Assessment of Correlation between Dual-Energy Ct (De-Ct)-Derived Iodine Concentration and Local Flourodeoxyglucose (Fdg) Uptake in Patients with Primary Non-Small-Cell Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Patients
2.2. Contrast-Enhanced DE-CT Imaging Protocol
2.3. 18F-FDG PET/CT Imaging Protocol
2.4. Delineation of Lesions and Image Assessment
2.5. Data Processing
2.6. Statistical Analysis
3. Results
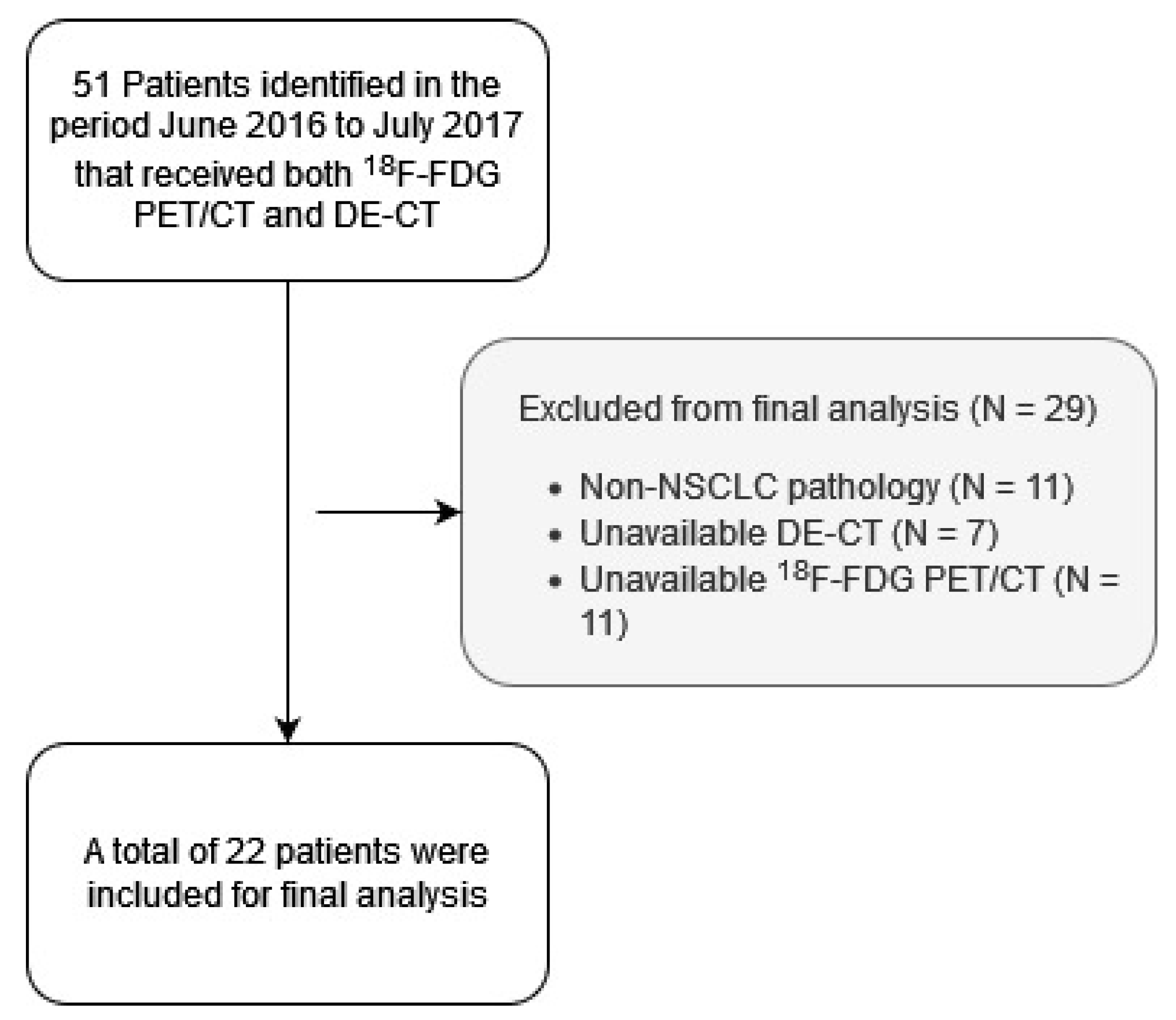
3.1. Patients
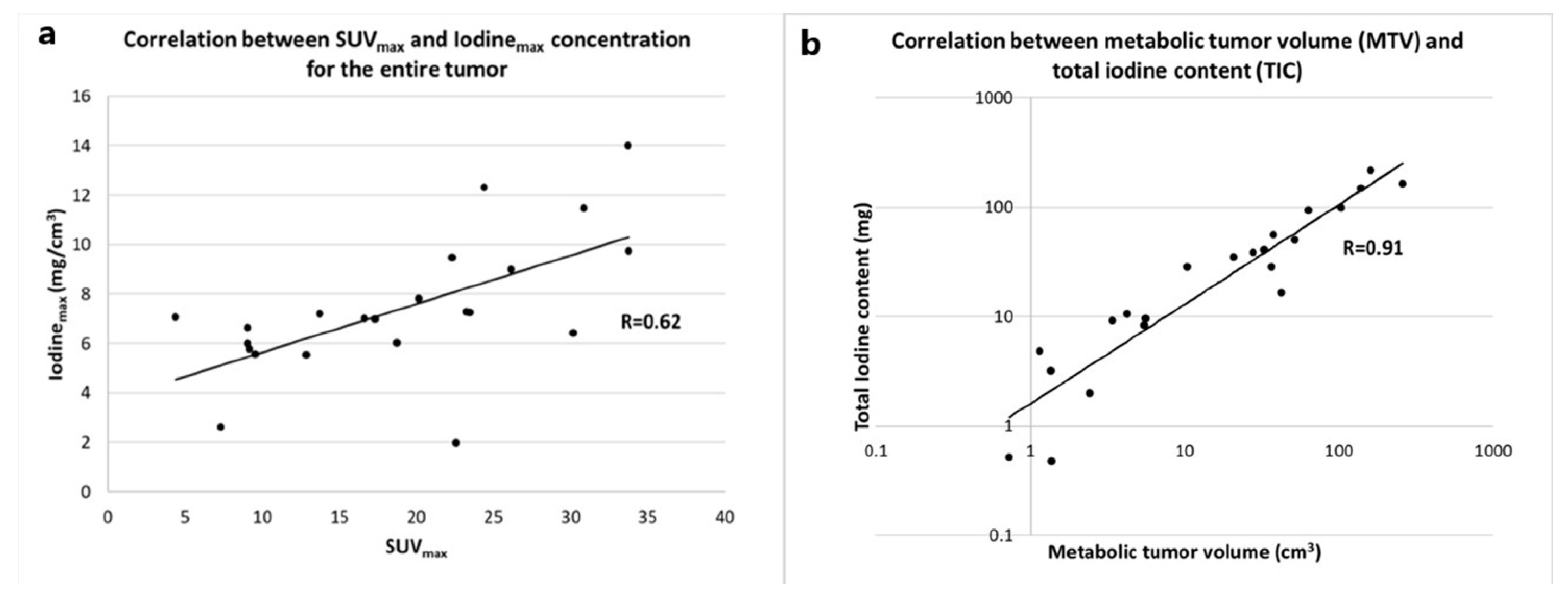
3.2. Correlation between Metabolic and Iodine-Related Parameters for the Whole Tumor
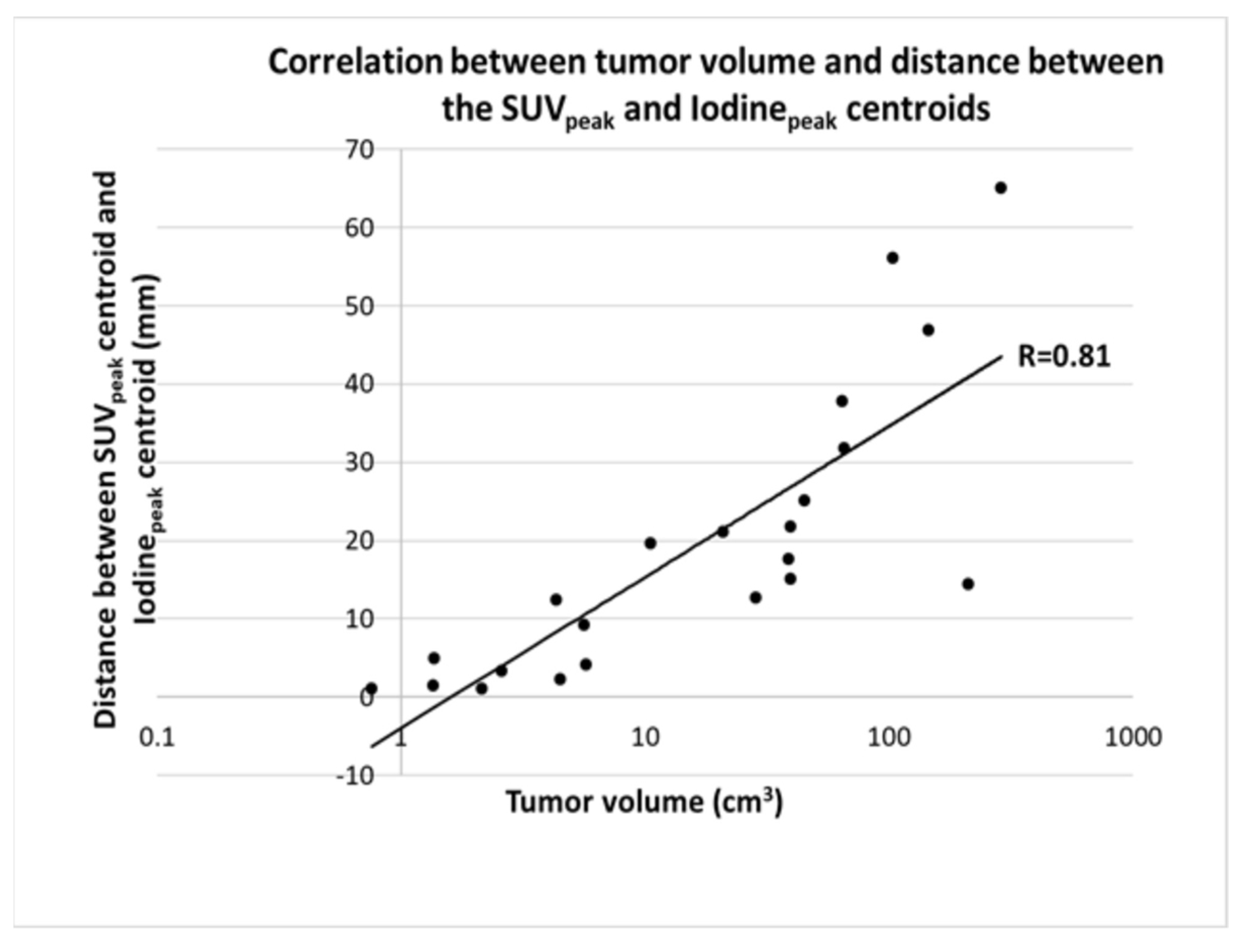
3.3. Correlation with Tumor Volume
3.4. Correlation of Max Iodine Concentration and SUVpeak
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Harders, S.W.; Madsen, H.H.; Rasmussen, T.R.; Hager, H.; Rasmussen, F. High Resolution Spiral CT for Determining the Malignant Potential of Solitary Pulmonary Nodules: Refining and Testing the Test. Acta Radiol. 2011, 52, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Verschakelen, J.A.; Bogaert, J.; Wever, W.D. Computed Tomography in Staging for Lung Cancer. Eur. Respir. J. 2002, 19, 40s–48s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochhegger, B.; Alves, G.R.T.; Irion, K.L.; Fritscher, C.C.; Fritscher, L.G.; Concatto, N.H.; Marchiori, E. PET/CT Imaging in Lung Cancer: Indications and Findings. J. Bras. Pneumol. 2015, 41, 264–274. [Google Scholar] [CrossRef]
- Harders, S.W.; Madsen, H.H.; Nellemann, H.M.; Rasmussen, T.R.; Thygesen, J.; Hager, H.; Andersen, N.T.; Rasmussen, F. Dynamic Contrast-Enhanced CT in Suspected Lung Cancer: Quantitative Results. Br. J. Radiol. 2013, 86, 20130257. [Google Scholar] [CrossRef] [Green Version]
- Harders, S.W.; Madsen, H.H.; Hjorthaug, K.; Arveschoug, A.K.; Rasmussen, T.R.; Meldgaard, P.; Hoejbjerg, J.A.; Pilegaard, H.K.; Hager, H.; Rehling, M.; et al. Mediastinal Staging in Non-Small-Cell Lung Carcinoma: Computed Tomography versus F-18-Fluorodeoxyglucose Positron-Emission Tomography and Computed Tomography. Cancer Imaging 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henzler, T.; Shi, J.; Jafarov, H.; Schoenberg, S.O.; Manegold, C.; Fink, C.; Schmid-Bindert, G. Functional CT Imaging Techniques for the Assessment of Angiogenesis in Lung Cancer. Transl. Lung Cancer Res. 2012, 1, 78–83. [Google Scholar]
- Kandathil, A.; Kay, F.U.; Butt, Y.M.; Wachsmann, J.W.; Subramaniam, R.M. Role of FDG PET/CT in the Eighth Edition of TNM Staging of Non-Small Cell Lung Cancer. Radiographics 2018, 38, 2134–2149. [Google Scholar] [CrossRef]
- Odisio, E.G.; Truong, M.T.; Duran, C.; de Groot, P.M.; Godoy, M.C. Role of Dual-Energy Computed Tomography in Thoracic Oncology. Radiol. Clin. North Am. 2018, 56, 535–548. [Google Scholar] [CrossRef]
- Miles, K.A.; Griffiths, M.R.; Fuentes, M.A. Standardized Perfusion Value: Universal CT Contrast Enhancement Scale That Correlates with FDG PET in Lung Nodules. Radiology 2001, 220, 548–553. [Google Scholar] [CrossRef]
- Schmid-Bindert, G.; Henzler, T.; Chu, T.Q.; Meyer, M.; Nance, J.W.; Schoepf, U.J.; Dinter, D.J.; Apfaltrer, P.; Krissak, R.; Manegold, C.; et al. Functional Imaging of Lung Cancer Using Dual Energy CT: How Does Iodine Related Attenuation Correlate with Standardized Uptake Value of 18FDG-PET-CT? Eur. Radiol. 2012, 22, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Miles, K.A.; Griffiths, M.R.; Keith, C.J. Blood Flow-Metabolic Relationships Are Dependent on Tumour Size in Non-Small Cell Lung Cancer: A Study Using Quantitative Contrast-Enhanced Computer Tomography and Positron Emission Tomography. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, U.; Nishihara, H.; Tsukamoto, E.; Morikawa, T.; Tamaki, N.; Miyasaka, K. Lung Tumors Evaluated with FDG-PET and Dynamic CT: The Relationship between Vascular Density and Glucose Metabolism. J. Comput. Assist. Tomogr. 2002, 26, 185–190. [Google Scholar] [CrossRef]
- Van Elmpt, W.; Das, M.; Hüllner, M.; Sharifi, H.; Zegers, K.; Reymen, B.; Lambin, P.; Wildberger, J.E.; Troost, E.G.C.; Veit-Haibach, P.; et al. Characterization of Tumor Heterogeneity Using Dynamic Contrast Enhanced CT and FDG-PET in Non-Small Cell Lung Cancer. Radiother. Oncol. 2013, 109, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Xu, Y.; Duan, J.; Li, C.; Sun, H.; Wang, W. Correlation of Iodine Uptake and Perfusion Parameters between Dual-Energy CT Imaging and First-Pass Dual-Input Perfusion CT in Lung Cancer. Medicine 2017, 96, e7479. [Google Scholar] [CrossRef] [PubMed]
- Kupik, O.; Metin, Y.; Eren, G.; Orhan Metin, N.; Arpa, M. A Comparison Study of Dual-Energy Spectral CT and 18F-FDG PET/CT in Primary Tumors and Lymph Nodes of Lung Cancer. Diagn. Interv. Radiol. 2021, 27, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Baxa, J.; Matouskova, T.; Ludvik, J.; Sedlmair, M.; Flohr, T.; Schmidt, B.; Bejcek, J.; Pesek, M.; Ferda, J. Single-Source Dual-Energy CT as a Part of 18F-FDG PET/CT: Direct Comparison of Iodine-Related and Metabolic Parameters in Non-Small Cell Lung Cancer. Anticancer Res. 2018, 38, 4131–4137. [Google Scholar] [CrossRef]
- Aoki, M.; Akimoto, H.; Sato, M.; Hirose, K.; Kawaguchi, H.; Hatayama, Y.; Seino, H.; Kakehata, S.; Tsushima, F.; Fujita, H.; et al. Impact of Pretreatment Whole-Tumor Perfusion Computed Tomography and 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Measurements on Local Control of Non-Small Cell Lung Cancer Treated with Stereotactic Body Radiotherapy. J. Radiat. Res. 2016, 57, 533–540. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE Guidelines. Saudi J. Anaesth. 2019, 13, S31–S34. [Google Scholar] [CrossRef]
- Shen, G.; Hu, S.; Kuang, A. Correlations of 18F-FDG Uptake on PET with Ki-67 Immunohistochemistry in Patients with Lung Cancer: A Meta-Analysis. J. Nucl. Med. 2016, 57, 131. [Google Scholar]
- Holmgren, L.; O’Reilly, M.S.; Folkman, J. Dormancy of Micrometastases: Balanced Proliferation and Apoptosis in the Presence of Angiogenesis Suppression. Nat. Med. 1995, 1, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical Applications of Research on Angiogenesis. N. Engl. J. Med. 1995, 333, 1757–1763. [Google Scholar] [CrossRef] [PubMed]



| Age | Years of Age | 73 (CI95% 69.8 to 76.2) |
|---|---|---|
| Days between PET/CT and SCE-CT | Number of days | 13 (CI95% 6.5 to 19.6) |
| Tumor size | Volume (cm3) | 51.49 (CI95% 18.16 to 84.83) |
| Gender | Male | 9 |
| Female | 13 | |
| Pathology | Adenocarcinoma | 19 |
| Squamous Cell Carcinoma | 2 | |
| Clinical stage | IA | 3 |
| IB | 3 | |
| IIA | 1 | |
| IIIA | 3 | |
| IIIB | 1 | |
| IIIC | 4 | |
| IVA | 1 | |
| IVB | 6 | |
| T stage | T1a | 0 |
| T1b | 3 | |
| T1c | 1 | |
| T2a | 4 | |
| T2b | 3 | |
| T3 | 5 | |
| T4 | 6 |
| Total Iodine Content | Iodinemean (mg/cm3) | Iodinemax (mg/cm3) | |||||
|---|---|---|---|---|---|---|---|
| Parameter | Threshold | R | p-Value | R | p-Value | R | p-Value |
| SUVmax | 0.61 | 0.002 | −0.53 | 0.012 | 0.62 | 0.001 | |
| SUVpeak | 0.68 | 0.001 | −0.44 | 0.039 | 0.73 | <0.001 | |
| SUVmean | 50% | 0.58 | 0.005 | −0.51 | 0.015 | 0.60 | 0.003 |
| 42% | 0.57 | 0.006 | −0.50 | 0.017 | 0.59 | 0.004 | |
| 30% | 0.54 | 0.010 | −0.47 | 0.027 | 0.57 | 0.006 | |
| 20% | 0.48 | 0.025 | −0.44 | 0.039 | 0.52 | 0.014 | |
| 10% | 0.37 | 0.092 | −0.39 | 0.070 | 0.42 | 0.051 | |
| MTV | 50% | 0.68 | 0.001 | −0.30 | 0.168 | 0.70 | <0.001 |
| 42% | 0.72 | <0.001 | −0.33 | 0.131 | 0.74 | <0.001 | |
| 30% | 0.80 | <0.001 | −0.37 | 0.088 | 0.80 | <0.001 | |
| 20% | 0.86 | <0.001 | −0.39 | 0.069 | 0.84 | <0.001 | |
| 10% | 0.92 | <0.001 | −0.40 | 0.064 | 0.86 | <0.001 | |
| SUV2.5 | 0.91 | <0.001 | −0.40 | 0.064 | 0.86 | <0.001 | |
| TLG | 50% | 0.63 | 0.002 | −0.31 | 0.158 | 0.69 | <0.001 |
| 42% | 0.66 | 0.001 | −0.33 | 0.137 | 0.72 | <0.001 | |
| 30% | 0.72 | <0.001 | −0.35 | 0.110 | 0.76 | <0.001 | |
| 20% | 0.75 | <0.001 | −0.36 | 0.097 | 0.78 | <0.001 | |
| 10% | 0.78 | <0.001 | −0.37 | 0.092 | 0.80 | <0.001 | |
| SUV2.5 | 0.77 | <0.001 | −0.37 | 0.012 | 0.80 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, M.B.; Drljevic-Nielsen, A.; Thygesen, J.; Kruis, M.F.; Hjorthaug, K.; Rasmussen, F.; Nijkamp, J.A. Assessment of Correlation between Dual-Energy Ct (De-Ct)-Derived Iodine Concentration and Local Flourodeoxyglucose (Fdg) Uptake in Patients with Primary Non-Small-Cell Lung Cancer. Tomography 2022, 8, 1770-1780. https://doi.org/10.3390/tomography8040149
Andersen MB, Drljevic-Nielsen A, Thygesen J, Kruis MF, Hjorthaug K, Rasmussen F, Nijkamp JA. Assessment of Correlation between Dual-Energy Ct (De-Ct)-Derived Iodine Concentration and Local Flourodeoxyglucose (Fdg) Uptake in Patients with Primary Non-Small-Cell Lung Cancer. Tomography. 2022; 8(4):1770-1780. https://doi.org/10.3390/tomography8040149
Chicago/Turabian StyleAndersen, Michael Brun, Aska Drljevic-Nielsen, Jesper Thygesen, Matthijs Ferdinand Kruis, Karin Hjorthaug, Finn Rasmussen, and Jasper Albertus Nijkamp. 2022. "Assessment of Correlation between Dual-Energy Ct (De-Ct)-Derived Iodine Concentration and Local Flourodeoxyglucose (Fdg) Uptake in Patients with Primary Non-Small-Cell Lung Cancer" Tomography 8, no. 4: 1770-1780. https://doi.org/10.3390/tomography8040149
APA StyleAndersen, M. B., Drljevic-Nielsen, A., Thygesen, J., Kruis, M. F., Hjorthaug, K., Rasmussen, F., & Nijkamp, J. A. (2022). Assessment of Correlation between Dual-Energy Ct (De-Ct)-Derived Iodine Concentration and Local Flourodeoxyglucose (Fdg) Uptake in Patients with Primary Non-Small-Cell Lung Cancer. Tomography, 8(4), 1770-1780. https://doi.org/10.3390/tomography8040149







