What Can Resting-State fMRI Data Analysis Explain about the Functional Brain Connectivity in Glioma Patients?
Abstract
:1. Introduction
2. Preoperative Evaluations
2.1. Network Localization
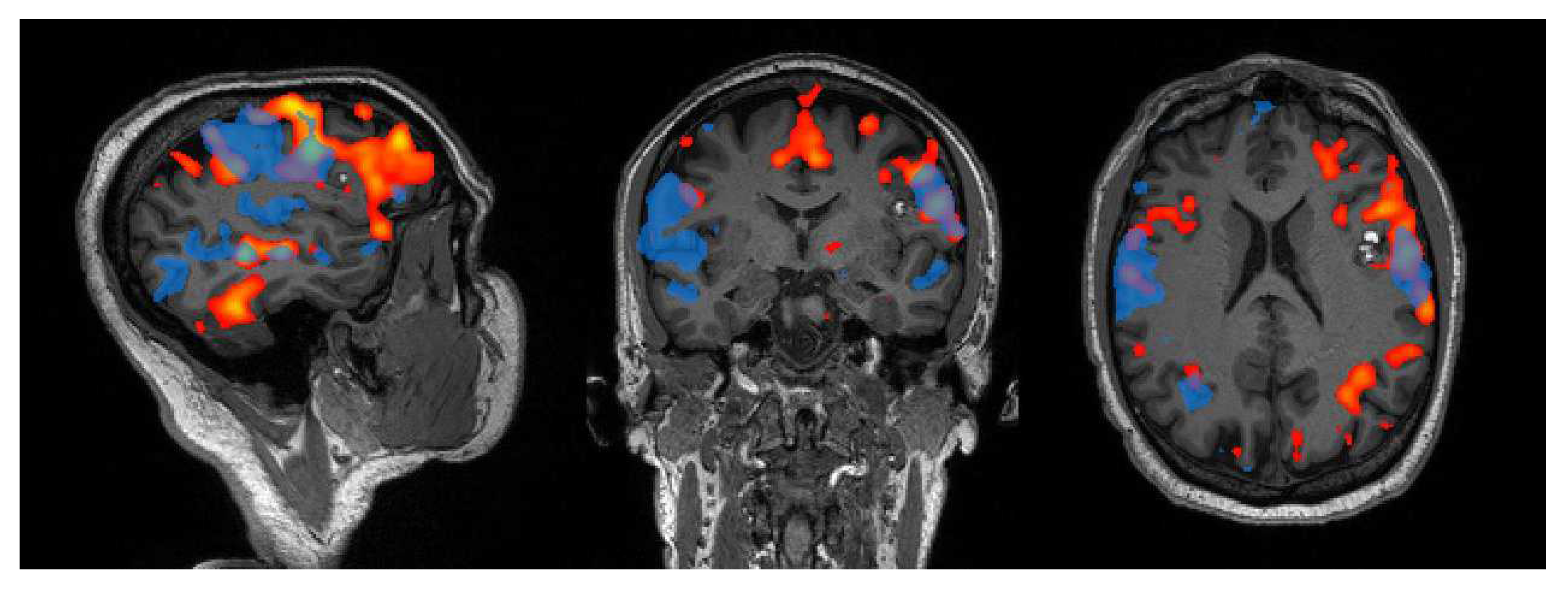
2.1.1. Motor Network
2.1.2. Language Network
2.2. Functional Connectivity Analysis
2.2.1. Language Network
2.2.2. Default Mode Network
2.2.3. Fronto-Parietal Network
2.2.4. Other Functional Networks
3. Longitudinal Evaluations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glover, G.H. Overview of Functional Magnetic Resonance Imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friston, K.J. Functional and Effective Connectivity: A Review. Brain Connect 2011, 1, 13–36. [Google Scholar] [CrossRef]
- Meier, M.P.; Ilmberger, J.; Fesl, G.; Ruge, M.I. Validation of functional motor and language MRI with direct cortical stimulation. Acta Neurochir. 2013, 155, 675–683. [Google Scholar] [CrossRef]
- Tyndall, A.J.; Reinhardt, J.; Tronnier, V.; Mariani, L.; Stippich, C. Presurgical motor, somatosensory and language fMRI: Technical feasibility and limitations in 491 patients over 13 years. Eur. Radiol. 2016, 27, 267–278. [Google Scholar] [CrossRef]
- Metwali, H.; Raemaekers, M.; Kniese, K.; Kardavani, B.; Fahlbusch, R.; Samii, A. Reliability of Functional Magnetic Resonance Imaging in Patients with Brain Tumors: A Critical Review and Meta-Analysis. World Neurosurg. 2019, 125, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Luna, L.P.; Sherbaf, F.G.; Sair, H.I.; Mukherjee, D.; Oliveira, I.B.; Köhler, C.A. Can Preoperative Mapping with Functional MRI Reduce Morbidity in Brain Tumor Resection? A Systematic Review and Meta-Analysis of 68 Observational Studies. Radiology 2021, 300, 338–349. [Google Scholar] [CrossRef]
- O’Connor, E.E.; Zeffiro, T.A. Why is Clinical fMRI in a Resting State? Front. Neurol. 2019, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Parkes, L.; Satterthwaite, T.D.; Bassett, D.S. Towards precise resting-state fMRI biomarkers in psychiatry: Synthesizing developments in transdiagnostic research, dimensional models of psychopathology, and normative neurodevelopment. Curr. Opin. Neurobiol. 2020, 65, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Rosazza, C.; Zacà, D.; Bruzzone, M.G. Pre-surgical Brain Mapping: To Rest or Not to Rest? Front. Neurol. 2018, 9, 520. [Google Scholar] [CrossRef] [Green Version]
- Azad, T.D.; Duffau, H. Limitations of functional neuroimaging for patient selection and surgical planning in glioma surgery. Neurosurg. Focus 2020, 48, E12. [Google Scholar] [CrossRef] [Green Version]
- Castellano, A.; Cirillo, S.; Bello, L.; Riva, M.; Falini, A. Functional MRI for Surgery of Gliomas. Curr. Treat. Opt. Neurol. 2017, 19, 34. [Google Scholar] [CrossRef]
- Hacker, C.D.; Roland, J.L.; Kim, A.H.; Shimony, J.S.; Leuthardt, E.C. Resting-state network mapping in neurosurgical practice: A review. Neurosurg. Focus 2019, 47, E15. [Google Scholar] [CrossRef] [Green Version]
- Smitha, K.A.; Raja, K.A.; Arun, K.M.; Rajesh, P.G.; Thomas, B.; Kapilamoorthy, T.R.; Kesavadas, C. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. Neuroradiol. J. 2017, 30, 305–317. [Google Scholar] [CrossRef]
- Lee, M.H.; Smyser, C.D.; Shimony, J.S. Resting-State fMRI: A Review of Methods and Clinical Applications. Am. J. Neuroradiol. 2013, 34, 1866–1872. [Google Scholar] [CrossRef] [Green Version]
- Vergara, V.M.; Mayer, A.R.; Damaraju, E.; Hutchison, K.; Calhoun, V.D. The effect of preprocessing pipelines in subject classification and detection of abnormal resting state functional network connectivity using group ICA. NeuroImage 2017, 145, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. FSL. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SPM12 Software–Statistical Parametric Mapping. Available online: http://www.fil.ion.ucl.ac.uk/spm/software/spm12/ (accessed on 13 December 2021).
- Bullmore, E.T.; Bassett, D.S. Brain Graphs: Graphical Models of the Human Brain Connectome. Annu. Rev. Clin. Psychol. 2011, 7, 113–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Ghinda, D.C.; Wu, J.-S.; Duncan, N.W.; Northoff, G. How much is enough—Can resting state fMRI provide a demarcation for neurosurgical resection in glioma? Neurosci. Biobehav. Rev. 2018, 84, 245–261. [Google Scholar] [CrossRef]
- Volz, L.J.; Kocher, M.; Lohmann, P.; Shah, N.J.; Fink, G.R.; Galldiks, N. Functional magnetic resonance imaging in glioma patients: From clinical applications to future perspectives. Q. J. Nucl. Med. Mol. Imaging 2018, 62, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Zacà, D.; Jovicich, J.; Corsini, F.; Rozzanigo, U.; Chioffi, F.; Sarubbo, S. ReStNeuMap: A tool for automatic extraction of resting-state functional MRI networks in neurosurgical practice. J. Neurosurg. 2019, 131, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Sharaev, M.; Smirnov, A.; Melnikova-Pitskhelauri, T.; Orlov, V.; Burnaev, E.; Pronin, I.; Pitskhelauri, D.; Bernstein, A. Functional Brain Areas Mapping in Patients with Glioma Based on Resting-State fMRI Data Decomposition. In Proceedings of the 2018 IEEE International Conference on Data Mining Workshops (ICDMW), Singapore, 17–20 November 2018; IEEE: Manhattan, NY, USA, 2018; pp. 292–298. [Google Scholar]
- Voets, N.L.; Plaha, P.; Jones, O.P.; Pretorius, P.; Bartsch, A. Presurgical Localization of the Primary Sensorimotor Cortex in Gliomas. Clin. Neuroradiol. 2021, 31, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Niu, C.; Wang, Y.; Cohen, A.D.; Liu, X.; Li, H.; Lin, P.; Chen, Z.; Min, Z.; Li, W.; Ling, X.; et al. Machine learning may predict individual hand motor activation from resting-state fMRI in patients with brain tumors in perirolandic cortex. Eur. Radiol. 2021, 31, 5253–5262. [Google Scholar] [CrossRef] [PubMed]
- Dierker, D.; Roland, J.L.; Kamran, M.; Rutlin, J.; Hacker, C.D.; Marcus, D.S.; Milchenko, M.; Miller-Thomas, M.M.; Benzinger, T.L.; Snyder, A.Z.; et al. Resting-state Functional Magnetic Resonance Imaging in Presurgical Functional Mapping: Sensorimotor Localization. Neuroimaging Clin. N. Am. 2017, 27, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, H.; Hameed, N.U.F.; Zhang, J.; Yuan, S.; Qiu, T.; Shen, D.; Wu, J. An automated method for identifying an independent component analysis-based language-related resting-state network in brain tumor subjects for surgical planning. Sci. Rep. 2017, 7, 13769. [Google Scholar] [CrossRef] [Green Version]
- Park, K.Y.; Lee, J.J.; Dierker, D.; Marple, L.M.; Hacker, C.D.; Roland, J.; Marcus, D.S.; Milchenko, M.; Miller-Thomas, M.M.; Benzinger, T.L.; et al. Mapping language function with task-based vs. resting-state functional MRI. PLoS ONE 2020, 15, e0236423. [Google Scholar] [CrossRef]
- Jin, L.; Li, C.; Zhang, Y.; Yuan, T.; Ying, J.; Zuo, Z.; Gui, S. The Functional Reorganization of Language Network Modules in Glioma Patients: New Insights From Resting State fMRI Study. Front. Oncol. 2021, 11, 159. [Google Scholar] [CrossRef]
- Jütten, K.; Mainz, V.; Delev, D.; Gauggel, S.; Binkofski, F.; Wiesmann, M.; Clusmann, H.; Na, C. Asymmetric tumor-related alterations of network-specific intrinsic functional connectivity in glioma patients. Hum. Brain Mapp. 2020, 41, 4549–4561. [Google Scholar] [CrossRef] [PubMed]
- Maniar, Y.; Peck, K.; Jenabi, M.; Gene, M.; Holodny, A. Functional MRI Shows Altered Deactivation and a Corresponding Decrease in Functional Connectivity of the Default Mode Network in Patients with Gliomas. Am. J. Neuroradiol. 2021, 42, 1505–1512. [Google Scholar] [CrossRef]
- Tordjman, M.; Madelin, G.; Gupta, P.K.; Cordova, C.; Kurz, S.C.; Orringer, D.; Golfinos, J.; Kondziolka, D.; Ge, Y.; Wang, R.L.; et al. Functional connectivity of the default mode, dorsal attention and fronto-parietal executive control networks in glial tumor patients. J. Neuro-Oncol. 2021, 152, 347–355. [Google Scholar] [CrossRef]
- Metwali, H.; Raemaekers, M.; Ibrahim, T.; Samii, A. Inter-Network Functional Connectivity Changes in Patients With Brain Tumors: A Resting-State Functional Magnetic Resonance Imaging Study. World Neurosurg. 2020, 138, e66–e71. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Dirkson, A.; Hamer, P.D.W.; van Geest, Q.; Hulst, H.E.; Barkhof, F.; Pouwels, P.J.; Geurts, J.J.; Reijneveld, J.C.; Douw, L. Connectomic profile and clinical phenotype in newly diagnosed glioma patients. NeuroImage Clin. 2017, 14, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.G.; Romero-Garcia, R.; Price, S.J.; Suckling, J. Global Effects of Focal Brain Tumors on Functional Complexity and Network Robustness: A Prospective Cohort Study. Neurosurgeon 2018, 84, 1201–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoecklein, V.M.; Stoecklein, S.; Galiè, F.; Ren, J.; Schmutzer, M.; Unterrainer, M.; Albert, N.L.; Kreth, F.-W.; Thon, N.; Liebig, T.; et al. Resting-state fMRI detects alterations in whole brain connectivity related to tumor biology in glioma patients. Neuro-Oncology 2020, 22, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Shi, Z.; Jiang, C.; Wang, K.; Chen, L.; Ai, L.; Zhang, L. Hemisphere-Specific Functional Remodeling and Its Relevance to Tumor Malignancy of Cerebral Glioma Based on Resting-State Functional Network Analysis. Front. Neurosci. 2021, 14, 611075. [Google Scholar] [CrossRef] [PubMed]
- Jütten, K.; Weninger, L.; Mainz, V.; Gauggel, S.; Binkofski, F.; Wiesmann, M.; Merhof, D.; Clusmann, H.; Na, C.-H. Dissociation of structural and functional connectomic coherence in glioma patients. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Van Lieshout, J.; Debaene, W.; Rapp, M.; Noordmans, H.J.; Rutten, G.-J. fMRI Resting-State Connectivity between Language and Nonlanguage Areas as Defined by Intraoperative Electrocortical Stimulation in Low-Grade Glioma Patients. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2021, 82, 357–363. [Google Scholar] [CrossRef]
- Hart, M.G.; Price, S.J.; Suckling, J. Connectome analysis for pre-operative brain mapping in neurosurgery. Br. J. Neurosurg. 2016, 30, 506–517. [Google Scholar] [CrossRef] [Green Version]
- Daniel, A.G.S.; Park, K.Y.; Roland, J.; Dierker, D.; Gross, J.; Humphries, J.B.; Hacker, C.D.; Snyder, A.Z.; Shimony, J.S.; Leuthardt, E.C. Functional connectivity within glioblastoma impacts overall survival. Neuro-Oncol 2021, 23, 412–421. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, N.; Yan, J.; Cheng, J.; Lu, J.; Wu, J. Tumor grade-related language and control network reorganization in patients with left cerebral glioma. Cortex 2020, 129, 141–157. [Google Scholar] [CrossRef]
- Cho, N.S.; Peck, K.K.; Gene, M.N.; Jenabi, M.; Holodny, A.I. Resting-state functional MRI language network connectivity differences in patients with brain tumors: Exploration of the cerebellum and contralesional hemisphere. Brain Imaging Behav. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, Y.; Jiang, T. Epilepsy enhance global efficiency of language networks in right temporal lobe gliomas. CNS Neurosci. Ther. 2021, 27, 363–371. [Google Scholar] [CrossRef]
- Liu, D.; Chen, J.; Hu, X.; Hu, G.; Liu, Y.; Yang, K.; Xiao, C.; Zou, Y.; Liu, H. Contralesional homotopic functional plasticity in patients with temporal glioma. J. Neurosurg. 2021, 134, 417–425. [Google Scholar] [CrossRef]
- Yuan, T.; Zuo, Z.; Ying, J.; Jin, L.; Kang, J.; Gui, S.; Wang, R.; Li, C. Structural and Functional Alterations in the Contralesional Medial Temporal Lobe in Glioma Patients. Front. Neurosci. 2020, 14, 10. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Vachha, B.; Laino, M.E.; Jenabi, M.; Flynn, J.R.; Zhang, Z.; Holodny, A.I.; Peck, K.K. Decreased Hand Motor Resting-State Functional Connectivity in Patients with Glioma: Analysis of Factors including Neurovascular Uncoupling. Radiology 2020, 294, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gohel, S.; Zhang, Z.; Hatzoglou, V.; Holodny, A.; Vachha, B. Glioma-Induced Disruption of Resting-State Functional Connectivity and Amplitude of Low-Frequency Fluctuations in the Salience Network. Am. J. Neuroradiol. 2021, 42, 551–558. [Google Scholar] [CrossRef]
- Almairac, F.; Deverdun, J.; Cochereau, J.; Coget, A.; Lemaitre, A.-L.; Moritz-Gasser, S.; Duffau, H.; Herbet, G. Homotopic redistribution of functional connectivity in insula-centered diffuse low-grade glioma. NeuroImage Clin. 2021, 29, 102571. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zhou, C.; Wang, Y.; Jiang, T. Contralesional functional network reorganization of the insular cortex in diffuse low-grade glioma patients. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Vassal, M.; Charroud, C.; Deverdun, J.; Le Bars, E.; Molino, F.; Bonnetblanc, F.; Boyer, A.; Dutta, A.; Herbet, G.; Moritz-Gasser, S.; et al. Recovery of functional connectivity of the sensorimotor network after surgery for diffuse low-grade gliomas involving the supplementary motor area. J. Neurosurg. 2017, 126, 1181–1190. [Google Scholar] [CrossRef] [Green Version]
- Sparacia, G.; Parla, G.; Re, V.L.; Cannella, R.; Mamone, G.; Carollo, V.; Midiri, M.; Grasso, G. Resting-State Functional Connectome in Patients with Brain Tumors Before and After Surgical Resection. World Neurosurg. 2020, 141, e182–e194. [Google Scholar] [CrossRef] [PubMed]
- Van Dokkum, L.; Gasser, S.M.; Deverdun, J.; Herbet, G.; Mura, T.; D’Agata, B.; Picot, M.; De Champfleur, N.M.; Duffau, H.; Molino, F.; et al. Resting state network plasticity related to picture naming in low-grade glioma patients before and after resection. NeuroImage Clin. 2019, 24, 102010. [Google Scholar] [CrossRef]
- Noll, K.R.; Chen, H.S.; Wefel, J.S.; Kumar, V.A.; Hou, P.; Ferguson, S.D.; Rao, G.; Johnson, J.M.; Schomer, D.F.; Suki, D.; et al. Alterations in Functional Connectomics Associated With Neurocognitive Changes Following Glioma Resection. Neurosurgeon 2020, 88, 544–551. [Google Scholar] [CrossRef]
- Nenning, K.-H.; Furtner, J.; Kiesel, B.; Schwartz, E.; Roetzer, T.; Fortelny, N.; Bock, C.; Grisold, A.; Marko, M.; Leutmezer, F.; et al. Distributed changes of the functional connectome in patients with glioblastoma. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Zhou, C.; Fan, X.; Jiang, T.; Wang, Y. Epilepsy-Related Brain Network Alterations in Patients With Temporal Lobe Glioma in the Left Hemisphere. Front. Neurol. 2020, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- De Baene, W.; Jansma, M.J.; Schouwenaars, I.T.; Rutten, G.-J.M.; Sitskoorn, M.M. Task-evoked reconfiguration of the fronto-parietal network is associated with cognitive performance in brain tumor patients. Brain Imaging Behav. 2020, 14, 2351–2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sighinolfi, G.; Mitolo, M.; Testa, C.; Martinoni, M.; Evangelisti, S.; Rochat, M.J.; Zoli, M.; Mazzatenta, D.; Lodi, R.; Tonon, C. What Can Resting-State fMRI Data Analysis Explain about the Functional Brain Connectivity in Glioma Patients? Tomography 2022, 8, 267-280. https://doi.org/10.3390/tomography8010021
Sighinolfi G, Mitolo M, Testa C, Martinoni M, Evangelisti S, Rochat MJ, Zoli M, Mazzatenta D, Lodi R, Tonon C. What Can Resting-State fMRI Data Analysis Explain about the Functional Brain Connectivity in Glioma Patients? Tomography. 2022; 8(1):267-280. https://doi.org/10.3390/tomography8010021
Chicago/Turabian StyleSighinolfi, Giovanni, Micaela Mitolo, Claudia Testa, Matteo Martinoni, Stefania Evangelisti, Magali Jane Rochat, Matteo Zoli, Diego Mazzatenta, Raffaele Lodi, and Caterina Tonon. 2022. "What Can Resting-State fMRI Data Analysis Explain about the Functional Brain Connectivity in Glioma Patients?" Tomography 8, no. 1: 267-280. https://doi.org/10.3390/tomography8010021





