Whole-Body MRI-Derived Adipose Tissue Characterization and Relationship to Pulmonary Function Impairment
Abstract
:1. Introduction
2. Materials and Methods
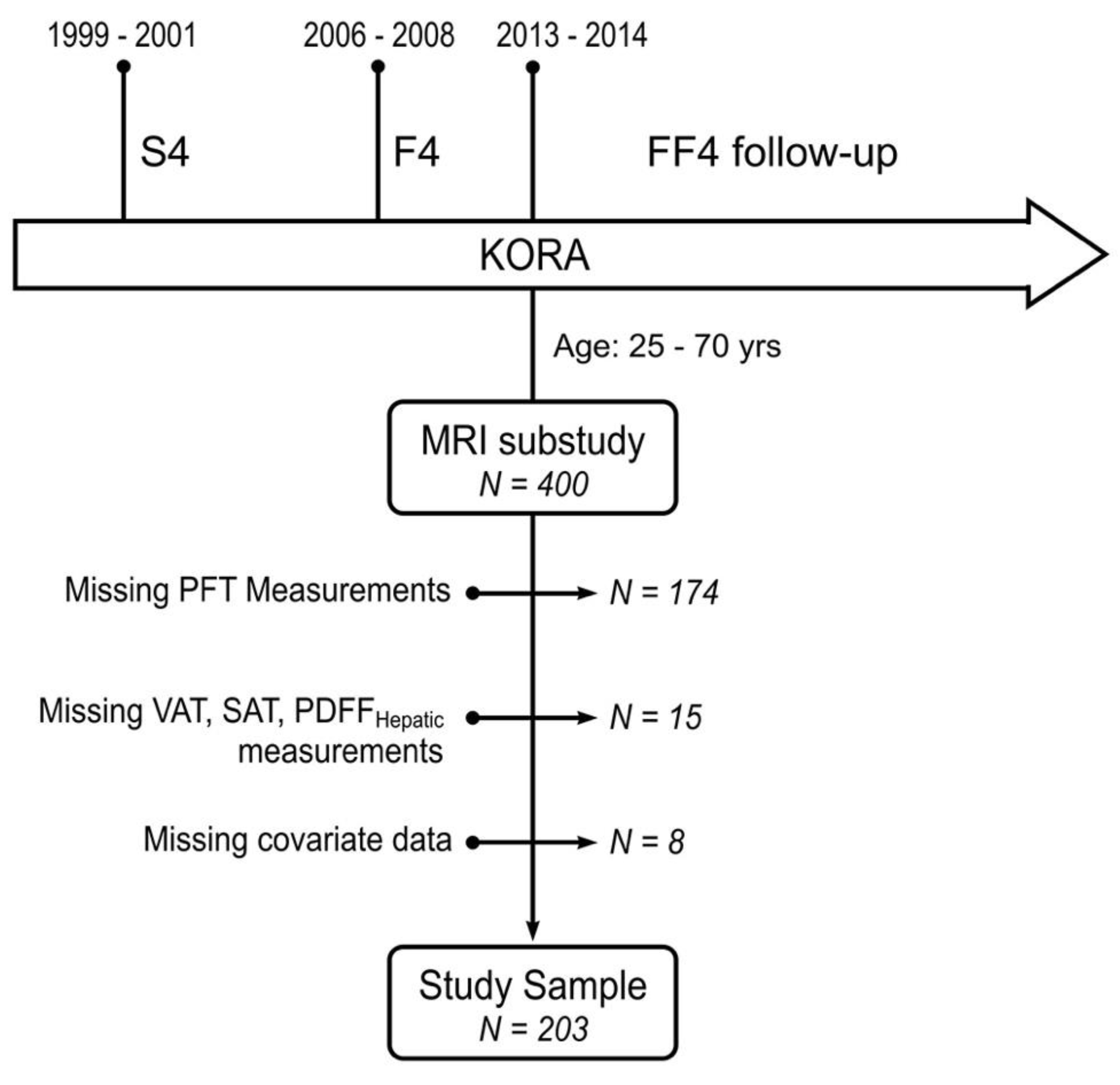
2.1. Study Design and Population
2.2. Clinical Characteristics
2.3. Whole-Body MR Imaging
2.4. Statistical Analysis
3. Results
Spirometric Parameters in Association with MR-Derived Adipose Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.-Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Cioffi, C.E.; Narayan, K.M.V.; Liu, K.; Uppal, K.; Jones, D.P.; Tran, V.; Yu, T.; Alvarez, J.A.; Bellissimo, M.P.; Maner-Smith, K.M.; et al. Hepatic fat is s stronger correlate of key clinical and molecular abnormalities than visceral abdominal subcutaneous fat in youth. BMJ Open Diabetes Res. Care 2020, 8, e001126. [Google Scholar] [CrossRef]
- Hamdy, O.; Porramatikul, S.; Al-Ozairi, E. Metabolic obesity: The paradox between visceral and subcutaneous fat. Curr. Diabetes Rev. 2006, 2, 367–373. [Google Scholar]
- Chen, W.-L.; Wang, C.-C.; Wu, L.-W.; Kao, T.-W.; Chan, J.Y.-H.; Chen, Y.-J.; Yang, Y.-H.; Chang, Y.-W.; Peng, T.-C. Relationship between lung function and metabolic syndrome. PLoS ONE 2014, 9, e108989. [Google Scholar] [CrossRef]
- Paek, Y.-J.; Jung, K.-S.; Hwang, Y.-I.; Lee, K.-S.; Lee, D.R.; Lee, J.U. Association between low pulmonary function and metabolic risk factors in Korean adults: The Korean National Health and Nutrition Survey. Metabolism 2010, 59, 1300–1306. [Google Scholar] [CrossRef]
- Oka, R.; Kobayasi, J.; Inazu, A.; Yagi, K.; Miyamoto, S.; Sakurai, M.; Nakamura, K.; Miura, K.; Nakagawa, H.; Yamagishi, M. Contribution of visceral adiposity and insulin resistance to metabolic risk factors in Japanese men. Metabolism 2010, 59, 748–754. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Breithaupt, K.; Muhajarine, N. Occurrence of chronic obstructive pulmonary disease among Canadians and sex-related risk factors. J. Clin. Epidemiol. 2000, 53, 755–761. [Google Scholar] [CrossRef]
- Chen, Y.; Dales, R.; Tang, M.; Krewski, D. Obesity may increase the incidence of asthma in women but not in men: Longitudinal observations from the Canadian National Population Health Surveys. Am. J. Epidemiol. 2002, 155, 191–197. [Google Scholar] [CrossRef]
- Thijs, W.; Dehnavi, R.A.; Hiemstra, P.S.; de Roos, A.; Melissant, C.F.; Janssen, K.; Tamsma, J.T.; Rabe, K.F. Association of lung function measurements and visceral fat in men with metabolic syndrome. Respir. Med. 2014, 108, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Unterborn, J. Pulmonary function testing in obesity, pregnancy, and extremes of body habitus. Clin. Chest Med. 2001, 22, 759–767. [Google Scholar] [CrossRef]
- Gan, W.Q.; Man, S.F.; Senthilselvan, A.; Sin, D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Lonardo, A.; Vinco, G.; Zoppini, G.; Lippi, G.; Bonora, E.; Loomba, R.; Tilg, H.; Byrne, C.D.; Fabbri, L.; et al. Association between non-alcoholic fatty liver disease and decreased lung function in adults: A systematic review and meta-analysis. Diabetes Metab. 2019, 45, 536–544. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Localization of fat depots and cardiovascular risk. Lipids Health Dis. 2018, 17, 218. [Google Scholar] [CrossRef] [Green Version]
- Yokoo, T.; Serai, S.D.; Pirasteh, A.; Bashir, M.R.; Hamilton, G.; Hernando, D.; Hu, H.H.; Hetterich, H.; Kühn, J.-P.; Kukuk, G.M.; et al. Linearity, Bias, and Precision of Hepatic Proton Density Fat Fraction Measurements by Using MR Imaging: A Meta-Analysis. Radiology 2018, 286, 486–498. [Google Scholar] [CrossRef]
- Hetterich, H.; Bayerl, C.; Peters, A.; Heier, M.; Linkohr, B.; Meisinger, C.; Auweter, S.; Kannengießer, S.A.R.; Kramer, H.; Ertl-Wagner, B.; et al. Feasibility of a three-step magnetic resonance imaging approach for the assessment of hepatic steatosis in an asymptomatic study population. Eur. Radiol. 2016, 26, 1895–1904. [Google Scholar] [CrossRef]
- Kim, S.R.; Lerman, L.O. Diagnostic imaging in the management of patients with metabolic syndrome. Transl. Res. 2018, 194, 1–18. [Google Scholar] [CrossRef]
- Bunnell, K.M.; Thaweethai, T.; Buckless, C.; Shinnick, D.J.; Torriani, M.; Foulkes, A.S.; Bredella, M.A. Body composition predictors of outcome in patients with COVID-19. Int J. Obes. 2021, 45, 2238–2243. [Google Scholar] [CrossRef]
- Chandarana, H.; Dane, B.; Mikheev, A.; Taffel, M.T.; Feng, Y.; Rusinek, H. Visceral adipose tissue in patients with COVID-19: Risk stratification for severity. Abdom. Radiol. 2021, 46, 818–825. [Google Scholar] [CrossRef]
- Bamberg, F.; Hetterich, H.; Rospleszcz, S.; Lorbeer, R.; Auweter, S.D.; Schlett, C.L.; Schafnitzel, A.; Bayerl, C.; Schindler, A.; Saam, T.; et al. Subclinical Disease Burden as Assessed by Whole-Body MRI in Subjects with Prediabetes, Subjects with Diabetes, and Normal Control Subjects from the General Population: The KORA-MRI Study. Diabetes 2017, 66, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Karrasch, S.; Flexeder, C.; Behr, J.; Holle, R.; Huber, R.M.; Jörres, R.A.; Nowak, D.; Peters, A.; Wichmann, H.-E.; Heinrich, J.; et al. Spirometric reference values for advanced age from a South german population. Respiration 2013, 85, 210–219. [Google Scholar] [CrossRef] [Green Version]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [Green Version]
- Mueller, J.; Karrasch, S.; Lorbeer, R.; Ivanovska, T.; Pomschar, A.; Kunz, W.G.; von Krüchten, R.; Peters, A.; Bamberg, F.; Schulz, H.; et al. Automated MR-based lung volume segmentation in population-based whole-body MR imaging: Correlation with clinical characteristics, pulmonary function testing and obstructive lung disease. Eur. Radiol. 2019, 29, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-year age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Würslin, C.; Machann, J.; Rempp, H.; Claussen, C.; Yang, B.; Schick, F. Topography mapping of whole body adipose tissue using A fully automated and standardized procedure. J. Magn. Reson. Imaging 2010, 31, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Storz, C.; Heber, S.D.; Rospleszcz, S.; Machann, J.; Sellner, S.; Nikolaou, K.; Lorbeer, R.; Gatidis, S.; Elser, S.; Peters, A.; et al. The role of visceral and subcutaneous adipose tissue measurements and their ratio by magnetic resonance imaging in subjects with prediabetes, diabetes and healthy controls from a general population without cardiovascular disease. Br. J. Radiol. 2018, 91, 20170808. [Google Scholar] [CrossRef] [PubMed]
- Lorbeer, R.; Bayerl, C.; Auweter, S.; Rospleszcz, S.; Lieb, W.; Meisinger, C.; Heier, M.; Peters, A.; Bamberg, F.; Hetterich, H. Association between MRI-derived hepatic fat fraction and blood pressure in participants without history of cardiovascular disease. J. Hypertens. 2017, 35, 737–744. [Google Scholar] [CrossRef]
- Canoy, D.; Luben, R.; Welch, A.; Bingham, S.; Wareham, N.; Day, N.; Khaw, K.T. Abdominal obesity and respiratory function in men and women in the EPIC-Norfolk Study, United Kingdom. Am. J. Epidemiol. 2004, 159, 1140–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochs-Balcom, H.M.; Grant, B.J.; Muti, P.; Sempos, C.T.; Freudenheim, J.L.; Trevisan, M.; Cassano, P.A.; Iacoviello, L.; Schünemann, H.J. Pulmonary function and abdominal adiposity in the general population. Chest 2006, 129, 853–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leone, N.; Courbon, D.; Thomas, F.; Bean, K.; Jégo, B.; Leynaert, B.; Guize, L.; Zureik, M. Lung function impairment and metabolic syndrome: The critical role of abdominal obesity. Am. J. Respir. Crit. Care Med. 2009, 179, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Choe, E.K.; Kang, H.Y.; Lee, Y.; Choi, S.H.; Kim, H.J.; Kim, J.S. The longitudinal association between changes in lung function and changes in abdominal visceral obesity in Korean non-smokers. PLoS ONE 2018, 13, e0193516. [Google Scholar] [CrossRef] [Green Version]
- Klopfenstein, B.J.; Kim, M.S.; Krisky, C.M.; Szumowski, J.; Rooney, W.D.; Purnell, J.Q. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br. J. Radiol. 2012, 85, e826–e830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, S.W.; Kim, S.Y.; Jung, J.Y.; Kang, Y.A.; Park, M.S.; Kim, Y.S.; Chang, J.; Ro, J.S.; Lee, Y.-H.; Lee, S.H. Relationship between obstructive lung disease and non-alcoholic fatty liver disease in the Korean population: Korea National Health and Nutrition Examination Survey, 2007–2010. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2603–2611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, D.-H.; Shim, J.-Y.; Lee, H.-R.; Moon, B.-S.; Park, B.-J.; Lee, Y.J. Relationship between non-alcoholic fatty liver disease and pulmonary function. Intern. Med. J. 2012, 42, 541–546. [Google Scholar] [CrossRef]
- Peng, T.-C.; Kao, T.-W.; Wu, L.-W.; Chen, Y.-J.; Chang, Y.-W.; Wang, C.-C.; Tsao, Y.-T.; Chen, W.-L. Association between Pulmonary Function and Nonalcoholic Fatty Liver Disease in the NHANES III Study. Medicine 2015, 94, e907. [Google Scholar] [CrossRef]
- Song, J.-U.; Jang, Y.; Lim, S.-Y.; Ryu, S.; Song, W.J.; Byrne, C.D.; Sung, K.-C. Decreased lung function is associated with risk of developing non-alcoholic fatty liver disease: A longitudinal cohort study. PLoS ONE 2019, 14, e0208736. [Google Scholar] [CrossRef] [PubMed]
- Takamura, T.; Misu, H.; Ota, T.; Kaneko, S. Fatty liver as a consequence and cause of insulin resistance: Lessons from type 2 diabetic liver. Endocr. J. 2012, 59, 745–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarus, R.; Sparrow, D.; Weiss, S.T. Baseline ventilatory function predicts the development of higher levels of fasting insulin and fasting insulin resistance index: The Normative Aging Study. Eur. Respir. J. 1998, 12, 641–645. [Google Scholar] [CrossRef] [Green Version]
- Reeder, S.B.; Cruite, I.; Hamilton, G.; Sirlin, C.B. Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and Spectroscopy. J. Magn. Reson. Imaging 2011, 34, 729–749. [Google Scholar] [CrossRef]
- Goehler, A.; Hsu, T.-M.H.; Seiglie, J.A.; Siedner, M.J.; Lo, J.; Triant, V.; Hsu, J.; Foulkes, A.; Bassett, I.; Khorasani, R.; et al. Visceral adiposity and severe COVID-19 disease: Application of an artificial intelligence algorithm to improve clinical risk prediction. Open Forum Infect. Dis. 2021, 8, ofab275. [Google Scholar] [CrossRef]
| Whole Sample | Subjects with OLD | Subjects without OLD | p-Value | |
|---|---|---|---|---|
| Total amount | 203 | 23 | 180 | |
| Age, years | 58.0 ± 5.8 | 56.6 ± 6.1 | 58.1 ± 5.7 | 0.22 |
| Men | 117 (57.6%) | 16 (69.6%) | 101 (56.1%) | 0.32 |
| Height, cm | 171.4 ± 10.0 | 173.8 ± 11.9 | 171.1 ± 9.7 | 0.22 |
| BMI, kg/m2 | 28.0 ± 4.4 | 27.0 ± 5.0 | 28.2 ± 4.3 | 0.21 |
| Body Surface Area, m2 | 1.9 ± 0.2 | 2.0 ± 0.2 | 1.9 ± 0.2 | 0.86 |
| Smoking | 0.53 | |||
| never-smoker | 79 (38.9%) | 7 (30.4%) | 72 (40.0%) | |
| ex-smoker | 79 (38.9%) | 9 (39.1%) | 70 (38.9%) | |
| smoker | 45 (22.2%) | 7 (30.4%) | 38 (21.1%) | |
| Pack-years (only ex-smoker and smoker) | 20.2 ± 20.4 | 25.5 ± 19.3 | 19.5 ± 20.5 | 0.27 |
| Glycemic status | 0.82 | |||
| normoglycemic | 122 (60.1%) | 13 (56.5%) | 109 (60.6%) | |
| prediabetes | 53 (26.1%) | 6 (26.1%) | 47 (26.1%) | |
| diabetes | 28 (13.8%) | 4 (17.4%) | 24 (13.3%) | |
| Physically active | 124 (61.1%) | 15 (65.2%) | 109 (60.6%) | 0.84 |
| Hypertension | 73 (36.0%) | 8 (34.8%) | 65 (36.1%) | 1.00 |
| Antihypertensive Medication | 55 (27.1%) | 5 (21.7%) | 50 (27.8%) | 0.72 |
| Lipid-lowering Medication | 25 (12.3%) | 2 (8.7%) | 23 (12.8%) | 0.75 |
| Total Cholesterol, mg/dL | 221.8 ± 37.4 | 219.5 ± 44.9 | 222.1 ± 36.4 | 0.76 |
| HDL Cholesterol, mg/dL | 62.5 ± 17.7 | 58.5 ± 15.6 | 63.0 ± 17.9 | 0.24 |
| LDL Cholesterol, mg/dL | 141.9 ± 34.3 | 140.5 ± 40.2 | 142.1 ± 33.6 | 0.84 |
| Triglycerides, mg/dL | 137.5 ± 90.9 | 154.6 ± 126.9 | 135.3 ± 85.4 | 0.34 |
| Alcohol consumption, g/d | 21.7 ± 27.1 | 19.4 ± 22.3 | 22.0 ± 27.7 | 0.66 |
| Pulmonary Function Test | ||||
| FEV1/FVC, % | 74.8 ± 7.7 | 59.7 ± 6.9 | 76.7 ± 5.2 | <0.001 |
| FEV1, L/s | 3.1 ± 0.8 | 2.6 ± 0.8 | 3.2 ± 0.8 | 0.001 |
| FVC, L | 4.2 ± 1.0 | 4.4 ± 1.1 | 4.2 ± 1.0 | 0.39 |
| Whole Sample | Presence of OLD | Absence of OLD | p-Value | |
|---|---|---|---|---|
| Visceral adipose tissue, L | 4.8 ± 2.7 | 4.2 ± 2.8 | 4.8 ± 2.7 | 0.31 |
| Subcutaneous adipose tissue, L | 8.1 ± 3.4 | 7.4 ± 3.8 | 8.2 ± 3.3 | 0.26 |
| Total abdominal adipose tissue, L | 12.9 ± 5.1 | 11.6 ± 5.8 | 13.1 ± 5.0 | 0.20 |
| PDFFhepatic, % (median[Q1, Q3]) | 4.7 [2.9, 13.1] | 3.9 [1.9, 6.1] | 5.4 [2.9, 14.5] | 0.04 |
| hsCRP, mg/L (median[Q1, Q3]) | 1.1 [0.6, 2.4] | 1.0 [0.7, 4.0] | 1.1 [0.6, 2.2] | 0.53 |
| FEV1 | FVC | FEV1/FVC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | β | 95%CI | p | β | 95%CI | p | β | 95%CI | p | |
| VAT | ||||||||||
| simple | −0.23 | [−0.34, −0.13] | <0.001 | −0.38 | [−0.49, −0.26] | <0.001 | 1.15 | [−0.36, 2.65] | 0.13 | |
| adjusted | −0.19 | [−0.30, −0.08] | <0.001 | −0.35 | [−0.48, −0.23] | <0.001 | 1.71 | [0.09, 3.33] | 0.04 | |
| +physAct | −0.19 | [−0.30, −0.08] | 0.001 | −0.34 | [−0.47, −0.22] | <0.001 | 1.68 | [0.04, 3.32] | 0.045 | |
| +obesity | −0.13 | [−0.25, −0.02] | 0.03 | −0.27 | [−0.40, −0.14] | <0.001 | 1.50 | [−0.22, 3.23] | 0.09 | |
| SAT | ||||||||||
| simple | −0.31 | [−0.41, −0.20] | <0.001 | −0.49 | [−0.60, −0.37] | <0.001 | 1.25 | [−0.34, 2.84] | 0.12 | |
| adjusted | −0.31 | [−0.42, −0.21] | <0.001 | −0.49 | [−0.60, −0.37] | <0.001 | 1.20 | [−0.45, 2.84] | 0.15 | |
| +physAct | −0.48 | [−0.60, −0.36] | <0.001 | −0.31 | [−0.42, −0.20] | <0.001 | 1.17 | [−0.49, 2.83] | 0.166 | |
| +obesity | −0.26 | [−0.39, −0.14] | <0.001 | −0.41 | [−0.55, −0.28] | <0.001 | 0.84 | [−1.06, 2.75] | 0.38 | |
| PDFFhepatic | ||||||||||
| simple | −0.09 | [−0.18, −0.01] | 0.03 | −0.24 | [−0.33, −0.15] | <0.001 | 2.16 | [1.01, 3.32] | <0.001 | |
| adjusted | −0.07 | [−0.17, 0.02] | 0.14 | −0.22 | [−0.33, −0.11] | <0.001 | 2.55 | [1.23, 3.86] | <0.001 | |
| +physAct | −0.07 | [−0.16, 0.03] | 0.180 | −0.21 | [−0.32, −0.11] | <0.001 | 2.54 | [1.21, 3.87] | <0.001 | |
| +obesity | −0.03 | [−0.12, 0.06] | 0.52 | −0.17 | [−0.27, −0.06] | 0.002 | 2.46 | [1.11, 3.81] | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüchten, R.v.; Rospleszcz, S.; Lorbeer, R.; Hasic, D.; Peters, A.; Bamberg, F.; Schulz, H.; Karrasch, S.; Schlett, C.L. Whole-Body MRI-Derived Adipose Tissue Characterization and Relationship to Pulmonary Function Impairment. Tomography 2022, 8, 560-569. https://doi.org/10.3390/tomography8020046
Krüchten Rv, Rospleszcz S, Lorbeer R, Hasic D, Peters A, Bamberg F, Schulz H, Karrasch S, Schlett CL. Whole-Body MRI-Derived Adipose Tissue Characterization and Relationship to Pulmonary Function Impairment. Tomography. 2022; 8(2):560-569. https://doi.org/10.3390/tomography8020046
Chicago/Turabian StyleKrüchten, Ricarda von, Susanne Rospleszcz, Roberto Lorbeer, Dunja Hasic, Annette Peters, Fabian Bamberg, Holger Schulz, Stefan Karrasch, and Christopher L. Schlett. 2022. "Whole-Body MRI-Derived Adipose Tissue Characterization and Relationship to Pulmonary Function Impairment" Tomography 8, no. 2: 560-569. https://doi.org/10.3390/tomography8020046
APA StyleKrüchten, R. v., Rospleszcz, S., Lorbeer, R., Hasic, D., Peters, A., Bamberg, F., Schulz, H., Karrasch, S., & Schlett, C. L. (2022). Whole-Body MRI-Derived Adipose Tissue Characterization and Relationship to Pulmonary Function Impairment. Tomography, 8(2), 560-569. https://doi.org/10.3390/tomography8020046






