Radiological Reporting of Urgencies Related to Medical Devices: Commentary on a Possible Systematic Approach
Abstract
:1. Introduction
2. Device Identification and Recognition
3. Device Integrity and Migration
4. Complication Reporting
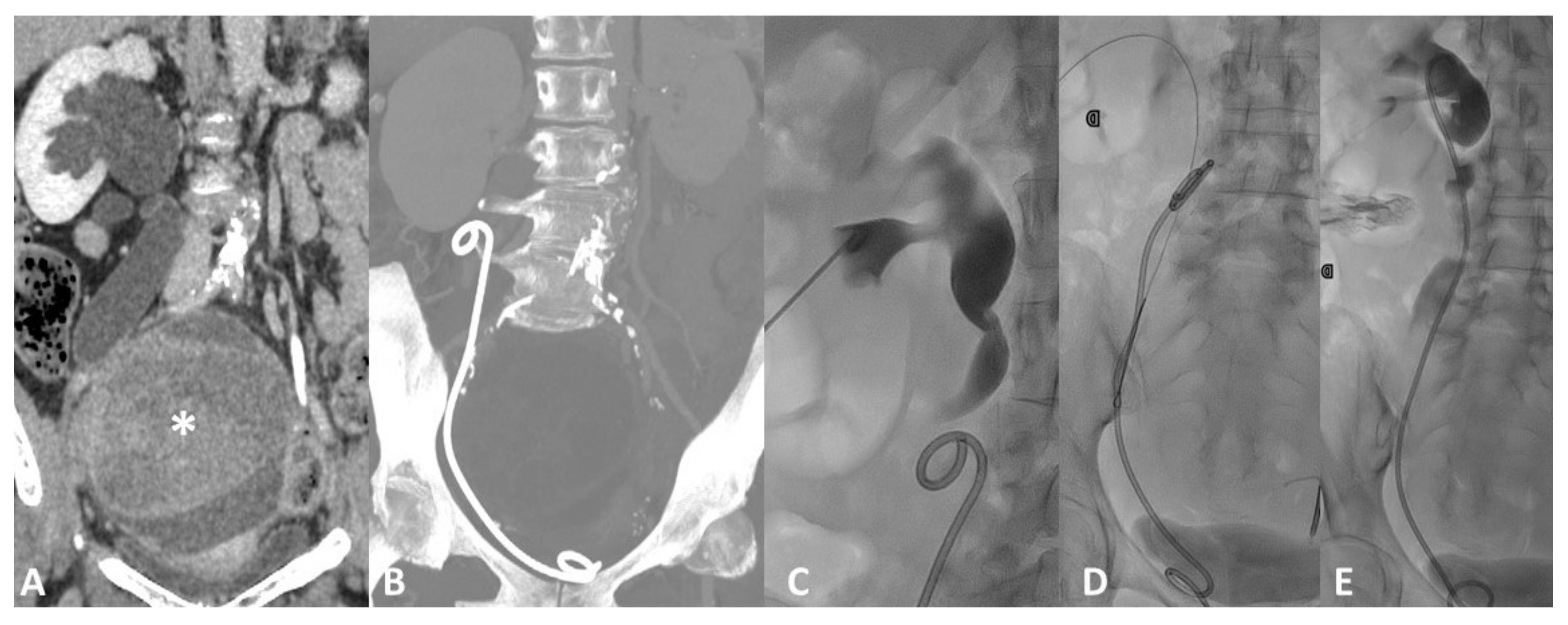
4.1. Vascular Injury
4.2. Parenchymal/Tissue Injury
4.3. Obstruction
4.4. Perforation
4.5. Infective/Inflammatory
5. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Official Journal of the European Union. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EE. 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32017R0745 (accessed on 27 June 2021).
- Lazzaro, A.; Corona, A.; Iezzi, L.; Quaresima, S.; Armisi, L.; Piccolo, I.; Medaglia, C.M.; Sbrenni, S.; Sileri, P.; Rosato, N.; et al. Radiofrequency-Based Identification Medical Device: An Evaluable Solution for Surgical Sponge Retrieval? Surg. Innov. 2017, 24, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Boortz, H.E.; Margolis, D.J.A.; Ragavendra, N.; Patel, M.K.; Kadell, B.M. Migration of intrauterine devices: Radiologic findings and implications for patient care. Radiographics 2012, 32, 335–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laaguili, J.; Mandour, C.; El Jebbouri, B.; Belhachmi, A.; Gazzaz, M.; El Mostarchid, B. Spontaneous expulsion of a paraspinal textiloma. Spine J. 2016, 16, e433–e434. [Google Scholar] [CrossRef] [PubMed]
- Neiva Machado, J.P.; Costa, J.C.; Costa, T. A Case Report on a Patient With Spontaneous Expulsion of Large Foreign Body (Dental Prothesis) without Complications. Arch. Bronconeumol. 2020, 56, 595. [Google Scholar] [CrossRef] [PubMed]
- Fysh, E.T.H.; Wrightson, J.M.; Lee, Y.C.G.; Rahman, N.M. Fractured indwelling pleural catheters. Chest 2012, 141, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Gyasi-Sarpong, C.K.; Maison, P.O.M.; Morhe, E.; Aboah, K.; Appiah, K.A.A.; Azorliade, R.; Baah-Nyamekye, K.; Otu-Boateng, K.; Amoah, G.; Antwi, I.; et al. Intravesical migration of an intrauterine device Urology. BMC Res. Notes 2016, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contegiacomo, A.; Amodeo, E.M.; Cina, A.; Distasi, C.; Iezzi, R.; Coppolino, D.; Attempati, N.; Manfredi, R. Renal artery embolization for iatrogenic renal vascular injuries management: 5 years’ experience. Br. J. Radiol. 2020, 93, 20190256. [Google Scholar] [CrossRef] [PubMed]
- Contegiacomo, A.; Conti, M.; Trombatore, P.; Dezio, M.; Muciaccia, M.; Lozupone, E.; Natale, L.; Manfredi, R. Radiological features and management of retained needles. Br. J. Radiol. 2020, 93, 20200316. [Google Scholar] [CrossRef] [PubMed]
- Weickert, U.; Venzke, T.; König, J.; Janssen, J.; Remberger, K.; Greiner, L. Why do bilioduodenal plastic stents become occluded? A clinical and pathological investigation on 100 consecutive patients. Endoscopy 2001, 33, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Avlund, T.H.; Erichsen, R.; Ravn, S.; Ciplys, Z.; Andersen, J.C.; Laurberg, S.; Iversen, L.H. The prognostic impact of bowel perforation following self-expanding metal stent as a bridge to surgery in colorectal cancer obstruction. Surg. Endosc. 2018, 32, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Abualruz, A.R.; O’Malley, R.; Ponnatapura, J.; Holbert, B.L.; Whitworth, P.; Tappouni, R.; Lalwani, N. MRI of common penile pathologies and penile prostheses. Abdom. Radiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Society, E. ESR paper on structured reporting in radiology. Insights Imaging 2018, 9, 1–7. [Google Scholar]
- Ganeshan, D.; Duong, P.A.T.; Probyn, L.; Lenchik, L.; McArthur, T.A.; Retrouvey, M.; Ghobadi, E.H.; Desouches, S.L.; Pastel, D.; Francis, I.R. Structured Reporting in Radiology. Acad. Radiol. 2018, 25, 66–73. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contegiacomo, A.; Conti, M.; Muciaccia, M.; Trombatore, P.; Dezio, M.; Lozupone, E.; Meduri, A.; Marano, R.; Natale, L.; Manfredi, R. Radiological Reporting of Urgencies Related to Medical Devices: Commentary on a Possible Systematic Approach. Tomography 2021, 7, 268-277. https://doi.org/10.3390/tomography7030024
Contegiacomo A, Conti M, Muciaccia M, Trombatore P, Dezio M, Lozupone E, Meduri A, Marano R, Natale L, Manfredi R. Radiological Reporting of Urgencies Related to Medical Devices: Commentary on a Possible Systematic Approach. Tomography. 2021; 7(3):268-277. https://doi.org/10.3390/tomography7030024
Chicago/Turabian StyleContegiacomo, Andrea, Marco Conti, Massimo Muciaccia, Pietro Trombatore, Michele Dezio, Emilio Lozupone, Agostino Meduri, Riccardo Marano, Luigi Natale, and Riccardo Manfredi. 2021. "Radiological Reporting of Urgencies Related to Medical Devices: Commentary on a Possible Systematic Approach" Tomography 7, no. 3: 268-277. https://doi.org/10.3390/tomography7030024
APA StyleContegiacomo, A., Conti, M., Muciaccia, M., Trombatore, P., Dezio, M., Lozupone, E., Meduri, A., Marano, R., Natale, L., & Manfredi, R. (2021). Radiological Reporting of Urgencies Related to Medical Devices: Commentary on a Possible Systematic Approach. Tomography, 7(3), 268-277. https://doi.org/10.3390/tomography7030024




