Dose Reduction and Diagnostic Performance of Tin Filter–Based Spectral Shaping CT in Patients with Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Data
2.2. CT Examination Technique and Reconstruction
2.3. Radiation Metrics of ST120kV and TF100kV Protocol
2.4. Assessment of Objective Image Quality
2.5. Assessment of Diagnostic Performance for Metastases
2.6. Assessment of Subjective Quality of TF100kV Images
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
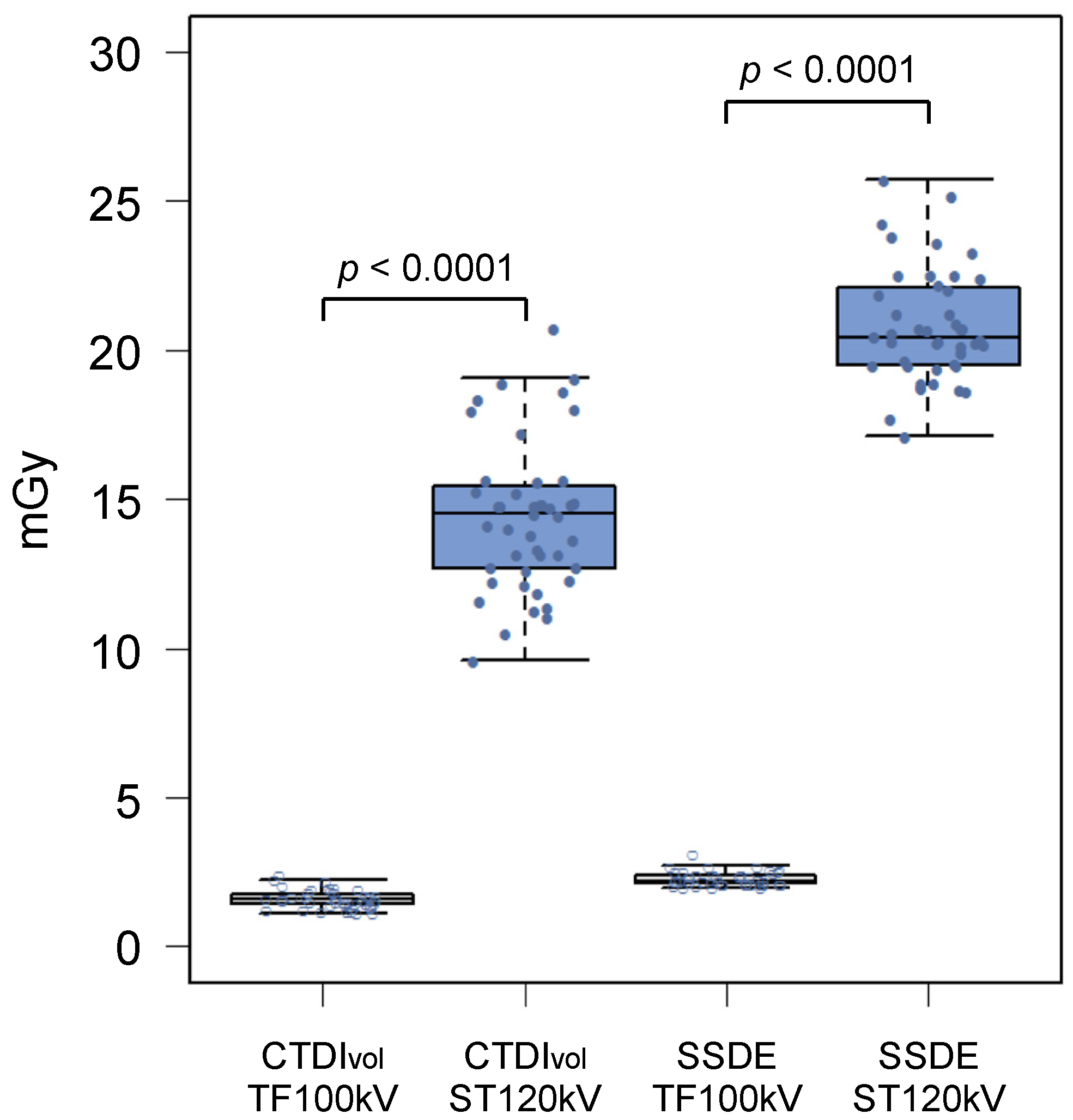
3.2. Radiation Dose
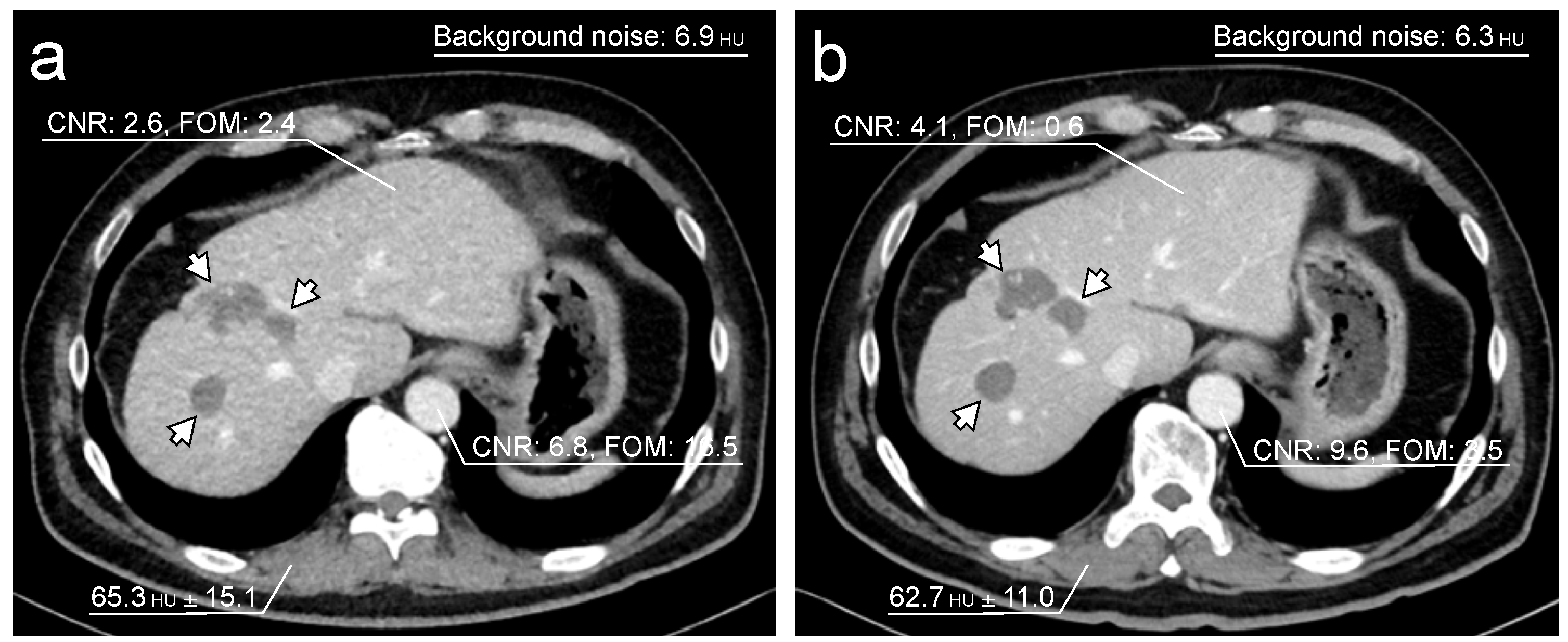
3.3. Objective Image Quality Assessment
3.4. Diagnostic Performance of Subjective Assessment
3.5. Subjective Quality of TF100kV Images
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer. Cancer Fact Sheets Colorectal Cancer Source: Globocan. 2020. Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 31 January 2022).
- Pita-Fernandez, S.; Alhayek-Ai, M.; Gonzalez-Martin, C.; Lopez-Calvino, B.; Seoane-Pillado, T.; Pertega-Diaz, S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: A systematic review and meta-analysis. Ann. Oncol. 2015, 26, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.; Augestad, K.M.; Cooper, G.S. Colorectal cancer surveillance: What’s new and what’s next. World J. Gastroenterol. 2014, 20, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Sloothen, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Kwiatkowski, F.; Sugarbaker, P.H.; Elias, D.; Levine, E.A.; De Simone, M.; Barone, R.; Yonemura, Y.; Cavaliere, F.; Quenet, F.; et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J. Clin. Oncol. 2004, 22, 3284–3292. [Google Scholar] [CrossRef]
- Pfannschmidt, J.; Dienemann, H.; Hoffmann, H. Surgical resection of pulmonary metastases from colorectal cancer: A systematic review of published series. Ann. Thorac. Surg. 2007, 84, 324–338. [Google Scholar] [CrossRef]
- Warwick, R.; Page, R. Resection of pulmonary metastases from colorectal carcinoma. Eur. J. Surg. Oncol. 2007, 33 (Suppl. S2), S59–S63. [Google Scholar] [CrossRef]
- de Jong, M.C.; Pulitano, C.; Ribero, D.; Strub, J.; Mentha, G.; Schulick, R.D.; Choti, M.A.; Aldrighetti, L.; Capussotti, L.; Pawlik, T.M. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: An international multi-institutional analysis of 1669 patients. Ann. Surg. 2009, 250, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Elias, D.; Gilly, F.; Boutitie, F.; Quenet, F.; Bereder, J.M.; Mansvelt, B.; Lorimier, G.; Dube, P.; Glehen, O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric French study. J. Clin. Oncol. 2010, 28, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.P.; Jackson, R.; Dunne, D.F.; Malik, H.Z.; Fenwick, S.W.; Poston, G.J.; Ghaneh, P. Systematic review and meta-analysis of follow-up after hepatectomy for colorectal liver metastases. Br. J. Surg 2012, 99, 477–486. [Google Scholar] [CrossRef]
- Bujanda, L.; Sarasqueta, C.; Hijona, E.; Hijona, L.; Cosme, A.; Gil, I.; Elorza, J.L.; Asensio, J.I.; Larburu, S.; Enriquez-Navascues, J.M.; et al. Colorectal cancer prognosis twenty years later. World J. Gastroenterol. 2010, 16, 862–867. [Google Scholar] [CrossRef]
- Brouwer, N.P.M.; Bos, A.; Lemmens, V.; Tanis, P.J.; Hugen, N.; Nagtegaal, I.D.; de Wilt, J.H.W.; Verhoeven, R.H.A. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int. J. Cancer 2018, 143, 2758–2766. [Google Scholar] [CrossRef]
- Kievit, J. Follow-up of patients with colorectal cancer: Numbers needed to test and treat. Eur. J. Cancer 2002, 38, 986–999. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Mangu, P.B.; Flynn, P.J.; Korde, L.; Loprinzi, C.L.; Minsky, B.D.; Petrelli, N.J.; Ryan, K.; Schrag, D.H.; Wong, S.L.; et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J. Clin. Oncol. 2013, 31, 4465–4470. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network [NCCN Guidelines]® Rectal Cancer, Version 2.2021. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 31 January 2022).
- National Comprehensive Cancer Network [NCCN Guidelines]® Colon Cancer, Version 3.2021. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 31 January 2022).
- Argiles, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: A randomized clinical trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef] [Green Version]
- Stintzing, S.; Modest, D.P.; Rossius, L.; Lerch, M.M.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.-E.; Heintges, T.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): A post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016, 17, 1426–1434. [Google Scholar] [CrossRef]
- Braun, F.M.; Johnson, T.R.; Sommer, W.H.; Thierfelder, K.M.; Meinel, F.G. Chest CT using spectral filtration: Radiation dose, image quality, and spectrum of clinical utility. Eur. Radiol. 2015, 25, 1598–1606. [Google Scholar] [CrossRef]
- Gordic, S.; Morsbach, F.; Schmidt, B.; Allmendinger, T.; Flohr, T.; Husarik, D.; Baumueller, S.; Raupach, R.; Stolzmann, P.; Leschka, S.; et al. Ultralow-dose chest computed tomography for pulmonary nodule detection: First performance evaluation of single energy scanning with spectral shaping. Investig. Radiol. 2014, 49, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Ludes, C.; Schaal, M.; Labani, A.; Jeung, M.Y.; Roy, C.; Ohana, M. Ultra-low dose chest CT: The end of chest radiograph? Presse Med. 2016, 45, 291–301. [Google Scholar] [CrossRef]
- Leyendecker, P.; Faucher, V.; Labani, A.; Noblet, V.; Lefebvre, F.; Magotteaux, P.; Ohana, M.; Roy, C. Prospective evaluation of ultra-low-dose contrast-enhanced 100-kV abdominal computed tomography with tin filter: Effect on radiation dose reduction and image quality with a third-generation dual-source CT system. Eur. Radiol. 2019, 29, 2107–2116. [Google Scholar] [CrossRef]
- Mozaffary, A.; Trabzonlu, T.A.; Kim, D.; Yaghmai, V. Comparison of tin filter-based spectral shaping CT and low-dose protocol for detection of urinary calculi. AJR Am. J. Roentgenol. 2019, 212, 808–814. [Google Scholar] [CrossRef]
- Petritsch, B.; Kosmala, A.; Weng, A.M.; Bley, T.A. Tin-filtered 100 kV ultra-low-dose CT of the paranasal sinus: Initial clinical results. PLoS ONE 2019, 14, e0216295. [Google Scholar] [CrossRef] [Green Version]
- Romanyukha, A.; Folio, L.; Lamart, S.; Simon, S.L.; Lee, C. Body size-specific effective dose conversion coefficients for Ct scans. Radiat. Prot. Dosim. 2016, 172, 428–437. [Google Scholar] [CrossRef] [Green Version]
- McCollough, C.; Bakalyar, D.M.; Bostani, M.; Brady, S.; Boedeker, K.; Boone, J.M.; Chen-Mayer, H.H.; Christianson, O.I.; Leng, S.; Li, B.; et al. Use of water equivalent diameter for calculating patient size and Size-Specific Dose Estimates (SSDE) in CT: The report of AAPM task group 220. AAPM Rep. 2014, 2014, 6–23. [Google Scholar]
- Brenner, D.J.; Hall, E.J. Computed tomography--an increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [Green Version]
- Mileto, A.; Guimaraes, L.S.; McCollough, C.H.; Fletcher, J.G.; Yu, L. State of the art in abdominal CT: The limits of iterative reconstruction algorithms. Radiology 2019, 293, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Suntharalingam, S.; Mikat, C.; Wetter, A.; Guberina, N.; Salem, A.; Heil, P.; Forsting, M.; Nassenstein, K. Whole-body ultra-low dose CT using spectral shaping for detection of osteolytic lesion in multiple myeloma. Eur. Radiol. 2018, 28, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Kanal, K.M.; Butler, P.F.; Sengupta, D.; Bhargavan-Chatfield, M.; Coombs, L.P.; Morin, R.L. U.S. Diagnostic reference levels and achievable doses for 10 adult ct examinations. Radiology 2017, 284, 120–133. [Google Scholar] [CrossRef] [Green Version]
- Japan Network for Research and Information on Medical Exposures. National Diagnostic Reference Levels in Japan. Japan 2020 DRLs. Available online: http://www.radher.jp/J-RIME/report/DRL2020_Engver.pdf (accessed on 31 January 2022).


| CT Parameters | ST120kV Protocol | TF100kV Protocol |
|---|---|---|
| Tin-filter a | off | on |
| kV | 120 kV | 100 kV |
| Quality reference mAs b | 320 mAs | 600 mAs |
| Rotation time | 0.5 s | 0.5 s |
| Beam collimation | 128 ch × 0.6 mm | 128 ch× 0.6 mm |
| Beam pitch | 0.6 | 0.5 |
| Kernel | Bf37 (Bl57 for lung area) | Bf37 (Bl57 for lung area) |
| Reconstruction technique | IR strength 2 for parenchymal images | IR strength 4 for parenchymal images |
| FBP for lung images | FBP for lung images |
| Variables | n (%) |
|---|---|
| Age (y) a | 65.1 (38–88) |
| Gender | |
| Male | 30 (68) |
| Female | 14 (32) |
| Total number of colorectal cancers b | 45 |
| Cecum/Appendix | 3 |
| Ascending colon | 4 |
| Transverse colon | 3 |
| Descending colon | 1 |
| Sigmoid colon | 12 |
| Rectum | 22 |
| Stage | |
| Ⅰ, Ⅱ | 10 (23) |
| Ⅲ, Ⅳ | 34 (77) |
| Disease-free and during follow-up | 26 (59) |
| During chemotherapy | 18 (41) |
| Total number of metastatic site c | 32, 30 |
| Lung metastasis c | 9, 9 |
| Liver metastasis c | 9, 8 |
| Lymph node metastasis c | 6, 5 |
| Peritoneal dissemination c | 5, 5 |
| Bone metastasis c | 3, 3 |
| Time from ST120kV images to TF100kV images (d) a | 156 (65–240) |
| ST120kV Protocol | TF100kV Protocol | Reduction Rate Using TF100kV Protocol (%) | p Value | |
|---|---|---|---|---|
| CTDIvol (mGy) a | 14.4 ± 2.50 | 1.60 ± 0.31 | 88.9 | <0.0001 |
| DLP (mGy·cm) b | 996.7 (886.2–1144.3) | 107.1 (95.9–125.5) | 89.3 | <0.0001 |
| Effective dose (mSv) b | 17.9 (16.0–20.6) | 1.93 (1.73–2.26) | 89.2 | <0.0001 |
| SSDE (mGy) a | 20.8 ± 1.90 | 2.29 ± 0.24 | 89.0 | <0.0001 |
| ST120kV Protocol | TF100kV Protocol | p Value | |
|---|---|---|---|
| CNR of liver | 2.76 (1.38–4.03) | 2.13 (1.42–2.73) | <0.0001 |
| CNR of abdominal aorta | 5.81 (4.76–7.52) | 4.08 (3.39–5.02) | <0.0001 |
| FOM of liver (mSv−1) | 0.36 (0.09–0.93) | 2.24 (0.89–4.07) | <0.0001 |
| FOM of abdominal aorta (mSv−1) | 1.81 (1.19–3.16) | 8.62 (5.98–14.1) | <0.0001 |
| Background noise | 6.04 (5.45–6.93) | 8.36 (7.61–8.86) | <0.0001 |
| Diagnostic Performance | Interreader Agreement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ST120kV | TF100kV | ST120kV | TF100kV | |||||||
| Diagnostic of | SN (%) | SP (%) | AC (%) | AUC a | SN (%) | SP (%) | AC (%) | AUC a | Kappa | Kappa |
| Lung metastasis | 89 [8/9] | 100 [35/35] | 98 [43/44] | 0.94 | 100 [9/9] | 100 [35/35] | 100 [44/44] | 1.0 | 0.59 | 0.66 |
| Liver metastasis | 67 [6/9] | 100 [35/35] | 93 [41/44] | 0.83 | 75 [6/8] | 100 [36/36] | 96 [42/44] | 0.88 | 0.72 | 0.44 |
| Lymph node metastasis | 83 [5/6] | 97 [37/38] | 96 [42/44] | 0.90 | 40 [2/5] | 100 [39/39] | 93 [41/44] | 0.70 | 0.39 | 0.33 |
| Peritoneal dissemination | 60 [3/5] | 95 [37/39] | 91 [40/44] | 0.77 | 60 [3/5] | 97 [38/39] | 93 [41/44] | 0.79 | 0.17 | 0.29 |
| Bone metastasis | 67 [2/3] | 100 [41/41] | 98 [43/44] | 0.83 | 67 [2/3] | 100 [41/41] | 98 [43/44] | 0.83 | 0.23 | 0.37 |
| Reader 1 | Reader 2 | |
|---|---|---|
| Lung metastasis | 4.00 (4.00–4.00) | 4.00 (4.00–4.00) |
| Liver metastasis | 3.68 (3.00–4.00) | 3.32 (3.00–4.00) |
| Lymph node metastasis | 3.89 (3.00–4.00) | 3.91 (3.00–4.00) |
| Peritoneal dissemination | 3.86 (3.00–4.00) | 3.91 (3.00–4.00) |
| Bone metastasis | 4.00 (4.00–4.00) | 4.00 (4.00–4.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, K.; Fujioka, T.; Mori, M.; Adachi, T.; Hiraishi, T.; Hada, H.; Ishikawa, T.; Tateishi, U. Dose Reduction and Diagnostic Performance of Tin Filter–Based Spectral Shaping CT in Patients with Colorectal Cancer. Tomography 2022, 8, 1079-1089. https://doi.org/10.3390/tomography8020088
Kimura K, Fujioka T, Mori M, Adachi T, Hiraishi T, Hada H, Ishikawa T, Tateishi U. Dose Reduction and Diagnostic Performance of Tin Filter–Based Spectral Shaping CT in Patients with Colorectal Cancer. Tomography. 2022; 8(2):1079-1089. https://doi.org/10.3390/tomography8020088
Chicago/Turabian StyleKimura, Koichiro, Tomoyuki Fujioka, Mio Mori, Takuya Adachi, Takumi Hiraishi, Hiroto Hada, Toshiaki Ishikawa, and Ukihide Tateishi. 2022. "Dose Reduction and Diagnostic Performance of Tin Filter–Based Spectral Shaping CT in Patients with Colorectal Cancer" Tomography 8, no. 2: 1079-1089. https://doi.org/10.3390/tomography8020088






