Changes of Atherosclerotic Plaque in Cerebral Artery Stenosis According to High-Resolution MR Imaging
Abstract
:1. Introduction
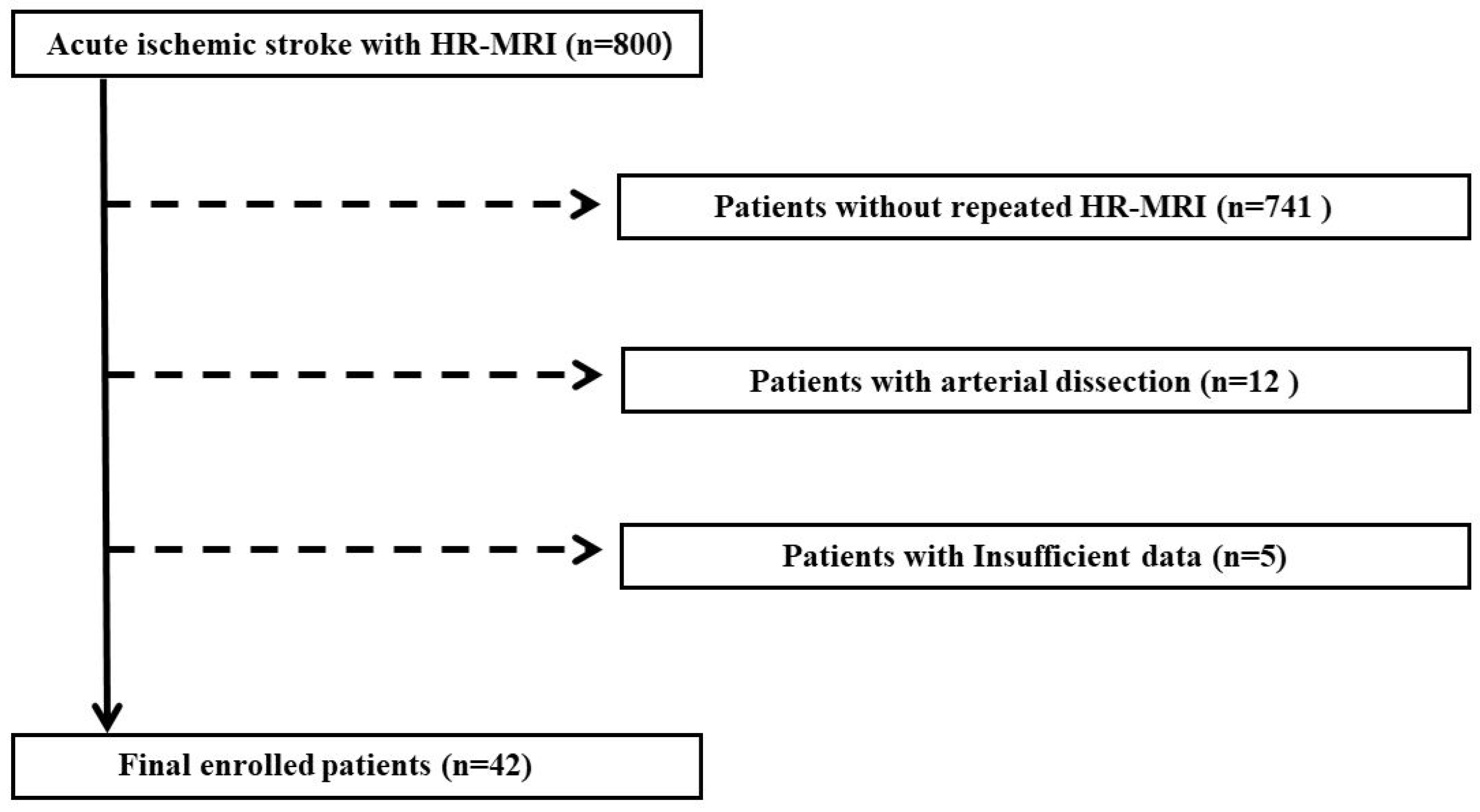
2. Materials and Methods
2.1. Study Population
2.2. Clinical Characteristics
2.3. Magnetic Resonance Imaging (MRI)
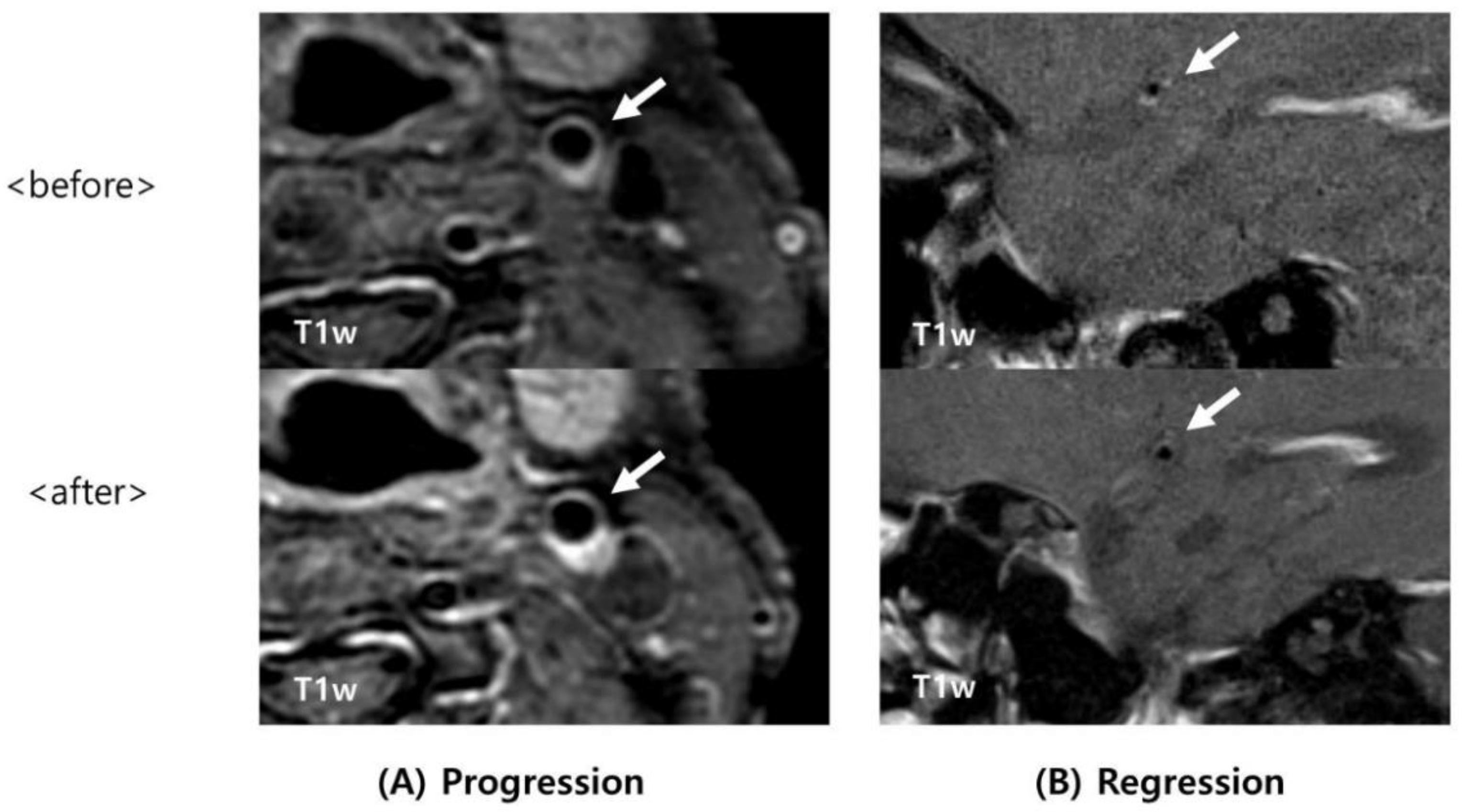
2.4. Image Analysis
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, J.M.; Hatsukami, T.S.; Ferguson, M.S.; Small, R.; Polissar, N.L.; Yuan, C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002, 106, 1368–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Baradaran, H.; Schweitzer, A.D.; Kamel, H.; Pandya, A.; Delgado, D.; Dunning, A.; Mushlin, A.I.; Sanelli, P.C. Carotid plaque MRI and stroke risk: A systematic review and meta-analysis. Stroke 2013, 44, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e563–e595. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Mandell, D.; Mossa-Basha, M.; Qiao, Y.; Hess, C.; Hui, F.; Matouk, C.; Johnson, M.; Daemen, M.; Vossough, A.; Edjlali, M.; et al. Intracranial vessel wall MRI: Principles and expert consensus recommendations of the American society of neuroradiology. Am. J. Neuroradiol. 2017, 38, 218–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, C.W.; Jahng, G.H.; Kim, E.J.; Choi, W.S.; Yang, D.M. High resolution wall and lumen MRI of the middle cerebral arteries at 3 tesla. Cerebrovasc. Dis. 2009, 27, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-L.; Deng, G.; Xie, B.; Ju, S.; Yang, M.; Chen, X.-H.; Teng, G.-J. High-resolution MRI of the vessel wall in patients with symptomatic atherosclerotic stenosis of the middle cerebral artery. J. Clin. Neurosci. 2015, 22, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-H.; Li, M.-L.; Gao, S.; Ni, J.; Zhou, L.-X.; Yao, M.; Peng, B.; Feng, F.; Jin, Z.; Cui, L.-Y. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis 2010, 212, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Li, J.; Zhao, M.; Zhang, X.; Degnan, A.J.; Mossa-Basha, M.; Saloner, D.; Lu, J.; Liu, Q.; Zhu, C. Progression of Plaque Burden of Intracranial Atherosclerotic Plaque Predicts Recurrent Stroke/Transient Ischemic Attack: A Pilot Follow-Up Study Using Higher-Resolution MRI. J. Magn. Reson. Imaging 2021, 54, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Mossy, J. Cerebral Infarcts and the Lesions of Intracranial and Extracranial Atherosclerosis. Arch Neurol. 1966, 14, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Wong, K.S.; Chen, X.Y. Intracranial atherosclerosis: From microscopy to high-resolution magnetic resonance imaging. J. Stroke 2017, 19, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Nighoghossian, N.; Derex, L.; Douek, P. The vulnerable carotid artery plaque: Current imaging methods and new perspectives. Stroke 2005, 36, 2764–2772. [Google Scholar] [CrossRef] [Green Version]
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.R.; Kruth, H.S.; Weber, D.K. Intraplaque Hemorrhage and Progression of Coronary Atheroma. 2003. Available online: www.nejm.org (accessed on 11 April 2022).
- Takaya, N.; Yuan, C.; Chu, B.; Saam, T.; Polissar, N.L.; Jarvik, G.P.; Isaac, C.; McDonough, J.; Natiello, C.; Small, R.; et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: A high-resolution magnetic resonance imaging study. Circulation 2005, 111, 2768–2775. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.G.; Lee, C.H.; Shin, B.S.; Chung, G.H.; Kwak, H.S. Characteristics of symptomatic basilar artery stenosis using high-resolution magnetic resonance imaging in ischemic stroke patients. J. Atheroscler. Thromb. 2021, 28, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Tell, G.S.; Polak, J.F.; Ward, B.J.; Kittner, S.J.; Savage, P.J.; Robbins, J. Relation of Smoking with Carotid Artery Wall Thickness and Stenosis in Older Adults the Cardiovascular Health Study. 1994. Available online: http://ahajournals.org (accessed on 20 November 2021).
- Inoue, T. Cigarette Smoking as a Risk Factor of Coronary Artery Disease and its Effects on Platelet Function. Tob. Induc. Dis. 2004, 2, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-L.; Leng, X.-Y.; Dong, Y.; Hou, X.-H.; Tong, L.; Ma, Y.-H.; Xu, W.; Cui, M.; Dong, Q.; Tan, L.; et al. Fasting glucose and HbA1c levels as risk factors for the presence of intracranial atherosclerotic stenosis. Ann. Transl. Med. 2019, 7, 804. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhao, H.; Liu, X.; Lu, Q.; Zhao, X.; Pu, J.; Xu, J. Elevated hemoglobin A1c Is Associated with Carotid Plaque Vulnerability: Novel Findings from Magnetic Resonance Imaging Study in Hypertensive Stroke Patients. Sci. Rep. 2016, 6, 33246. [Google Scholar] [CrossRef] [Green Version]

| Subjects (n = 42) | |
|---|---|
| Age, years | 63.10 ± 9.15 |
| Male sex (%) | 35 (83.3) |
| Interval of HR-MRI (months) | 16.88 ± 12.53 |
| Distribution of artery stenosis | |
| Basilar artery (%) | 9 (21.4) |
| Proximal ICA (%) | 26 (61.9) |
| Middle cerebral artery (%) | 7 (16.7) |
| Intracranial artery stenosis (%) | 16 (38.1) |
| Intraplaque hemorrhage (IPH) (%) | 20 (47.6) |
| Stroke or TIA (%) | 24 (57.1) |
| Symptomatic stenosis (%) * | 16 (38.1) |
| Antiplatelet agent use | |
| No antiplatelet (%) | 4 (9.5) |
| Mono antiplatelet (%) | 17 (40.5) |
| Dual antiplatelet (%) | 21 (50.0) |
| Statin intensity | |
| No statin (%) | 2 (4.8) |
| Moderate intensity (%) | 27 (64.3) |
| High intensity (%) | 13 (30.9) |
| Hypertension (%) | 32 (76.2) |
| Diabetes mellitus (DM) (%) | 19 (45.2) |
| Uncontrolled DM (%) † | 7 (36.8) |
| Dyslipidemia (%) | 9 (21.4) |
| Coronary artery disease (%) | 6 (14.3) |
| Atrial fibrillation (%) | 2 (4.8) |
| Previous stroke (%) | 3 (7.1) |
| Smoking (%) | 14 (33.3) |
| Alcohol drinking (%) | 19 (45.2) |
| Stenosis without Regression (n = 27) | Stenosis with Regression (n = 15) | p-Value | |
|---|---|---|---|
| Male | 23 (85.2) | 12 (80.0) | 0.666 |
| Age, years | 65.70 ± 9.08 | 58.40 ± 7.43 | 0.011 |
| Interval of HR-MRI (months) | 16.26 ± 10.85 | 18.00 ± 15.45 | 0.671 |
| Location (each artery, 100%) | |||
| Basilar artery (n = 9) | 6 (66.7) | 3 (33.3) | |
| Proximal ICA (n = 26) | 20 (76.9) | 6 (23.1) | 0.009 |
| Middle cerebral artery (n = 7) | 1 (14.3) | 6 (85.7) | |
| Intracranial artery stenosis | 7 (25.9) | 9 (60.0) | 0.029 |
| Symptomatic stenosis * | 11 (40.7) | 5 (33.3) | 0.636 |
| Intraplaque hemorrhage (IPH) Regression of IPH | 14 (51.9) 3 (11.1) | 6 (40.0) 6 (40.0) | 0.461 0.029 |
| Stroke or TIA | 13 (48.1) | 11 (73.3) | 0.114 |
| Antiplatelet agent use | |||
| No antiplatelet | 4 (14.8) | 0 (0) | |
| Mono antiplatelet | 12 (44.4) | 5 (33.3) | 0.149 |
| Dual antiplatelet | 11 (40.7) | 10 (66.7) | |
| Statin intensity | |||
| No statin Moderate intensity High intensity | 2 (7.4) 15 (55.6) 10 (37.0) | 0 (0) 12 (80.0) 3 (20.0) | 0.233 |
| Hypertension Diabetes mellitus (DM) | 21 (77.8) 12 (44.4) | 11 (73.3) 7 (46.7) | 0.746 0.89 |
| Uncontrolled DM † | 6 (50.0) | 1 (14.3) | 0.173 |
| Dyslipidemia | 7 (25.9) | 2 (13.3) | 0.341 |
| Coronary artery disease | 4 (15.4) | 2 (13.3) | 0.858 |
| Atrial fibrillation | 2 (7.7) | 0 (0) | 0.305 |
| Smoking | 12 (44.4) | 2 (13.3) | 0.04 |
| Alcohol drinking | 13 (50.0) | 6 (40.0) | 0.536 |
| Previous stroke | 2 (7.7) | 1 (6.7) | 0.903 |
| Laboratory test | |||
| Hb | 11.34 ± 5.47 | 12.59 ± 4.59 | 0.459 |
| WBC (×1000) | 7.49 ± 2.37 | 8.26 ± 1.87 | 0.309 |
| PLT (×1000) | 216.96 ± 65.30 | 243.43 ± 62.69 | 0.233 |
| HbA1c (initial) ‡ | 7.28 ± 1.24 | 8.30 ± 1.05 | 0.084 |
| HbA1c (follow up) ‡ | 7.68 ± 1.72 | 6.70 ± 0.34 | 0.08 |
| Change of HbA1c | 0.4 ± 1.34 | −1.60 ± 1.05 | 0.004 |
| FDP | 2.09 ± 1.28 | 1.58 ± 1.85 | 0.412 |
| D-dimer | 0.42 ± 0.31 | 0.31 ± 0.19 | 0.266 |
| BUN | 17.63 ± 6.44 | 16.36 ± 5.36 | 0.539 |
| Creatinine | 0.78 ± 0.45 | 0.76 ± 0.29 | 0.842 |
| GFR | 82.56 ± 18.30 | 90.16 ± 15.81 | 0.266 |
| Uric acid | 6.04 ± 1.58 | 4.52 ± 0.54 | 0.055 |
| Total cholesterol | 168.04 ± 33.14 | 172.79 ± 40.17 | 0.696 |
| Triglyceride | 178.43 ± 172.57 | 151.58 ± 77.93 | 0.614 |
| High-density lipoprotein (HDL) | 41.05 ± 9.39 | 41.00 ± 8.77 | 0.989 |
| Low-density lipoprotein (LDL) | 102.77 ± 28.19 | 111.85 ± 30.94 | 0.381 |
| Stenosis without Progression (n = 35) | Stenosis with Progression (n = 7) | p-Value | |
|---|---|---|---|
| Male | 29 (82.9) | 6 (85.7) | 0.853 |
| Age, years | 61.86 ± 9.14 | 69.29 ± 6.65 | 0.048 |
| Interval of HR-MRI (months) | 16.83 ± 13.27 | 17.14 ± 8.59 | 0.953 |
| Location (each artery, 100%) | |||
| Basilar artery (n = 9) | 8 (88.9) | 1 (11.1) | |
| Proximal ICA (n = 26) | 20 (76.9) | 6 (23.1) | 0.306 |
| Middle cerebral artery (n = 7) | 7 (100.0) | 0 (0) | |
| Intracranial artery stenosis | 15 (42.9) | 1 (14.3) | 0.222 |
| Symptomatic stenosis * | 12 (34.3) | 4 (57.1) | 0.397 |
| Intraplaque hemorrhage (IPH) Progression of IPH | 13 (37.1) 1 (2.9) | 7 (100) 6 (85.7) | 0.003 <0.001 |
| Stroke or TIA | 20 (57.1) | 4 (57.1) | 1 |
| Antiplatelet agent use | |||
| No antiplatelet | 3 (8.6) | 1 (14.3) | |
| Mono antiplatelet | 14 (40.0) | 3 (42.9) | 0.862 |
| Dual antiplatelet | 18 (51.4) | 3 (42.9) | |
| Statin intensity | |||
| No statin Moderate intensity High intensity | 1 (2.9) 25 (71.4) 9 (25.7) | 1 (14.3) 2 (28.6) 4 (57.1) | 0.077 |
| Hypertension Diabetes mellitus (DM) | 25 (71.4) 14 (40.0) | 7 (100) 5 (71.4) | 0.105 0.214 |
| Uncontrolled DM † | 4 (28.6) | 3 (60.0) | 0.211 |
| Dyslipidemia | 6 (17.1) | 3 (42.9) | 0.13 |
| Coronary artery disease | 4 (11.8) | 2 (28.6) | 0.268 |
| Atrial fibrillation | 2 (6.2) | 0 (0) | 1 |
| Smoking | 11 (31.4) | 3 (42.9) | 0.668 |
| Alcohol drinking | 18 (51.4) | 1 (16.7) | 0.191 |
| Previous stroke | 3 (8.8) | 0 (0) | 1 |
| Laboratory test | |||
| Hb | 11.69 ± 5.35 | 12.32 ± 4.29 | 0.771 |
| WBC (×1000) | 7.91 ± 2.17 | 7.26 ± 2.39 | 0.491 |
| PLT (×1000) | 230.50 ± 62.85 | 211.86 ± 75.79 | 0.501 |
| HbA1c (initial) ‡ | 7.90 ± 1.25 | 6.96 ± 1.06 | 0.154 |
| HbA1c (follow up) ‡ | 6.97 ± 1.24 | 8.28 ± 1.67 | 0.08 |
| Change of HbA1c | −0.93 ± 1.13 | 1.32 ± 1.49 | 0.003 |
| FDP | 1.76 ± 1.58 | 2.63 ± 0.81 | 0.299 |
| D-dimer | 0.32 ± 0.19 | 0.69 ± 0.46 | 0.206 |
| BUN | 16.58 ± 5.79 | 19.71 ± 6.82 | 0.218 |
| Creatinine | 0.77 ± 0.40 | 0.81 ± 0.38 | 0.816 |
| GFR | 86.68 ± 18.50 | 78.69 ± 13.67 | 0.298 |
| Uric acid | 5.19 ± 1.41 | 6.78 ± 1.34 | 0.053 |
| Total cholesterol | 165.03 ± 34.92 | 190.85 ± 31.75 | 0.081 |
| Triglyceride | 169.00 ± 153.03 | 190.86 ± 31.75 | 0.081 |
| High-density lipoprotein (HDL) | 40.82 ± 9.27 | 41.86 ± 8.69 | 0.791 |
| Low-density lipoprotein (LDL) | 101.96 ± 30.37 | 122.86 ± 15.88 | 0.09 |
| <Regression> | ||||
|---|---|---|---|---|
| Crude OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | |
| Age | 0.90 (0.83–0.98) | 0.019 | 0.87 (0.79–0.97) | 0.014 |
| Regression of IPH | 5.33 (1.09–25.99) | 0.038 | 10.13 (1.31–78.57) | 0.027 |
| Smoking | 0.19 (0.04–1.02) | 0.053 | 0.11 (0.01–0.83) | 0.033 |
| <Progression> | ||||
| Crude OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | |
| Age | 1.10 (0.99–1.22) | 0.061 | 1.01 (0.84–1.23) | 0.897 |
| Progression of IPH | 204.00 (11.17–3724.26) | <0.001 | 115.80 (3.77–3554.18) | 0.007 |
| LDL | 1.03 (0.99–1.06) | 0.1 | 1.02 (0.96–1.09) | 0.495 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, M.S.; Lee, K.H.; Cho, S.Y.; Im, Y.-J.; Shin, B.-S.; Kang, H.G. Changes of Atherosclerotic Plaque in Cerebral Artery Stenosis According to High-Resolution MR Imaging. Tomography 2022, 8, 1690-1701. https://doi.org/10.3390/tomography8040141
Baek MS, Lee KH, Cho SY, Im Y-J, Shin B-S, Kang HG. Changes of Atherosclerotic Plaque in Cerebral Artery Stenosis According to High-Resolution MR Imaging. Tomography. 2022; 8(4):1690-1701. https://doi.org/10.3390/tomography8040141
Chicago/Turabian StyleBaek, Min Soo, Kang Hoon Lee, Seong Yoon Cho, Yong-Jin Im, Byoung-Soo Shin, and Hyun Goo Kang. 2022. "Changes of Atherosclerotic Plaque in Cerebral Artery Stenosis According to High-Resolution MR Imaging" Tomography 8, no. 4: 1690-1701. https://doi.org/10.3390/tomography8040141






