Diffusion Restriction Comparison between Gleason 4 Fused Glands and Cribriform Glands within Patient Using Whole-Mount Prostate Pathology as Ground Truth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
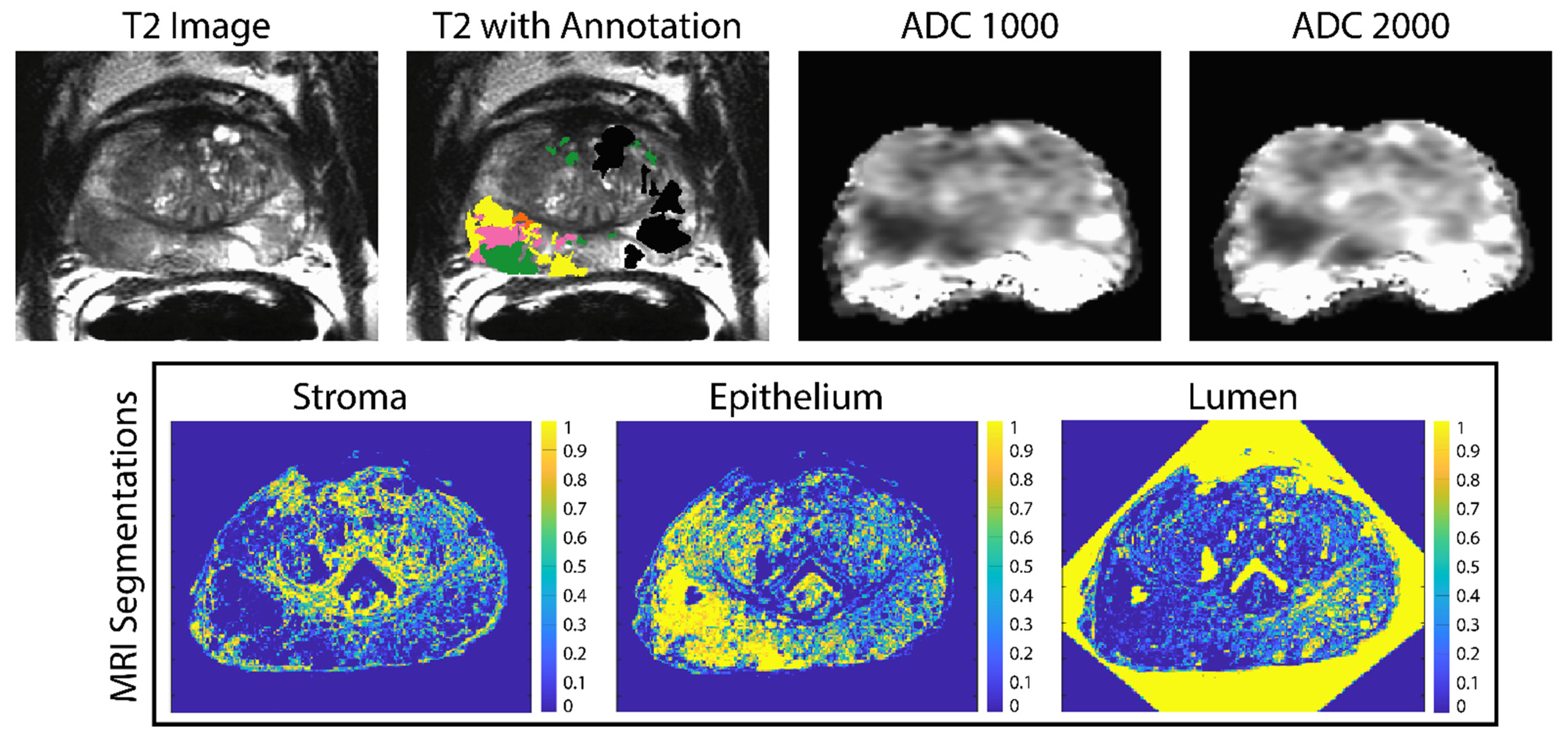
2.2. Imaging and MRI Pre-Processing
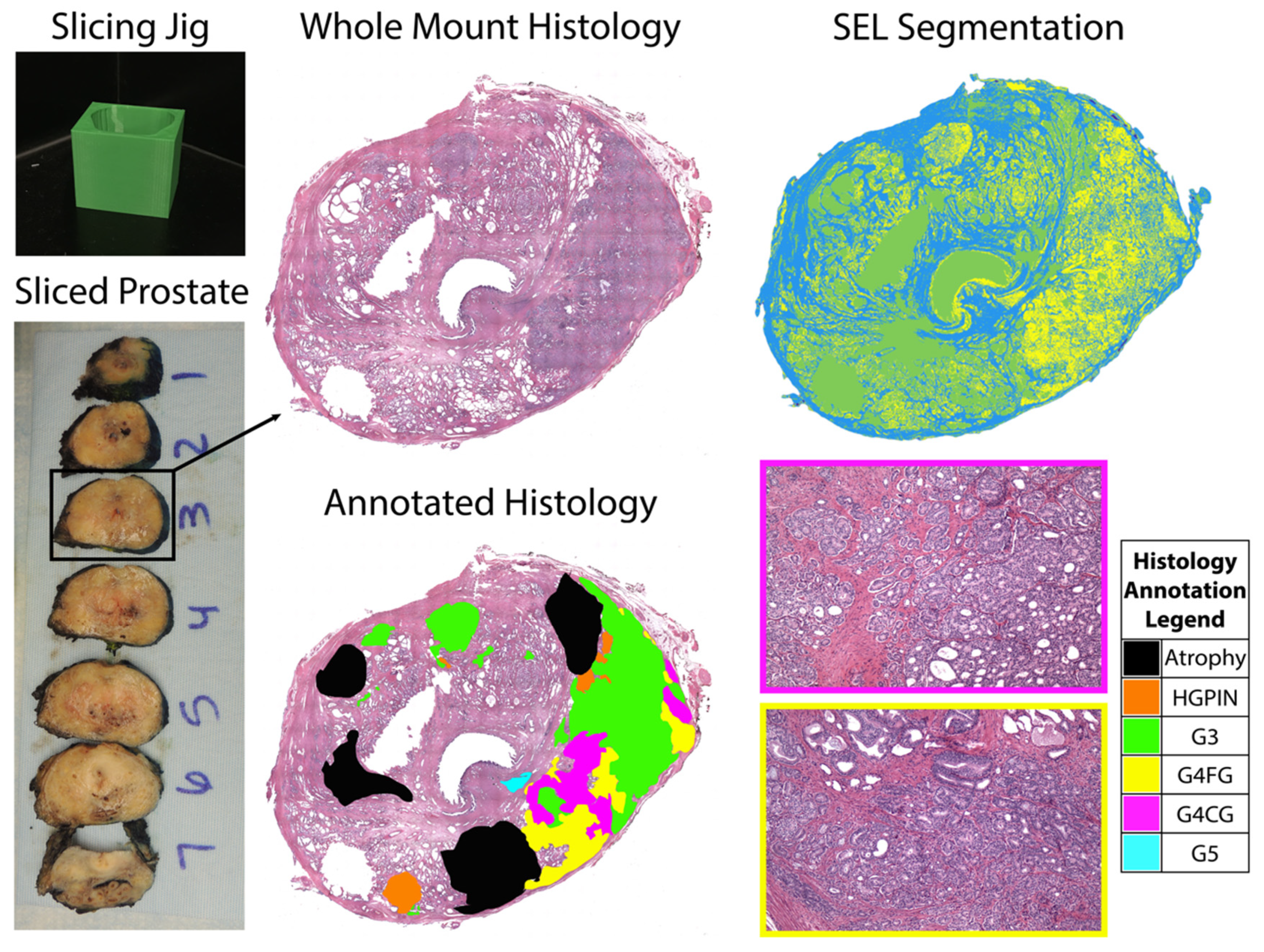
2.3. Surgery and Tissue Sectioning
2.4. Tissue Segmentation and Annotation
2.5. Histology Co-Registration
2.6. Linear Regression Models
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Epstein, J.I.; Zelefsky, M.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, G.P.; Chen, W.; Trevathan, S.; Hermans, M. Long-Term Follow-Up after Prostatectomy for Prostate Cancer and the Need for Active Monitoring. Prostate Cancer 2020, 2020, 7196189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iczkowski, K.A.; Torkko, K.C.; Kotnis, G.R.; Wilson, R.S.; Huang, W.; Wheeler, T.M.; Abeyta, A.M.; La Rosa, F.G.; Cook, S.; Werahera, P.N.; et al. Digital Quantification of Five High-Grade Prostate Cancer Patterns, Including the Cribriform Pattern, and Their Association with Adverse Outcome. Am. J. Clin. Pathol. 2011, 136, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Hassan, O.; Matoso, A. Clinical significance of subtypes of Gleason pattern 4 prostate cancer. Transl. Androl. Urol. 2018, 7, S477–S483. [Google Scholar] [CrossRef]
- Montironi, R.; Cimadamore, A.; Gasparrini, S.; Mazzucchelli, R.; Santoni, M.; Massari, F.; Cheng, L.; Lopez-Beltran, A.; Scarpelli, M. Prostate cancer with cribriform morphology: Diagnosis, aggressiveness, molecular pathology and possible relationships with intraductal carcinoma. Expert Rev. Anticancer Ther. 2018, 18, 685–693. [Google Scholar] [CrossRef]
- Erho, N.; Crisan, A.; Vergara, I.A.; Mitra, A.P.; Ghadessi, M.; Buerki, C.; Bergstralh, E.J.; Kollmeyer, T.; Fink, S.; Haddad, Z.; et al. Discovery and Validation of a Prostate Cancer Genomic Classifier that Predicts Early Metastasis Following Radical Prostatectomy. PLoS ONE 2013, 8, e66855. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.S.; Morgan, T.M.; Wallington, D.G.; Chinnaiyan, A.M.; Spratt, D.E.; Mehra, R. Correlation between cribriform/intraductal prostatic adenocarcinoma and percent Gleason pattern 4 to a 22-gene genomic classifier. Prostate 2020, 80, 146–152. [Google Scholar] [CrossRef]
- Iczkowski, K.A.; Paner, G.P.; Van der Kwast, T. The New Realization About Cribriform Prostate Cancer. Adv. Anat. Pathol. 2018, 25, 31–37. [Google Scholar] [CrossRef]
- Dong, F.; Yang, P.; Wang, C.; Wu, S.; Xiao, Y.; McDougal, W.S.; Young, R.H.; Wu, C.-L. Architectural Heterogeneity and Cribriform Pattern Predict Adverse Clinical Outcome for Gleason Grade 4 Prostatic Adenocarcinoma. Am. J. Surg. Pathol. 2013, 37, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Kir, G.; Sarbay, B.; Gümüş, E.; Topal, C. The association of the cribriform pattern with outcome for prostatic adenocarcinomas. Pathol.-Res. Pract. 2014, 210, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Trudel, D.; Downes, M.R.; Sykes, J.; Kron, K.J.; Trachtenberg, J.; van der Kwast, T.H. Prognostic impact of intraductal carcinoma and large cribriform carcinoma architecture after prostatectomy in a contemporary cohort. Eur. J. Cancer 2014, 50, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Kweldam, C.F.; Wildhagen, M.F.; Steyerberg, E.W.; Bangma, C.H.; Van der Kwast, T.; Van Leenders, G.J.L.H. Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer. Mod. Pathol. 2015, 28, 457–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hambrock, T.; Somford, D.M.; Huisman, H.J.; Van Oort, I.M.; Witjes, J.A.; De Kaa, C.A.H.-V.; Scheenen, T.; Barentsz, J.O. Relationship between Apparent Diffusion Coefficients at 3.0-T MR Imaging and Gleason Grade in Peripheral Zone Prostate Cancer. Radiology 2011, 259, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Vos, E.K.; Kobus, T.; Litjens, G.J.; Hambrock, T.; de Kaa, C.A.H.-V.; Barentsz, J.O.; Maas, M.C.; Scheenen, T.W. Multiparametric Magnetic Resonance Imaging for Discriminating Low-Grade from High-Grade Prostate Cancer. Investig. Radiol. 2015, 50, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Wichtmann, B.D.; Zöllner, F.G.; Attenberger, U.I.; Schönberg, S.O. Multiparametric MRI in the Diagnosis of Prostate Cancer: Physical Foundations, Limitations, and Prospective Advances of Diffusion-Weighted MRI. RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der Bildgebenden Verfahren 2021, 193, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Tewes, S.; Mokov, N.; Hartung, D.; Schick, V.; Peters, I.; Schedl, P.; Pertschy, S.; Wacker, F.; Voshage, G.; Hueper, K. Standardized Reporting of Prostate MRI: Comparison of the Prostate Imaging Reporting and Data System (PI-RADS) Version 1 and Version 2. PLoS ONE 2016, 11, e0162879. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.A.; Hotker, A.M.; Goldman, D.A.; Moskowitz, C.S.; Gondo, T.; Matsumoto, K.; Ehdaie, B.; Woo, S.; Fine, S.W.; Reuter, V.E.; et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: Critical evaluation using whole-mount pathology as standard of reference. Eur. Radiol. 2016, 26, 1606–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallis, C.J.D.; Haider, M.A.; Nam, R.K. Role of mpMRI of the prostate in screening for prostate cancer. Transl. Androl. Urol. 2017, 6, 464–471. [Google Scholar] [CrossRef] [Green Version]
- Ghai, S.; Haider, M.A. Multiparametric-MRI in diagnosis of prostate cancer. Indian J. Urol. 2015, 31, 194–201. [Google Scholar] [CrossRef]
- Arnold, C.W.; Wallace, W.D.; Chen, S.; Oh, A.; Abtin, F.; Genshaft, S.; Binder, S.; Aberle, D.; Enzmann, D. A Web-based System for Integrating and Correlating Radiology and Pathology Findings During Cancer Diagnosis. Acad. Radiol. 2016, 23, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.S.; Milbach, N.; Hurrell, S.L.; Cochran, E.; Connelly, J.; Bovi, J.; Schultz, C.; Mueller, W.M.; Rand, S.D.; Schmainda, K.M.; et al. Progressing Bevacizumab-Induced Diffusion Restriction Is Associated with Coagulative Necrosis Surrounded by Viable Tumor and Decreased Overall Survival in Patients with Recurrent Glioblastoma. Am. J. Neuroradiol. 2016, 37, 2201–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, D.L.; Van der Kwast, T.; Evans, A.J.; Plotkin, A.; Trachtenberg, J.; Wilson, B.C.; Haider, M.A. Prostate Tissue Composition and MR Measurements: Investigating the Relationships between ADC, T2, Ktrans, Ve, and Corresponding Histologic Features. Radiology 2010, 255, 485–494. [Google Scholar] [CrossRef]
- McGarry, S.D.; Brehler, M.; Bukowy, J.D.; Bs, A.K.L.; Bs, S.A.B.; Bs, S.R.D.; Banerjee, A.; Bs, S.L.H.; Malyarenko, D.; Chenevert, T.L.; et al. Multi-Site Concordance of Diffusion-Weighted Imaging Quantification for Assessing Prostate Cancer Aggressiveness. J. Magn. Reson. Imaging 2021. [Google Scholar] [CrossRef]
- Shah, V.; Pohida, T.; Turkbey, B.; Mani, H.; Merino, M.; Pinto, P.A.; Choyke, P.; Bernardo, M. A method for correlatingin vivoprostate magnetic resonance imaging and histopathology using individualized magnetic resonance -based molds. Rev. Sci. Instrum. 2009, 80, 104301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGarry, S.D.; Bukowy, J.D.; Iczkowski, K.A.; Unteriner, J.G.; Duvnjak, P.; Lowman, A.K.; Jacobsohn, K.; Hohenwalter, M.; Griffin, M.O.; Barrington, A.W.; et al. Gleason Probability Maps: A Radiomics Tool for Mapping Prostate Cancer Likelihood in MRI Space. Tomography 2019, 5, 127–134. [Google Scholar] [CrossRef]
- McGarry, S.D.; Hurrell, S.L.; Kaczmarowski, A.L.; Cochran, E.J.; Connelly, J.; Rand, S.D.; Schmainda, K.M.; LaViolette, P.S. Magnetic Resonance Imaging-Based Radiomic Profiles Predict Patient Prognosis in Newly Diagnosed Glioblastoma Before Therapy. Tomography 2016, 2, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Hemal, A.K.; Team, V. Vattikuti Institute Prostatectomy: A Technique of Robotic Radical Prostatectomy: Experience in More than 1000 Cases. J. Endourol. 2004, 18, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Jeong, W.; Peabody, J.O.; Hemal, A.K.; Menon, M. Robot-Assisted Radical Prostatectomy: Inching toward Gold Standard. Urol. Clin. N. Am. 2014, 41, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Cox, R. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, S.L.; McGarry, S.D.; Kaczmarowski, A.; Iczkowski, K.A.; Jacobsohn, K.; Hohenwalter, M.D.; Hall, W.A.; See, W.A.; Banerjee, A.; Charles, D.K.; et al. Optimized b-value selection for the discrimination of prostate cancer grades, including the cribriform pattern, using diffusion weighted imaging. J. Med. Imaging 2017, 5, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGarry, S.D.; Hurrell, S.L.; Iczkowski, K.A.; Hall, W.; Kaczmarowski, A.L.; Banerjee, A.; Keuter, T.; Jacobsohn, K.; Bukowy, J.D.; Nevalainen, M.T.; et al. Radio-pathomic Maps of Epithelium and Lumen Density Predict the Location of High-Grade Prostate Cancer. Int. J. Radiat. Oncol. 2018, 101, 1179–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGarry, S.D.; Bukowy, J.D.; Iczkowski, K.A.; Lowman, A.K.; Brehler, M.; Bobholz, S.; Nencka, A.; Barrington, A.; Jacobsohn, K.; Unteriner, J.; et al. Radio-pathomic mapping model generated using annotations from five pathologists reliably distinguishes high-grade prostate cancer. J. Med. Imaging 2020, 7, 054501. [Google Scholar] [CrossRef] [PubMed]
- Bobholz, S.A.; Lowman, A.K.; Barrington, A.; Brehler, M.; McGarry, S.; Cochran, E.J.; Connelly, J.; Mueller, W.M.; Agarwal, M.; O’Neill, D.; et al. Radiomic Features of Multiparametric MRI Present Stable Associations with Analogous Histological Features in Patients with Brain Cancer. Tomography 2020, 6, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Barbur, V.A.; Montgomery, D.C.; Peck, E.A. Introduction to Linear Regression Analysis. Statistician 1994, 43, 339. [Google Scholar] [CrossRef]
- Yan, X.; Su, X.G. Linear regression analysis: Theory and computing. In Linear Regression Analysis: Theory and Computing; World Scientific Publishing Co. Pte. Ltd.: Hackensack, NJ, USA, 2009. [Google Scholar]
- Glasserman, P. Linear Regression; Columbia Business School: New York, NY, USA, 2001. [Google Scholar]
- Oskolkov, N. How Linear Mixed Model Works; Towards Data Science: Toronto, ON, Canada, 2020. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M. Linear Mixed-Effects Models: Basic Concepts and Examples. In Mixed-Effects Models in S and S-PLUS, 1st ed.; Springer: New York, NY, USA, 2000; pp. 3–56. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
| Recruited Patients (n = 48) | Study Cohort (n = 22) | |
|---|---|---|
| Age at RP, years (mean, SD) | 61 (5.9) | 63 (4.3) |
| Race (n, %) | ||
| African American | 7 (15) | 3 (13) |
| White/Caucasian | 40 (83) | 18 (82) |
| Other | 1 (2) | 1 (5) |
| Preoperative PSA, ng/mL (n, %) | ||
| ≤10 | 38 (79) | 14 (64) |
| 10.1–20.0 | 8 (17) | 6 (27) |
| ≥20 | 2 (40 | 2 (9) |
| Grade group at RP (n, %) | ||
| 6 | 9 (19) | 1 (5) |
| 3 + 4 | 22 (46) | 11 (50) |
| 4 + 3 | 6 (12) | 4 (18) |
| 8 | 8 (17) | 4 (18) |
| ≥9 | 3 (6) | 2 (9) |
| pT (n, %) | ||
| 1 | 33 (69) | 14 (64) |
| 2 | 11 (23) | 6 (27) |
| 3 | 4 (8) | 2 (9) |
| Gleason 4 Subtypes (n, %) | ||
| Cribriform glands | 27 (56) | 22 (100) |
| Fused glands | 40 (83) | 22 (100) |
| ADC1000 | Fixed effect | Estimate | 95% CI | t-value | Pr (>|t|) |
| Intercept | 1.274 | (1.176, 1.373) | 25.88 | <0.0001 | |
| Cribriform vs. Fused Glands | −0.096 | (−0.103, −0.089) | −26.682 | <0.0001 | |
| Random effect | Std Dev | 95% CI | |||
| Subject (Intercept) | 0.231 | (0.172, 0.313) | |||
| Residual | 0.354 | (0.352, 0.355) | |||
| ACD2000 | Fixed effect | Estimate | 95% CI | t-value | Pr (>|t|) |
| Intercept | 0.933 | (0.862, 1.003) | 26.601 | <0.0001 | |
| Cribriform vs. Fused Glands | −0.062 | (−0.066, −0.057) | −26.428 | <0.0001 | |
| Random effect | Std Dev | 95% CI | |||
| Subject (Intercept) | 0.164 | (0.123, 0.223) | |||
| Residual | 0.229 | (0.228, 0.230) | |||
| Lumen | Fixed effect | Estimate | 95% CI | t-value | Pr (>|t|) |
| Intercept | 0.0515 | (0.041, 0.062) | 9.755 | <0.0001 | |
| Cribriform vs. Fused Glands | 0.0173 | (0.015, 0.02) | 12.295 | <0.0001 | |
| Random effect | Std Dev | 95% CI | |||
| Subject (Intercept) | 0.0244 | (0.018, 0.033) | |||
| Residual | 0.138 | (0.138, 0.139) | |||
| Stroma | Fixed effect | Estimate | 95% CI | t-value | Pr (>|t|) |
| Intercept | 0.831 | (0.799, 0.863) | 51.682 | <0.0001 | |
| Cribriform vs. Fused Glands | 0.094 | (0.088, 0.099) | 35.276 | <0.0001 | |
| Random effect | Std Dev | 95% CI | |||
| Subject (Intercept) | 0.0749 | (0.056, 0.102) | |||
| Residual | 0.260 | (0.259, 0.261) | |||
| Epithelium | Fixed effect | Estimate | 95% CI | t-value | Pr (>|t|) |
| Intercept | 0.118 | (0.088, 0.147) | 7.971 | <0.0001 | |
| Cribriform vs. Fused Glands | −0.111 | (−0.115, −0.106) | −50.11 | <0.0001 | |
| Random effect | Std Dev | 95% CI | |||
| Subject (Intercept) | 0.0689 | (0.051, 0.094) | |||
| Residual | 0.217 | (0.216, 0.218) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duenweg, S.R.; Fang, X.; Bobholz, S.A.; Lowman, A.K.; Brehler, M.; Kyereme, F.; Iczkowski, K.A.; Jacobsohn, K.M.; Banerjee, A.; LaViolette, P.S. Diffusion Restriction Comparison between Gleason 4 Fused Glands and Cribriform Glands within Patient Using Whole-Mount Prostate Pathology as Ground Truth. Tomography 2022, 8, 635-643. https://doi.org/10.3390/tomography8020053
Duenweg SR, Fang X, Bobholz SA, Lowman AK, Brehler M, Kyereme F, Iczkowski KA, Jacobsohn KM, Banerjee A, LaViolette PS. Diffusion Restriction Comparison between Gleason 4 Fused Glands and Cribriform Glands within Patient Using Whole-Mount Prostate Pathology as Ground Truth. Tomography. 2022; 8(2):635-643. https://doi.org/10.3390/tomography8020053
Chicago/Turabian StyleDuenweg, Savannah R., Xi Fang, Samuel A. Bobholz, Allison K. Lowman, Michael Brehler, Fitzgerald Kyereme, Kenneth A. Iczkowski, Kenneth M. Jacobsohn, Anjishnu Banerjee, and Peter S. LaViolette. 2022. "Diffusion Restriction Comparison between Gleason 4 Fused Glands and Cribriform Glands within Patient Using Whole-Mount Prostate Pathology as Ground Truth" Tomography 8, no. 2: 635-643. https://doi.org/10.3390/tomography8020053
APA StyleDuenweg, S. R., Fang, X., Bobholz, S. A., Lowman, A. K., Brehler, M., Kyereme, F., Iczkowski, K. A., Jacobsohn, K. M., Banerjee, A., & LaViolette, P. S. (2022). Diffusion Restriction Comparison between Gleason 4 Fused Glands and Cribriform Glands within Patient Using Whole-Mount Prostate Pathology as Ground Truth. Tomography, 8(2), 635-643. https://doi.org/10.3390/tomography8020053







