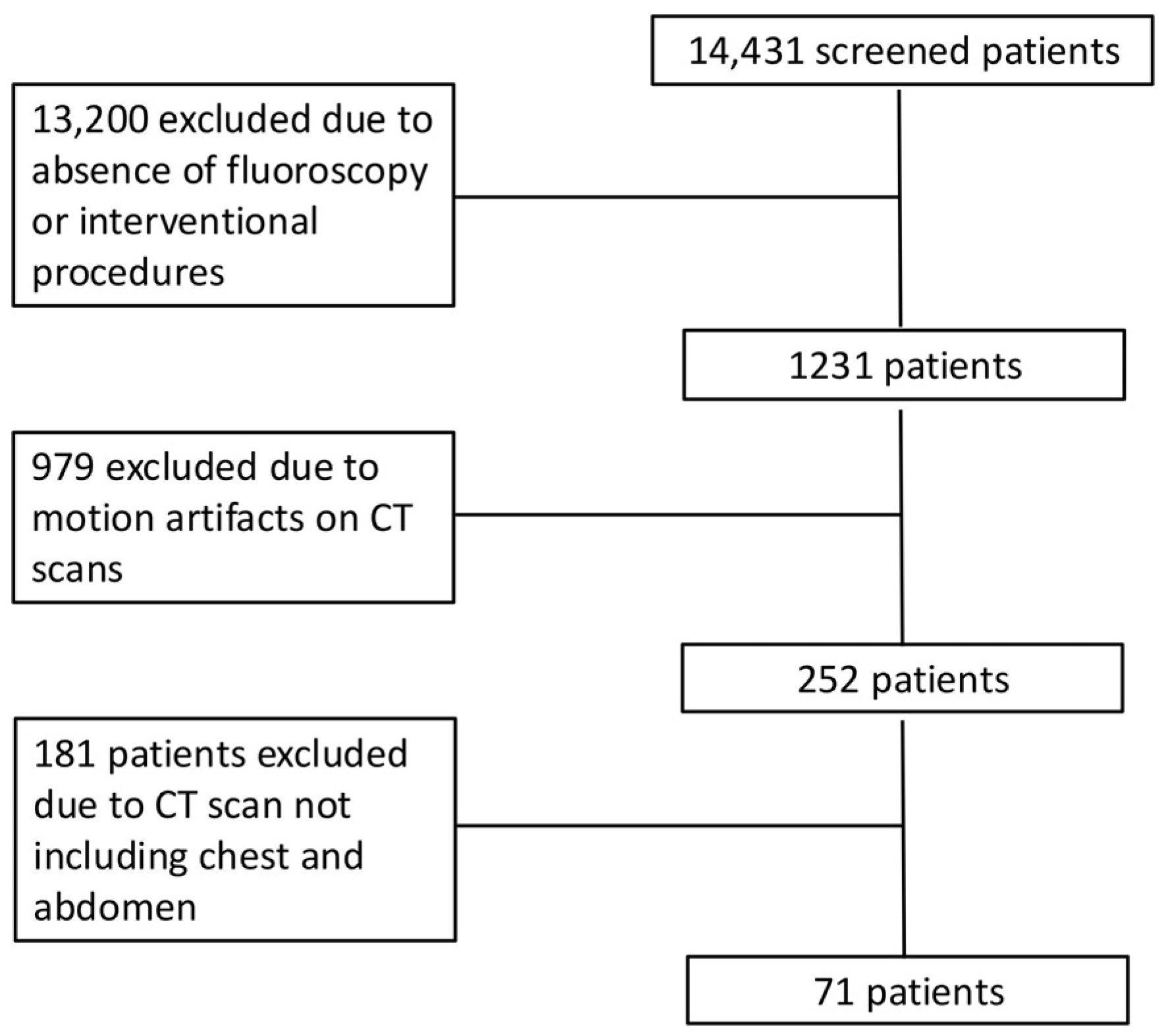
2.1. Patient Population
2.2. CT Scanning Protocols
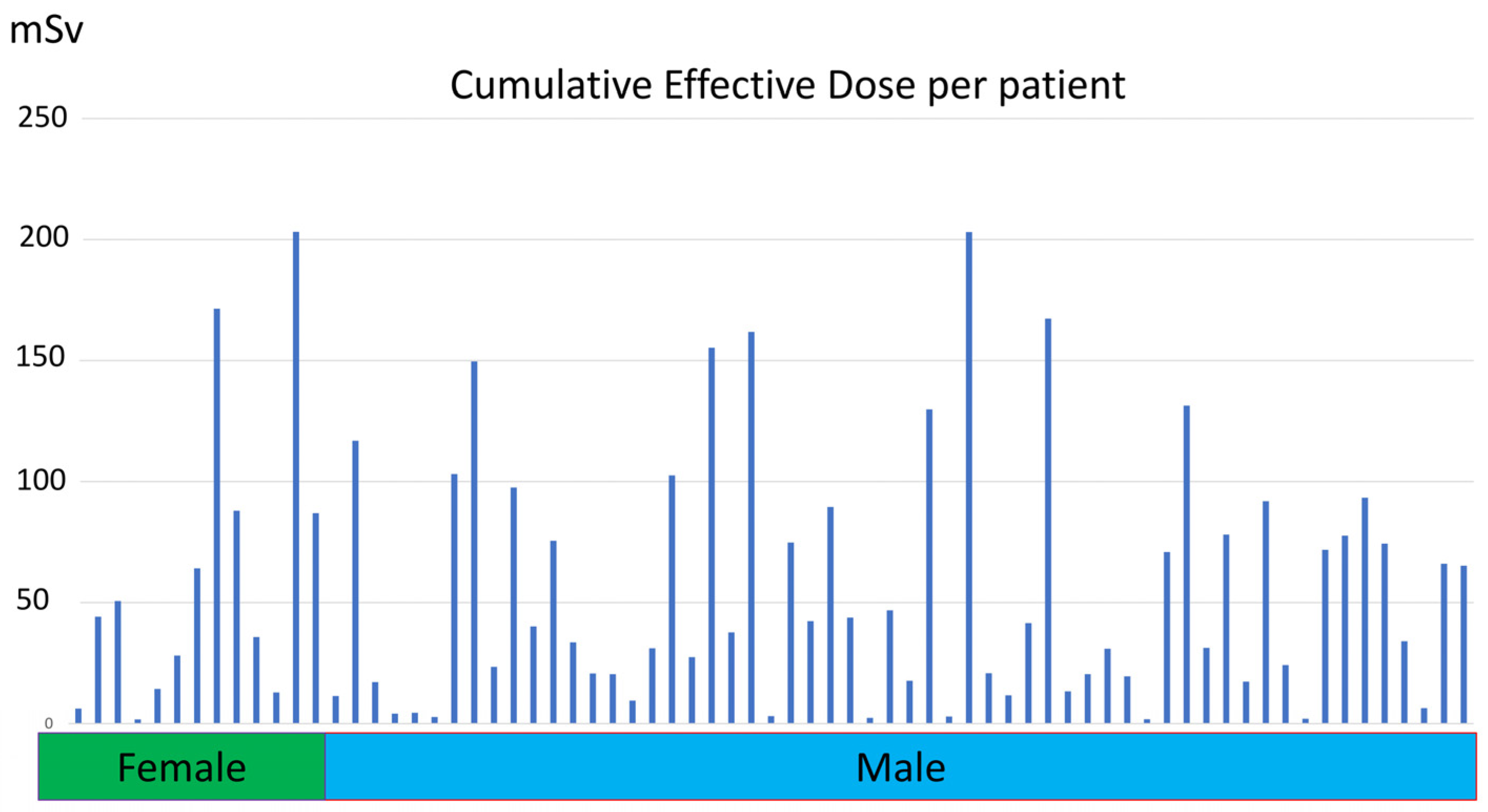
2.3. Cumulative Effective Dose
2.4. Radiation-Induced Oncogenic Risk
2.5. Statistical Analysis
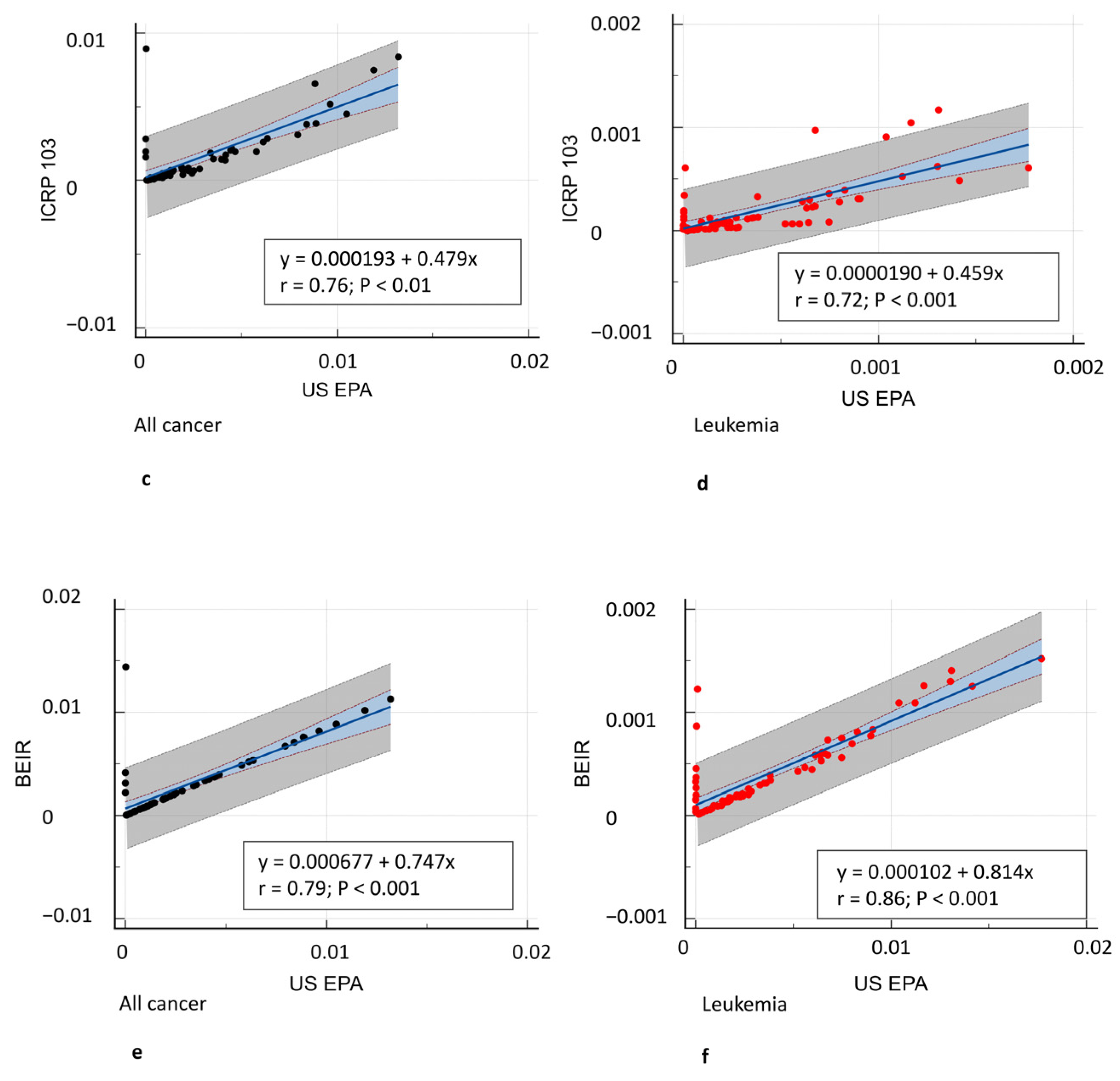
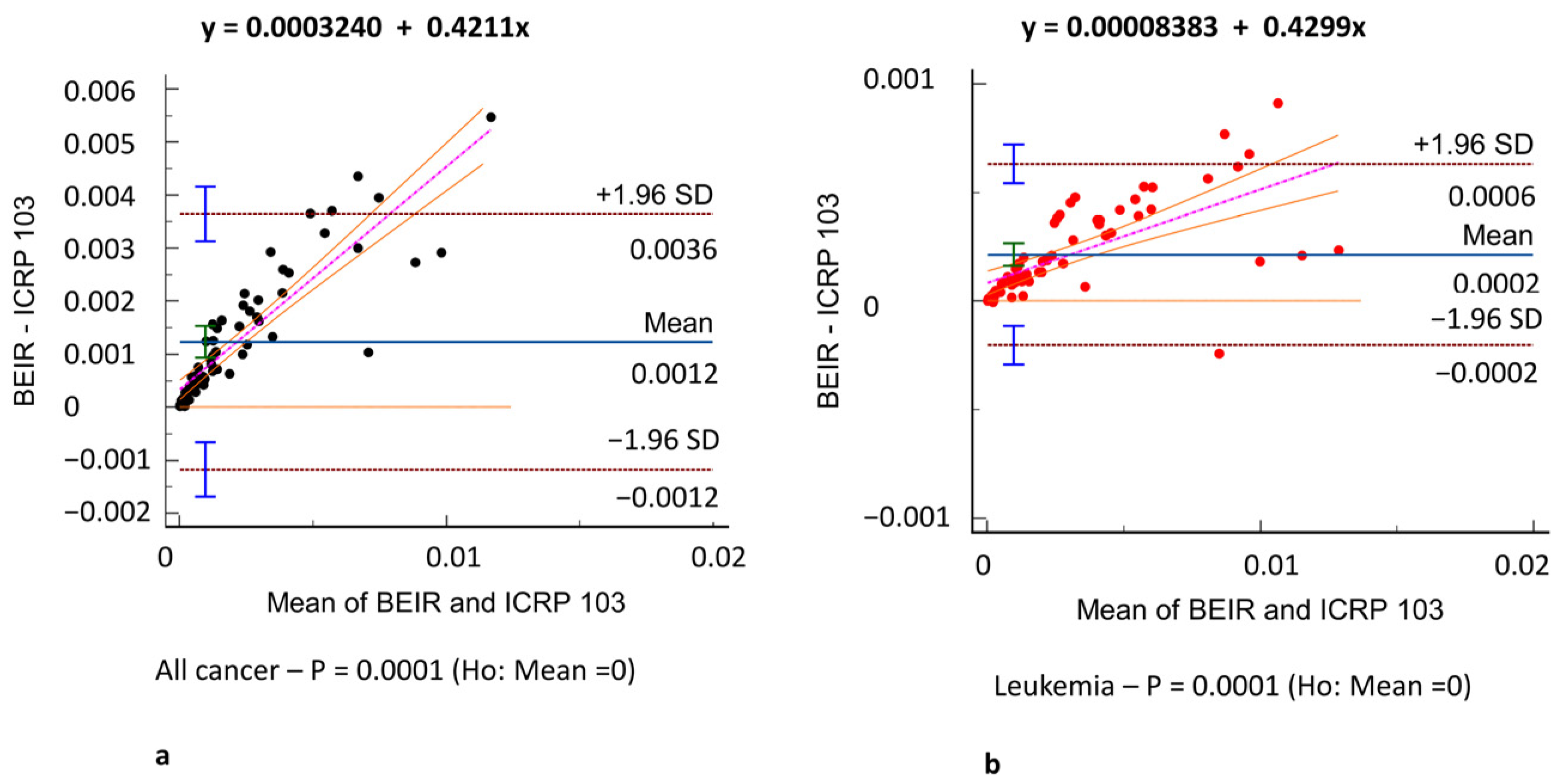
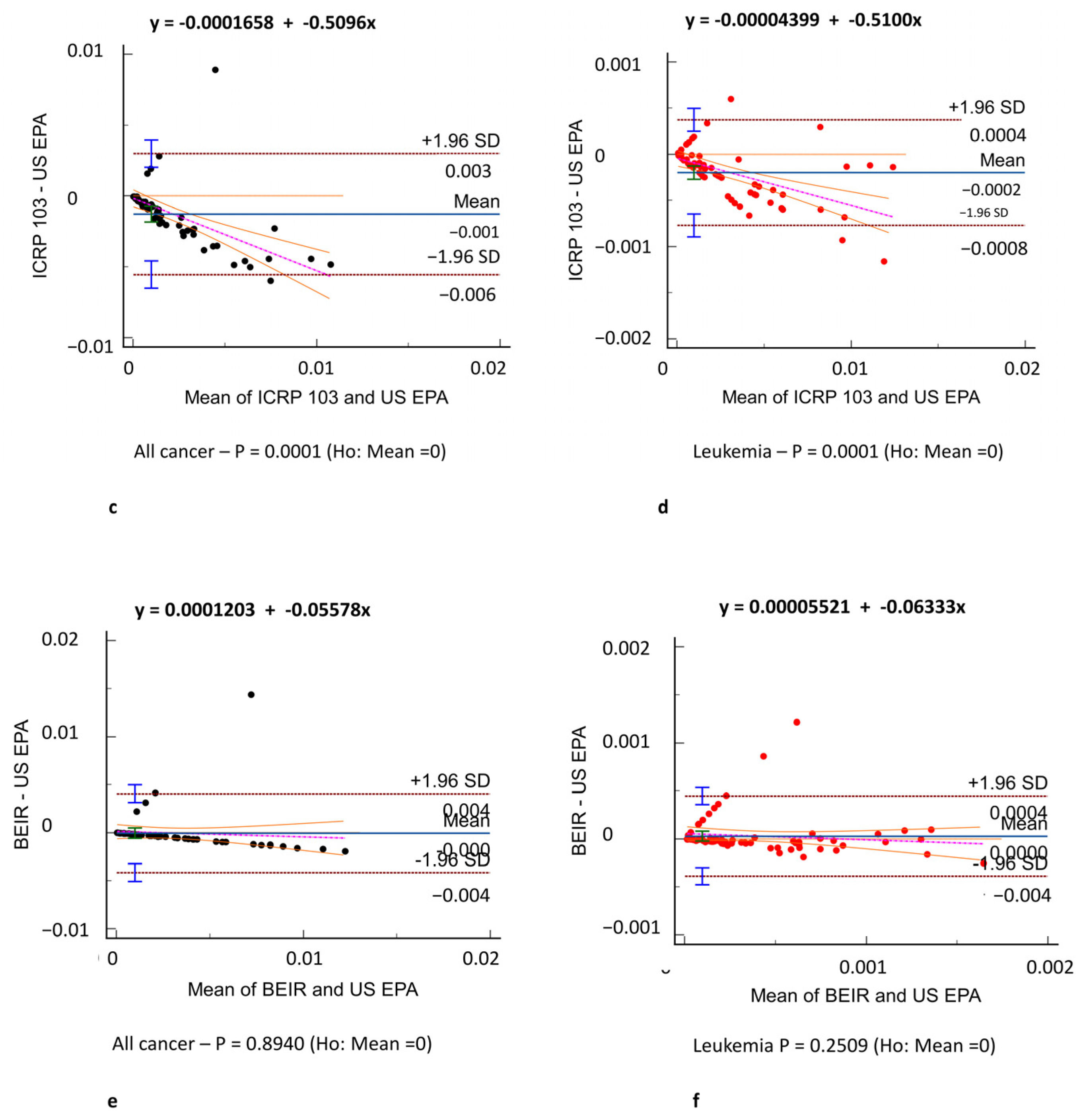
3. Results
3.1. Patient Characteristics
3.2. Cumulative Effective Dose
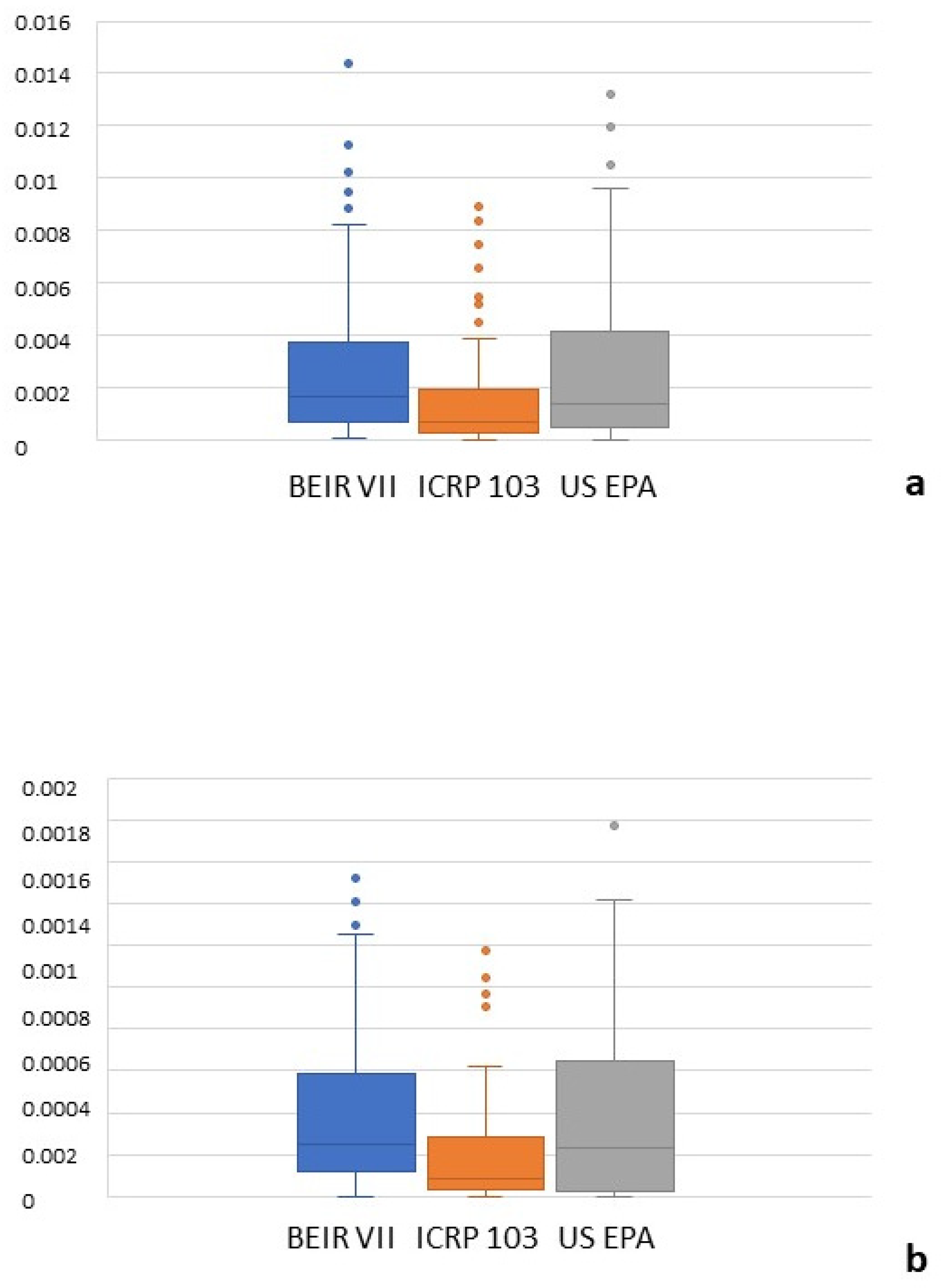
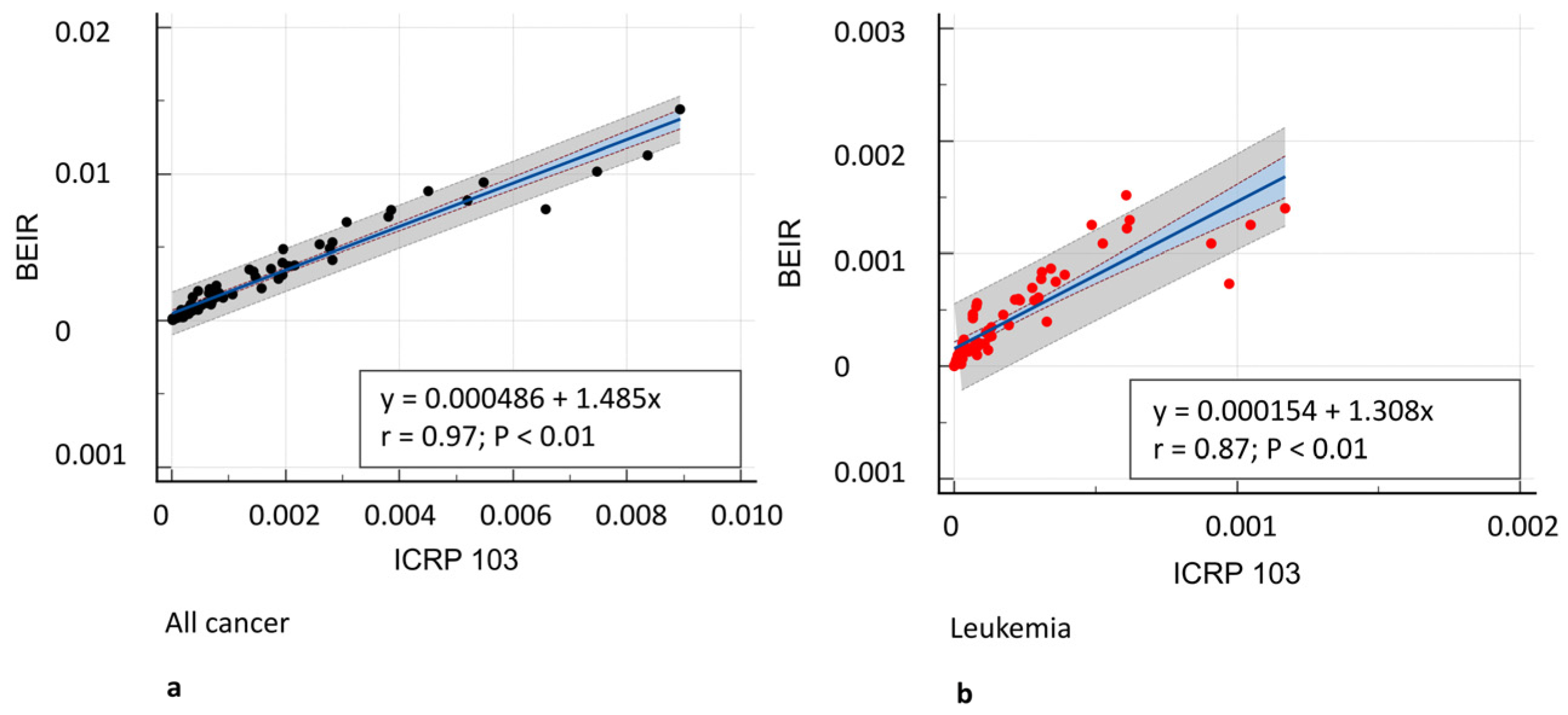
3.3. Oncogenic Risk
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOR | Additional oncogenic risk |
| BEIR VII | Biological Effects of Ionizing Radiation seventh report |
| CTDIvol | Volume computed tomography index |
| ICRP 103 | International Commission on Radiological Protection 103 |
| ICC | Intraclass correlation coefficient |
| ICU | Intensive care unit |
| IQR | Interquartile range |
| LAR | Lifetime attributable risk |
| US EPA | United States Environmental Protection Agency |
References
- Mahesh, M.; Ansari, A.J.; Mettler, F.A. Patient Exposure from Radiologic and NuclearMedicine Procedures in the United States and Worldwide:2009–2018. Radiology 2023, 307, e221263. [Google Scholar] [CrossRef] [PubMed]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources, effects, and risks of ionizing radiation. In Evaluation of Medical Exposure to Ionizing Radiation; Scientific Annex A. 2020/2021 Report to the General Assembly with annexes; United Nations: New York, NY, USA, 2022; Volume 1. [Google Scholar]
- Toy, D.; Siegel, M.D.; Rubinowitz, A.N. Imaging in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2022, 43, 899–923. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Liao, X.; Kang, Y.; Zhang, J.; Jia, L. Radiation Exposure to Staff in Intensive Care Unit with Portable CT Scanner. Biomed. Res. Int. 2016, 2016, 5656480. [Google Scholar] [CrossRef]
- Krishnan, S.; Moghekar, A.; Duggal, A.; Yella, J.; Narechania, S.; Ramachandran, V.; Mehta, A.; Adhi, F.; Vijayan, A.K.C.; Han, X.; et al. Radiation Exposure in the Medical ICU: Predictors and Characteristics. Chest 2018, 153, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, I. Greetings: 50 years of Atomic Bomb Casualty Commission–Radiation Effects Research Foundation studies. Proc. Natl. Acad. Sci. USA 1998, 95, 5424–5425. [Google Scholar] [CrossRef]
- Kodama, K.; Mabuchi, K.; Shigematsu, I. A Long-Term Cohort Study of the Atomic-Bomb Survivors. J. Epidemiol. 1996, 6, 95–105. [Google Scholar] [CrossRef]
- Ozasa, K. Epidemiological research on radiation-induced cancer in atomic bomb survivors. J. Radiat. Res. 2016, 57, i112–i117. [Google Scholar] [CrossRef]
- Kodama, K.; Ozasa, K.; Okubo, T. Radiation and cancer risk in atomic-bomb survivors. J. Radiol. Prot. 2012, 32, N51–N54. [Google Scholar] [CrossRef]
- Ozasa, K.; Grant, E.J.; Kodama, K. Japanese Legacy Cohorts: The Life Span Study Atomic Bomb Survivor Cohort and Survivors’ Offspring. J. Epidemiol. 2018, 28, 162–169. [Google Scholar] [CrossRef]
- Calabrese, E.J. The linear No-Threshold (LNT) dose response model: A comprehensive assessment of its historical and scientific foundation. Chem. Biol. Interact. 2019, 301, 6–25. [Google Scholar] [CrossRef]
- National Research Council of the National Academies. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2; The National Academies Press: Washington, DC, USA, 2006; p. 245. [Google Scholar]
- Berrington de Gonzalez, A.; Iulian Apostoaei, A.; Veiga, L.H.; Rajaraman, P.; Thomas, B.A.; Owen Hoffman, F.; Gilbert, E.; Land, C. RadRAT: A radiation risk assessment tool for lifetime cancer risk projection. J. Radiol. Prot. 2012, 32, 205–222. [Google Scholar] [CrossRef] [PubMed]
- ICRP 2007. The 2007 Recommendations of the International Commission on Radiological Protection; ICRP Publication 103; Elsevier: Oxford, UK, 2007. [Google Scholar]
- US Environmental Protection Agency. EPA Radiogenic Cancer Risk Models and Projections for the U.S. Population; EPA Report 402-R-11-001 2011; US Environmental Protection Agency: Washington, DC, USA, 2011; pp. 16–48.
- World Health Organization. Health Risk Assessment from the Nuclear Accident After the 2011 Great East Japan Earthquake and Tsunami Based on a Preliminary Dose Estimation; World Health Organization: Geneva, Switzerland, 2013; pp. 125–129. [Google Scholar]
- ICRP 2010. Conversion Coefficients for Radiological Protection Quantities for External Radiation Exposures; ICRP Publication 116; International Commission of Radiological Protection: Oxford, UK, 2010. [Google Scholar]
- Petoussi-Henss, N.; Bolch, W.E.; Eckerman, K.F.; Endo, A.; Hertel, N.; Hunt, J.; Menzel, H.G.; Pelliccioni, M.; Schlattl, H.; Zankl, M. ICRP Publication 116—The first ICRP/ICRU application of the male and female adult reference computational phantoms. Phys. Med. Biol. 2014, 59, 5209–5224. [Google Scholar] [CrossRef] [PubMed]
- Boone, J.M.; McNitt-Gray, M.F.; Hernandez, A.M. Monte Carlo Basics for Radiation Dose Assessment in Diagnostic Radiology. J. Am. Coll. Radiol. 2017, 14, 793–794. [Google Scholar] [CrossRef]
- Deak, P.D.; Smal, Y.; Kalender, W.A. Multisection CT Protocols: Sex- and Age-specific Conversion Factors Used to Determine Effective Dose from Dose-Length Product. Radiology 2010, 257, 158–166. [Google Scholar] [CrossRef]
- Rääf, C.; Markovic, N.; Tondel, M.; Wålinder, R.; Isaksson, M. Introduction of a method to calculate cumulative age- and gender-specific lifetime attributable risk (LAR) of cancer in populations after a large-scale nuclear power plant accident. PLoS ONE 2020, 15, e0228549. [Google Scholar] [CrossRef]
- Brenner, D.J.; Doll, R.; Goodhead, D.T.; Hall, E.J.; Land, C.E.; Little, J.B.; Lubin, J.H.; Preston, D.L.; Preston, R.J.; Puskin, J.S.; et al. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc. Natl. Acad. Sci. USA 2003, 100, 13761–13766. [Google Scholar] [PubMed]
- Samei, E.; Järvinen, H.; Kortesniemi, M.; Simantirakis, G.; Goh, C.; Wallace, A.; Vano, E.; Bejan, A.; Rehani, M.; Vassileva, J. Medical imaging dose optimisation from ground up: Expert opinion of an international summit. J. Radiol. Prot. 2018, 38, 967–989. [Google Scholar] [CrossRef]
- Abbott, A. Researchers pin down risks of low-dose radiation. Nature 2015, 523, 17–18. [Google Scholar]
- Tubiana, M.; Aurengo, A.; Averbeck, D.; Masse, R. Recent reports on the effect of low doses of ionizing radiation and its dose–effect relationship. Radiat. Environ. Biophys. 2006, 44, 245–251. [Google Scholar] [CrossRef]
- Richardson, D.B.; Leuraud, K.; Laurier, D.; Gillies, M.; Haylock, R.; Kelly-Reif, K.; Bertke, S.; Daniels, R.D.; Thierry-Chef, I.; Moissonnier, M.; et al. Cancer mortality after low dose exposure to ionising radiation in workers in France, the United Kingdom, and the United States (INWORKS): Cohort study. BMJ Br. Med. J. 2023, 382, e074520. [Google Scholar]
- Cardis, E.; Vrijheid, M.; Blettner, M.; Gilbert, E.; Hakama, M.; Hill, C.; Howe, G.; Kaldor, J.; Muirhead, C.R.; Schubauer-Berigan, M.; et al. Risk of cancer after low doses of ionising radiation: Retrospective cohort study in 15 countries. BMJ Br. Med. J. 2025, 331, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, M.; Byrnes, G.; Cardis, E.; Bernier, M.-O.; Blettner, M.; Dabin, J.; Engels, H.; Istad, T.S.; Johansen, C.; Kaijser, M.; et al. Brain cancer after radiation exposure from CT examinations of children and young adults: Results from the EPI-CT cohort study. Lancet Oncol. 2023, 24, 45–53. [Google Scholar] [CrossRef]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Craft, A.W.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Duport, P. A database of cancer induction by low dose radiation in mammals: Overview and initial observations. Int. J. Low Radiat. 2003, 1, 120–131. [Google Scholar] [CrossRef]
- Breckow, J. Linear-no-threshold is a radiation-protection standard rather than a mechanistic effect model. Radiat. Environ. Biophys. 2006, 44, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Little, M.P.; Wakeford, R.; Tawn, E.J.; Bouffler, S.D.; Berrington de Gonzalez, A. Risks associated with low doses and low dose rates of ionizing radiation: Why linearity may be (almost) the best we can do. Radiology 2009, 251, 6–12. [Google Scholar] [CrossRef]
- Ko, M.; Lao, X.Y.; Kapadia, R.; Elmore, E.; Radpath, J.L. Neoplastic transformation in vitro by low doses of ionizing radiation: Role of adaptive response and bystander effects. Mutat. Res. 2006, 597, 11–17. [Google Scholar] [CrossRef]
- Feinendegen, L.E. Evidence for benefical low level of radiation effects and radiation hormesis. Br. J. Radiol. 2005, 78, 3–7. [Google Scholar] [CrossRef]
- Slovis, B.H.; Shah, K.H.; Yeh, D.D.; Seethala, R.; Kaafarani, H.M.A.; Eikermann, M.; Raja, A.S.; Lee, J. Significant but reasonable radiation exposure from computed tomography-related medical imaging in the ICU. Emerg. Radiol. 2016, 23, 141–146. [Google Scholar] [CrossRef]
- Yee, M.V.; Barron, R.A.; Knobloch, T.A.; Pandey, U.; Twyford, C.; Freebairn, R.C. Radiation exposure of ventilated trauma patients in intensive care. Eur. J. Emerg. Med. 2012, 19, 231–234. [Google Scholar] [CrossRef]
- Börjesson, J.; Latifi, A.; Friman, O.; Beckman, M.O.; Oldner, A.; Labruto, F. Accuracy of low-dose chest CT in intensive care patients. Emerg. Radiol. Emerg. Radiol. 2011, 18, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Wassipaul, C.; Kifjak, D.; Milos, R.I.; Prayer, F.; Roehrich, S.; Winter, M.; Beer, L.; Watzenboeck, M.L.; Pochepnia, S.; Weber, M.; et al. Ultra-low-dose vs. standard-of-care-dose CT of the chest in patients with postCOVID-19 conditions—A prospective intra-patient multi-reader study. Eur. Radiol. 2024, 34, 7244–7254. [Google Scholar] [PubMed]
- WRITING/STEERING COMMITTEE MEMBERS; Douglas, P.; Carr, J.J.; Cerqueira, M.D.; Cummings, J.E.; Gerber, T.C.; Mukherjee, D.; Taylor, A.J. Developing an Action Plan for Patient Radiation Safety in Adult Cardiovascular Medicine. Circ. Cardiovasc. Imaging 2012, 5, 400–414. [Google Scholar] [CrossRef]
- Söderberg, M.; Gunnarsson, M. Automatic exposure control in computed tomography—An evaluation of systems from different manufacturers. Acta Radiol. 2010, 51, 625–634. [Google Scholar] [CrossRef]
- Najjar, R. Radiology’s Ionising Radiation Paradox: Weighing the Indispensable Against the Detrimental in Medical Imaging. Cureus 2023, 15, e41623. [Google Scholar] [CrossRef]
| BEIR VII | ICRP 103 | US EPA | |
|---|---|---|---|
| Epidemiological data | Solid cancer: Japanese atomic bomb survivor morbidity data from the period 1958–1998 | ||
| Leukemia: Japanese atomic bomb survivor mortality from the period 1950–2000 | |||
| Reference population | USA | Europe, USA, Asia | USA |
| Excess absolute risk/excess relative risk | 0.3/0.7 | 0.5/0.5 | 0.3/0.7 |
| Dose-response model | Linear no-threshold | ||
| Minimal tumoral latency period | 5 years for solid tumors 2 years for leukemia | ||
| Dose and dose rate effectiveness factor | 1.5 | 2 | 1.5 |
| Lifetime attributable risk | Weighted geometric mean | Weighted arithmetic mean | Weighted arithmetic mean |
| Characteristics | |
| Age (years) | 66 (56–71) |
| Male | 66 (55–71) |
| Female | 61 (58–73) |
| Sex | |
| Male | 58 |
| Female | 13 |
| Hospitalization (days) | 31 (15–70) |
| Male | 30 (21–70) |
| Female | 20 (15–26) |
| Reason for hospitalization | |
| Major thoracic surgery | 19 |
| Major abdominal surgery | 27 |
| Solid organ transplant | 20 |
| Major trauma | 5 |
| Cancer Categories | BEIR VII | ICRP 103 | US EPA | p # | ICC (95% CI) |
|---|---|---|---|---|---|
| Bladder | 20.75 (8.78–46.89) | 7.22 (2.96–20.93) | 10.55 (4.07–28.23) | 0.0001 | 0.87 (0.81–0.91) |
| Bone | * | * | 0.29 (0.001–1.66) | ||
| Breast | 10.55 (3.95–27.44) | 17.37 (1.63–23.62) | * | 0.003 | 0.9 (0.67–0.97) |
| Colon | 25.77 (12.23–66.67) | 7.14 (3.09−17.74) | 25.69 (12.2–67.85) | 0.0001 | 0.85 (0.79–0.90) |
| Kidney | * | * | 20.45 (9.36–50.41) | ||
| Liver | 3.6 (1.37–9.24) | 2.79 (1.24–7.48) | 6.18 (3.19–17.04) | 0.0001 | 0.88 (0.83–0.92) |
| Lung | 30.81 (13.43–74) | 23.35 (9.75–60.48) | 31.06 (13.5–78.79) | 0.0001 | 0.98 (0.97–0.98) |
| Esophagus | * | 4 (1.71–8.88) | * | ||
| Ovary | 6.93 (2.29–13.32) | 3.99 (0.69–6.1) | * | 0.001 | 0.7 (0.17–0.91) |
| Prostate | 4.37 (0.65–15.26) | * | 9.46 (1.39–34.68) | 0.001 | 0.71 (0.58–0.81) |
| Skin | * | * | 29.43 (11.43–72.64) | ||
| Stomach | 5.26 (2.13–15.27) | 4.14 (1.75–13.63) | 11.46 (4.93–27.16) | 0.001 | 0.87 (0.81–0.91) |
| Thyroid | 0.10 (0.02–0.5) | 0.46 (0.35–2.03) | 4.57 (1.52–8.71) | 0.001 | 0.38 (0.23–0.53) |
| Uterus | 3.50 (1.14–6.78) | * | * | ||
| Other solid tumors | 28.93 (11.16–71.35) | 10.34 (3.09–30.84) | 6.43 (2.16–12.17) | 0.001 | 0.9 (0.86–0.93) |
| Leukemia | 24.66 (12.9–58.8) | 8.22 (3.02–27.93) | 23.34 (3.47–64.37) | 0.001 | 0.83 (0.76–0.88) |
| All solid tumors | 132.80 (53.76–316.2) | * | 86.78 (16.66–296.53) | 0.001 | 0.97 (0.88–0.99) |
| All cancers | 162.08 (70.6–371.4) | 69.05 (30.35–195.37) | 139.68 (50.51–416.16) | 0.001 | 0.91 (0.87–0.94) |
| BEIR VII | ICRP 103 | US EPA | p # | ICC (95% CI) | |
|---|---|---|---|---|---|
| All-cancer, male | 161.86 (72.82–376.95) | 65.97 (30.9–187.03) | 188.88 (81.73–418.07) | 0.001 | 0.91 (0.87–0.94) |
| All-cancer, female | 220.46 (746.82–4148.07) | 175.69 (48.93–280.85) | 60.91 (42–134.19) | 0.018 | 0.44 (0.2–0.94) |
| Leukemia, male | 29.47 (14.04–65.36) | 8.22 (3.09–28.33) | 28.36 (14.88–67.68) | 0.0001 | 0.85 (0.79–0.90) |
| Leukemia, female | 19.87 (68.82–366.38) | 11.97 (2.33–18.75) | 12.86 (6.11–25.61) | 0.00034 | 0.38 (0.1–0.80) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quaia, E.; Zanon, C.; Torchio, R.; Dughiero, F.; De Monte, F.; Paiusco, M. Variability Between Radiation-Induced Cancer Risk Models in Estimating Oncogenic Risk in Intensive Care Unit Patients. Tomography 2025, 11, 42. https://doi.org/10.3390/tomography11040042
Quaia E, Zanon C, Torchio R, Dughiero F, De Monte F, Paiusco M. Variability Between Radiation-Induced Cancer Risk Models in Estimating Oncogenic Risk in Intensive Care Unit Patients. Tomography. 2025; 11(4):42. https://doi.org/10.3390/tomography11040042
Chicago/Turabian StyleQuaia, Emilio, Chiara Zanon, Riccardo Torchio, Fabrizio Dughiero, Francesca De Monte, and Marta Paiusco. 2025. "Variability Between Radiation-Induced Cancer Risk Models in Estimating Oncogenic Risk in Intensive Care Unit Patients" Tomography 11, no. 4: 42. https://doi.org/10.3390/tomography11040042
APA StyleQuaia, E., Zanon, C., Torchio, R., Dughiero, F., De Monte, F., & Paiusco, M. (2025). Variability Between Radiation-Induced Cancer Risk Models in Estimating Oncogenic Risk in Intensive Care Unit Patients. Tomography, 11(4), 42. https://doi.org/10.3390/tomography11040042









