Stereogram of the Living Heart, Lung, and Adjacent Structures
Abstract
:1. Introduction
2. Concept and Types of Binocular Stereopsis
3. Preparation of a Stereogram
4. Representative Images
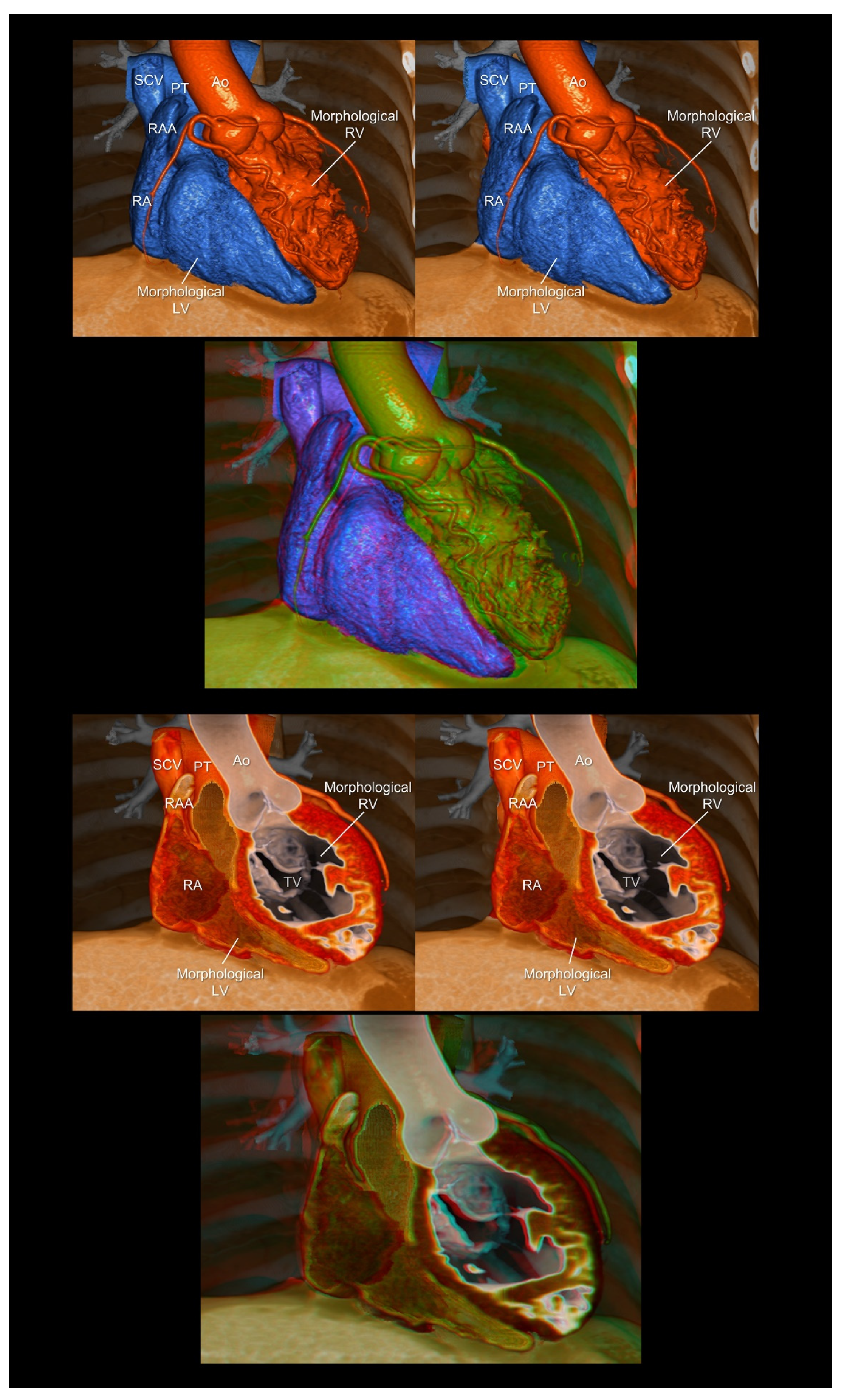
4.1. Attitudinal Position of the Living Heart within the Chest
4.2. Coronary Arteries
4.3. Coronary Veins
4.4. Pulmonary Arteries
4.5. Valvar Heart Diseases
4.6. Congenital Heart Disease
4.7. Cardiac Mass
4.8. Virtual Procedural Simulation
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soliman, O.I.; Kirschbaum, S.W.; van Dalen, B.M.; van der Zwaan, H.B.; Delavary, B.M.; Vletter, W.B.; van Geuns, R.-J.M.; Cate, F.J.T.; Geleijnse, M.L. Accuracy and reproducibility of quantitation of left ventricular function by real-time three-dimensional echocardiography versus cardiac magnetic resonance. Am. J. Cardiol. 2008, 102, 778–783. [Google Scholar] [CrossRef]
- Kim, J.; Cohen, S.B.; Atalay, M.K.; Maslow, A.D.; Poppas, A. Quantitative Assessment of Right Ventricular Volumes and Ejection Fraction in Patients with Left Ventricular Systolic Dysfunction by Real Time Three-Dimensional Echocardiography versus Cardiac Magnetic Resonance Imaging. Echocardiography 2015, 32, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Spicer, D.E.; Anderson, R.H. Revisiting the Anatomy of the Living Heart. Circ. J. 2016, 80, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tretter, J.T.; Gupta, S.K.; Izawa, Y.; Nishii, T.; Mori, S. Virtual Dissection: Emerging as the Gold Standard of Analyzing Living Heart Anatomy. J. Cardiovasc. Dev. Dis. 2020, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Izawa, Y.; Mori, S.; Tretter, J.T.; Quintessenza, J.A.; Toh, H.; Toba, T.; Watanabe, Y.; Kono, A.K.; Okada, K.; Hirata, K.-I. Normative Aortic Valvar Measurements in Adults Using Cardiac Computed Tomography—A Potential Guide to Further Sophisticate Aortic Valve-Sparing Surgery. Circ. J. 2021, 85, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Harake, D.; Gnanappa, G.K.; Alvarez, S.G.; Whittle, A.; Punithakumar, K.; Boechler, P.; Noga, M.; Khoo, N.S. Stereoscopic Display Is Superior to Conventional Display for Three-Dimensional Echocardiography of Congenital Heart Anatomy. J. Am. Soc. Echocardiogr. 2020, 33, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Shivkumar, K. Real three-dimensional cardiac imaging using leading-edge holographic display. Clin. Anat. 2021, 34, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Nagatani, Y.; Matsubayashi, Y.; Mori, Y.; Wakisaka, H.; Lee, J.; Minamidate, N.; Takashima, N.; Kinoshita, T.; Suzuki, T. A Virtual-Reality Imaging Analysis of the Dynamic Aortic Root Anatomy. Ann. Thorac. Surg. 2021, 112, 2077–2083. [Google Scholar] [CrossRef]
- Vukicevic, M.; Mosadegh, B.; Min, J.K.; Little, S.H. Cardiac 3D Printing and its Future Directions. JACC Cardiovasc. Imaging 2017, 10, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Valverde, I.; Gomez-Ciriza, G.; Hussain, T.; Suárez-Mejías, C.; Velasco-Forte, M.N.; Byrne, N.; Ordoñez, A.; Gonzalez-Calle, A.; Anderson, D.; Hazekamp, M.G.; et al. Three-dimensional printed models for surgical planning of complex congenital heart defects: An international multicentre study. Eur. J. Cardiothorac. Surg. 2017, 52, 1139–1148. [Google Scholar] [CrossRef]
- Anwar, S.; Singh, G.K.; Miller, J.; Sharma, M.; Manning, P.; Billadello, J.J.; Eghtesady, P.; Woodard, P.K. 3D Printing is a Transformative Technology in Congenital Heart Disease. JACC Basic Transl. Sci. 2018, 3, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Wheatstone, C., XVIII. Contributions to the physiology of vision.—Part the first. On some remarkable, and hitherto unobserved, phenomena of binocular vision. Philos. Trans. R. Soc. Lond. 1838, 128, 371–394. [Google Scholar] [CrossRef]
- DeWitt, L.M. Observations on the sino-ventricular connecting system of the mammalian heart. Anat. Rec. 1909, 3, 475–497. [Google Scholar] [CrossRef] [Green Version]
- Mori, S.; Izawa, Y.; Nishii, T. Simple Stereoscopic Display of 3-Dimensional Living Heart Anatomy Relevant to Electrophysiological Practice. JACC Clin. Electrophysiol. 2020, 6, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Shivkumar, K. Stereoscopic three-dimensional anatomy of the heart: Another legacy of Dr. Wallace A. McAlpine. Anat. Sci. Int. 2021, 96, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Gupta, P. Anaglyph stereo virtual dissection: A novel inexpensive method for stereoscopic visualisation of intracardiac anatomy on CT angiogram. Cardiol. Young 2021, 31, 1958–1961. [Google Scholar] [CrossRef] [PubMed]
- Barlow, H.B.; Blakemore, C.; Pettigrew, J.D. The neural mechanism of binocular depth discrimination. J. Physiol. 1967, 193, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Hubel, D.H.; Wiesel, T.N. Stereoscopic vision in macaque monkey. Cells sensitive to binocular depth in area 18 of the macaque monkey cortex. Nature 1970, 225, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Loukas, M. The importance of attitudinally appropriate description of cardiac anatomy. Clin. Anat. 2009, 22, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Daubert, J.C.; Ritter, P.; Le Breton, H.; Gras, D.; Leclercq, C.; Lazarus, A.; Mugica, J.; Mabo, P.; Cazeau, S. Permanent left ventricular pacing with transvenous leads inserted into the coronary veins. Pacing Clin. Electrophysiol. 1998, 21 Pt 2, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Baman, T.S.; Ilg, K.J.; Gupta, S.K.; Good, E.; Chugh, A.; Jongnarangsin, K.; Pelosi, F., Jr.; Ebinger, M.; Crawford, T.; Oral, H.; et al. Mapping and ablation of epicardial idiopathic ventricular arrhythmias from within the coronary venous system. Circ. Arrhythm Electrophysiol. 2010, 3, 274–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreidieh, B.; Rodríguez-Mañero, M.; Schurmann, P.; Ibarra-Cortez, S.H.; Dave, A.S.; Valderrábano, M. Retrograde Coronary Venous Ethanol Infusion for Ablation of Refractory Ventricular Tachycardia. Circ. Arrhythmia Electrophysiol. 2016, 9, e004352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izawa, Y.; Mori, S.; Nishii, T.; Matsuzoe, H.; Imada, H.; Suehiro, H.; Nakayama, K.; Matsumoto, K.; Tanaka, H.; Fujiwara, S.; et al. Optimal image reconstruction using multidetector-row computed tomography to facilitate cardiac resynchronization therapy. Echocardiography 2017, 34, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Miyagawa, K.; Nakayama, K.; Kinutani, H.; Shinke, T.; Okada, K.; Okita, Y.; Hirata, K.I.; Emoto, N. Balloon pulmonary angioplasty: An additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. EuroIntervention 2014, 10, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P.; Leon, M.B.; Adams, D.H.; Webb, J.G.; Farivar, R.S. Transcatheter Therapy for Mitral Regurgitation Clinical Challenges and Potential Solutions. Circulation 2017, 136, 404–417. [Google Scholar] [CrossRef]
- Schäfers, H.J.; Bierbach, B.; Aicher, D. A new approach to the assessment of aortic cusp geometry. J. Thorac. Cardiovasc. Surg. 2006, 132, 436–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunihara, T.; Aicher, D.; Rodionycheva, S.; Groesdonk, H.-V.; Langer, F.; Sata, F.; Schäfers, H.-J. Preoperative aortic root geometry and postoperative cusp configuration primarily determine long-term outcome after valve-preserving aortic root repair. J. Thorac. Cardiovasc. Surg. 2012, 143, 1389–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, S.; Fukuzawa, K.; Takaya, T.; Takamine, S.; Ito, T.; Fujiwara, S.; Nishii, T.; Kono, A.K.; Yoshida, A.; Hirata, K. Clinical cardiac structural anatomy reconstructed within the cardiac contour using multidetector-row computed tomography: The arrangement and location of the cardiac valves. Clin. Anat. 2016, 29, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Tretter, J.T.; Izawa, Y.; Spicer, D.E.; Okada, K.; Anderson, R.H.; Quintessenza, J.A.; Mori, S. Understanding the Aortic Root Using Computed Tomographic Assessment: A Potential Pathway to Improved Customized Surgical Repair. Circ. Cardiovasc. Imaging 2021, 14, e013134. [Google Scholar] [CrossRef]
- Izumi, C.; Eishi, K.; Ashihara, K.; Arita, T.; Otsuji, Y.; Kunihara, T.; Komiya, T.; Shibata, T.; Seo, Y.; Daimon, M.; et al. JCS/JSCS/JATS/JSVS 2020 Guidelines on the Management of Valvular Heart Disease. Circ. J. 2020, 84, 2037–2119. [Google Scholar] [CrossRef]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Nørgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR): An Expert Consensus Document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc. Imaging 2019, 12, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-J.; van Arsdell, G.S. 3D Printing in Surgical Management of Double Outlet Right Ventricle. Front. Pediatr. 2017, 5, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, N.; Kasdi, R.; Coles, J.G.; Yoo, S.-J. Use of 3-dimensionally printed heart models in the planning and simulation of surgery in patients with Raghib syndrome (coronary sinus defect with left superior vena cava). JTCVS Tech. 2020, 2, 135–138. [Google Scholar] [CrossRef]
- Kilner, P.J.; Geva, T.; Kaemmerer, H.; Trindade, P.T.; Schwitter, J.; Webb, G.D. Recommendations for cardiovascular magnetic resonance in adults with congenital heart disease from the respective working groups of the European Society of Cardiology. Eur. Heart J. 2010, 31, 794–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e698–e800. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Anderson, R.H.; Nishii, T.; Matsumoto, K.; Loomba, R.S. Isomerism in the setting of the so-called “heterotaxy”: The usefulness of computed tomographic analysis. Ann. Pediatr. Cardiol. 2017, 10, 175–186. [Google Scholar] [CrossRef]
- Nagasawa, A.; Mori, S.; Akita, T.; Yamada, H.; Oki, T.; Nishii, T.; Yamashita, T.; Okita, Y.; Hirata, K.I. Giant Coronary Arterial Aneurysm of the Proximal Left Anterior Descending Artery as the Cause of Wide Splitting of the Second Heart Sound. Intern. Med. 2018, 57, 1111–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasamatsu, A.; Takaya, T.; Mori, S.; Kashio, K.; Takahashi, H.; Ito, T.; Takamine, S.; Fujiwara, S.; Nishii, T.; Kono, A.K.; et al. Reconstruction of an extracardiac aortocoronary collateral and simulation of selective angiography with multidetector-row computed tomography. Circulation 2015, 131, e476–e479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutroneo, G.; Bruschetta, D.; Trimarchi, F.; Cacciola, A.; Cinquegrani, M.; Duca, A.; Rizzo, G.; Alati, E.; Gaeta, M.; Milardi, D. In Vivo CT Direct Volume Rendering: A Three-Dimensional Anatomical Description of the Heart. Pol. J. Radiol. 2016, 81, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackett, M.; Proctor, M. Three-Dimensional Display Technologies for Anatomical Education: A Literature Review. J. Sci. Educ. Technol. 2016, 25, 641–654. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izawa, Y.; Nishii, T.; Mori, S. Stereogram of the Living Heart, Lung, and Adjacent Structures. Tomography 2022, 8, 824-841. https://doi.org/10.3390/tomography8020068
Izawa Y, Nishii T, Mori S. Stereogram of the Living Heart, Lung, and Adjacent Structures. Tomography. 2022; 8(2):824-841. https://doi.org/10.3390/tomography8020068
Chicago/Turabian StyleIzawa, Yu, Tatsuya Nishii, and Shumpei Mori. 2022. "Stereogram of the Living Heart, Lung, and Adjacent Structures" Tomography 8, no. 2: 824-841. https://doi.org/10.3390/tomography8020068
APA StyleIzawa, Y., Nishii, T., & Mori, S. (2022). Stereogram of the Living Heart, Lung, and Adjacent Structures. Tomography, 8(2), 824-841. https://doi.org/10.3390/tomography8020068





