Relationship between Baseline [18F]FDG PET/CT Semiquantitative Parameters and BRCA Mutational Status and Their Prognostic Role in Patients with Invasive Ductal Breast Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Selection
2.2. The [18F]FDG PET/CT Acquisition and Interpretation
2.3. Statistical Analysis
2.4. Survival Analysis
3. Results
3.1. Patients Characteristics
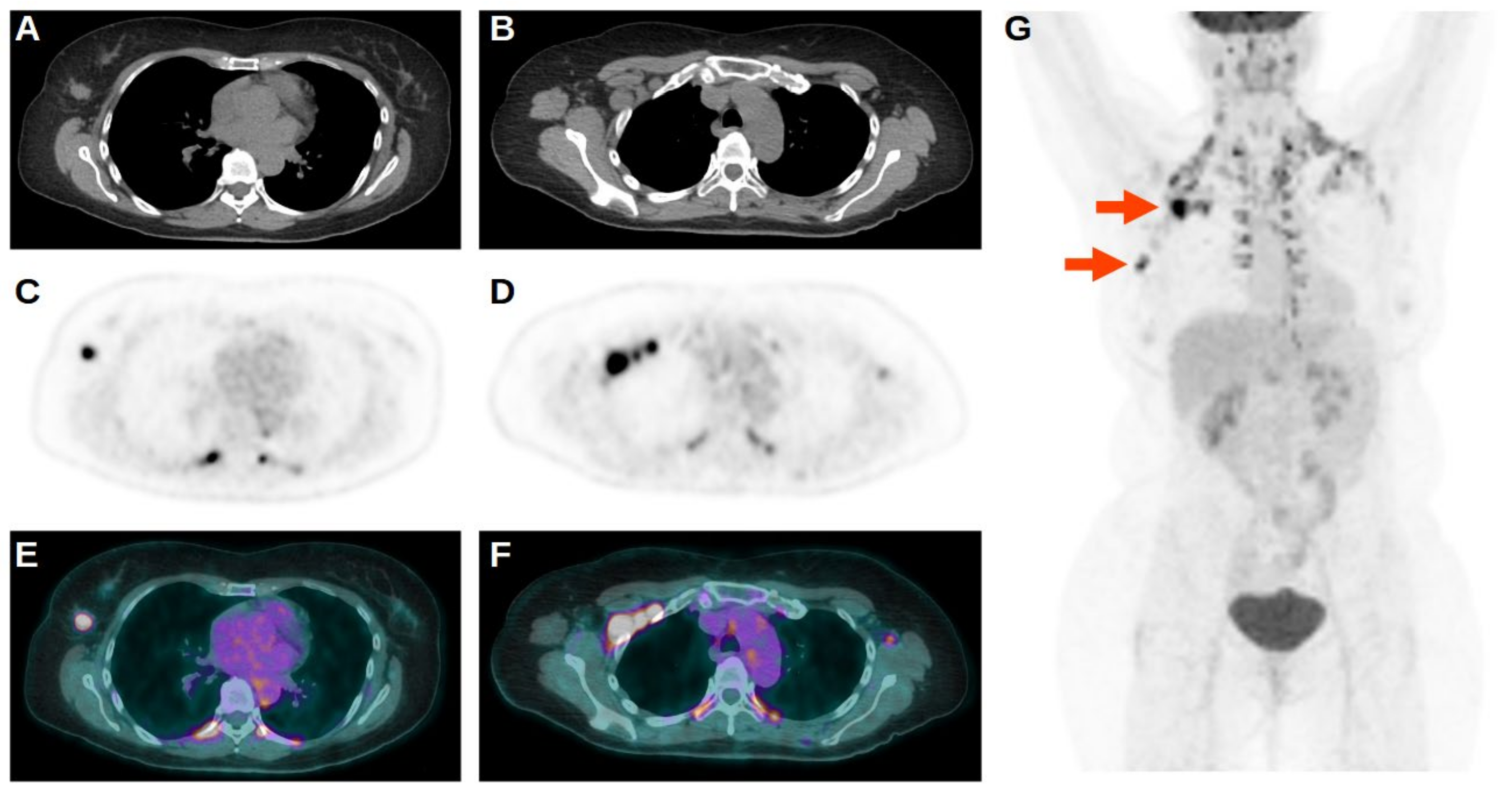
3.2. The 18F-FDG PET/CT Results
3.3. Prognostic Value of 18F-FDG PET/CT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef] [PubMed]
- Rojas, K.; Stuckey, A. Breast Cancer Epidemiology and Risk Factors. Clin. Obstet. Gynecol. 2016, 59, 651–672. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.S.; Clark, A.S.; Tchou, J.; Zhang, P.; Freedman, G.M. Clinical Diagnosis and Management of Breast Cancer. J. Nucl. Med. 2016, 57 (Suppl. 1), 9S–16S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewer, H.R.; Jones, M.E.; Schoemaker, M.J.; Ashworth, A.; Swerdlow, A.J. Family history and risk of breast cancer: An analysis accounting for family structure. Breast Cancer Res. Treat. 2017, 165, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.J.; Silver, D.; Cantor, S.; Livingston, D.M.; Scully, R. BRCA 1, BRCA2, and Rad51 operative in a common DNA damage response pathway. Cancer Res. 1999, 59, 1752–1756. [Google Scholar]
- Bordeleau, L.; Panchal, S.; Goodwin, P. Prognosis of BRCA-associated breast cancer: A summary of evidence. Breast Cancer Res. Treat. 2010, 119, 13–24. [Google Scholar] [CrossRef]
- Kim, E.K.; Park, S.Y.; Kim, S.W. Clinicopathological characteristics of BRCA associated breast cancer in Asian patients. J. Pathol. Transl. Med. 2020, 54, 265–275. [Google Scholar] [CrossRef]
- Huszno, J.; Kołosza, Z.; Grzybowska, E. BRCA1 mutation in breast cancer patients: Analysis of prognostic factors and survival. Oncol. Lett. 2019, 17, 1986–1995. [Google Scholar] [CrossRef] [Green Version]
- Copson, E.R.; Maishman, T.C.; Tapper, W.J.; Cutress, R.I.; Greville-Heygate, S.; Altman, D.G.; Eccles, B.; Gerty, S.; Durcan, L.T.; Jones, L.; et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol. 2018, 19, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Van den Broek, A.J.; Schmidt, M.K.; van’t Veer, L.J.; Tollenaar, R.A.; van Leeuwen, F.E. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: What’s the evidence? A systematic review with meta-analysis. PLoS ONE 2015, 10, e0120189. [Google Scholar] [CrossRef] [Green Version]
- Baretta, Z.; Mocellin, S.; Goldin, E.; Olopade, O.I.; Huo, D. Effect of BRCA germline mutations on breast cancer prognosis: A systematic review and meta-analysis. Medicine 2016, 95, e4975. [Google Scholar] [CrossRef]
- Jafari, S.H.; Saadatpour, Z.; Salmaninejad, A.; Momeni, F.; Mokhtari, M.; Nahand, J.S.; Rahmati, M.; Mirzaei, H.; Kianmehr, M. Breast cancer diagnosis: Imaging techniques and biochemical markers. J. Cell Physiol. 2018, 233, 5200–5213. [Google Scholar] [CrossRef]
- Albano, D.; Gatta, R.; Marini, M.; Rodella, C.; Camoni, L.; Dondi, F.; Giubbini, R.; Bertagna, F. Role of 18F-FDG PET/CT Radiomics Features in the Differential Diagnosis of Solitary Pulmonary Nodules: Diagnostic Accuracy and Comparison between Two Different PET/CT Scanners. J. Clin. Med. 2021, 10, 5064. [Google Scholar] [CrossRef]
- Albano, D.; Dondi, F.; Schumacher, R.F.; D’Ippolito, C.; Porta, F.; Giubbini, R.; Bertagna, F. Clinical and Prognostic Role of 18F-FDG PET/CT in Pediatric Ewing Sarcoma. J. Pediatr. Hematol. Oncol. 2020, 42, e79–e86. [Google Scholar] [CrossRef]
- Bellini, P.; Albano, D.; Dondi, F.; Bertagna, F.; Giubbini, R. Different glucose metabolism behavior relating to histotypes in synchronous breast cancers evaluated by [18F]FDG PET-CT. Nucl. Med. Rev. 2022, 25, 64–65. [Google Scholar] [CrossRef]
- Albano, D.; Dondi, F.; Gazzilli, M.; Giubbini, R.; Bertagna, F. Meta-Analysis of the Diagnostic Performance of 18F-FDG-PET/CT Imaging in Native Valve Endocarditis. JACC Cardiovasc. Imaging 2021, 14, 1063–1065. [Google Scholar] [CrossRef]
- Dondi, F.; Albano, D.; Cerudelli, E.; Gazzilli, M.; Giubbini, R.; Treglia, G.; Bertagna, F. Radiolabelled PSMA PET/CT or PET/MRI in hepatocellular carcinoma (HCC): A systematic review. Clin. Transl. Imaging 2020, 8, 461–467. [Google Scholar] [CrossRef]
- Groheux, D.; Cochet, A.; Humbert, O.; Alberini, J.L.; Hindié, E.; Mankoff, D. ¹⁸F-FDG PET/CT for Staging and Restaging of Breast Cancer. J. Nucl. Med. 2016, 57 (Suppl. 1), 17S–26S. [Google Scholar] [CrossRef] [Green Version]
- Paydary, K.; Seraj, S.M.; Zadeh, M.Z.; Emamzadehfard, S.; Shamchi, S.P.; Gholami, S.; Werner, T.J.; Alavi, A. The Evolving Role of FDG-PET/CT in the Diagnosis, Staging, and Treatment of Breast Cancer. Mol. Imaging Biol. 2019, 21, 1–10. [Google Scholar] [CrossRef]
- Özdemir, S.; Sılan, F.; Akgün, M.Y.; Aracı, N.; Çırpan, İ.; Koç Öztürk, F.; Özdemir, Ö. Prognostic Prediction of BRCA Mutations by 18F-FDG PET/CT SUVmax in Breast Cancer. Mol. Imaging Radionucl. Ther. 2021, 30, 158–168. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Fujioka, T.; Katsuta, L.; Tsuchiya, J.; Kubota, K.; Kasahara, M.; Oda, G.; Nakagawa, T.; Onishi, I.; Tateishi, U. Diagnostic performance of time-of-flight PET/CT for evaluating nodal metastasis of the axilla in breast cancer. Nucl. Med. Commun. 2019, 40, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Sasada, S.; Masumoto, N.; Kimura, Y.; Kajitani, K.; Emi, A.; Kadoya, T.; Okada, M. Identification of Axillary Lymph Node Metastasis in Patients with Breast Cancer Using Dual-Phase FDG PET/CT. Am. J. Roentgenol. 2019, 213, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Noh, W.C.; Kim, H.A.; Kim, E.K.; Park, K.W.; Lee, S.S.; Choi, J.H.; Han, K.W.; Byun, B.H.; Lim, I.; et al. The Relationship Between Estrogen Receptor, Progesterone Receptor and Human Epidermal Growth Factor Receptor 2 Expression of Breast Cancer and the Retention Index in Dual Phase (18)F-FDG PET/CT. Nucl. Med. Mol. Imaging 2016, 50, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Cerci, S.M.; Bozkurt, K.K.; Eroglu, H.E.; Cerci, C.; Erdemoglu, E.; Bulbul, P.T.; Cetin, M.; Cetin, R.; Ciris, I.M.; Bulbul, M. Evaluation of the association between HIF-1α and HER-2 expression, hormone receptor status, Ki-67 expression, histology and tumor FDG uptake in breast cancer. Oncol. Lett. 2016, 12, 3889–3895. [Google Scholar] [CrossRef] [Green Version]
- Caresia Aroztegui, A.P.; García Vicente, A.M.; Alvarez Ruiz, S.; Delgado Bolton, R.C.; Orcajo Rincon, J.; Garcia Garzon, J.R.; de Arcocha Torres, M.; Garcia-Velloso, M.J. Oncology Task Force of the Spanish Society of Nuclear Medicine and Molecular Imaging. 18F-FDG PET/CT in breast cancer: Evidence-based recommendations in initial staging. Tumour Biol. 2017, 39, 1010428317728285. [Google Scholar] [CrossRef] [Green Version]
- Pak, K.; Seok, J.W.; Kim, H.Y.; Nguyen, T.L.; Kim, K.; Kim, S.J.; Kim, I.J.; Hopper, J. Prognostic value of metabolic tumor volume and total lesion glycolysis in breast cancer: A meta-analysis. Nucl. Med. Commun. 2020, 41, 824–829. [Google Scholar] [CrossRef]

| Characteristic | n (%) |
|---|---|
| Age (mean ± SD, range) | 45 ± 13, 23–86 |
| Size (mean ± SD, range) (mm) | 27.7 ± 12.8, 10.0–81.0 |
| Grading | |
| G2 | 12 (29.3%) |
| G3 | 29 (70.7%) |
| Breast | |
| Left | 24 (58.5%) |
| Right | 17 (41.5%) |
| BRCA mutation | |
| No | 28 (68.3%) |
| BRCA1 | 7 (17.1%) |
| BRCA2 | 6 (14.6%) |
| Ki-67 expression (mean ± SD, range) (%) | 46.83 ± 25.6, 5.0–98.0 |
| ER expression (mean ± SD, range) (%) | 52.29 ± 46.1, 0.0–100.0 |
| PR expression (mean ± SD, range) (%) | 19.85 ± 31.9; 0.0–98.0 |
| HER2 status | |
| Negative | 27 (65.9%) |
| Positive | 14 (34.1%) |
| AJCC stage | |
| I | 7 (17.1%) |
| II | 17 (41.5%) |
| III | 8 (19.5%) |
| IV | 9 (21.9%) |
| Nodal metastasis | |
| Yes | 25 (61.0%) |
| No | 16 (39.0%) |
| Distant metastasis | |
| Yes | 2 (4.9%) |
| No | 39 (95.1%) |
| Therapy | |
| Surgery, ChT | 6 (14.6%) |
| Surgery, ChT, RT | 10 (24.4%) |
| Surgery, ChT, RT, IT | 4 (9.8%) |
| Surgery, ChT, RT, IT, OT | 4 (9.8%) |
| Surgery, ChT, RT, OT | 4 (9.8%) |
| Surgery, ChT, IT | 2 (4.9%) |
| Surgery, ChT, IT, OT | 5 (12.1%) |
| Surgery, ChT, OT | 6 (14.6%) |
| PET/CT parameters | |
| SUVmax (mean ± SD, range) | 9.19 ± 6.15, 1.74–27.88 |
| SUVmean (mean ± SD, range) | 5.43 ± 3.91, 1.02–18.55 |
| SUVlbm (mean ± SD, range) | 6.77 ± 4.67, 1.22–19.53 |
| SUVbsa (mean ± SD, range) | 2.57 ± 1.73, 0.47–7.17 |
| S-L (mean ± SD, range) | 3.65 ± 2.29, 0.73–8.77 |
| S-BP (mean ± SD, range) | 4.68 ± 3.21, 0.87–14.28 |
| MTV (mean ± SD, range) | 9.46 ± 9.28, 1.30–42.10 |
| TLG (mean ± SD, range) | 70.52 ± 90.36, 5.70–463.40 |
| Relapse or progression | |
| Yes | 9 (22.0%) |
| No | 32 (78.0%) |
| Death | |
| Yes | 2 (4.9%) |
| No | 39 (95.1%) |
| PFS months (mean ± SD, range) | 53.90 ± 30.24, 3.15–150.98 |
| OS months (mean ± SD, range) | 57.48 ± 25.91, 10.63–150.98 |
| SUVmax | p-Value | SUVmean | p-Value | SUVlbm | p-Value | SUVbsa | p-Value | S-L | p-Value | S-BP | p-Value | MTV | p-Value | TLG | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AJCC Stage | 0.463 | 0.379 | 0.367 | 0.391 | 0.368 | 0.324 | 0.006 | 0.008 | ||||||||
| I | 11.60 | 5.11 | 8.99 | 3.42 | 4.39 | 5.08 | 2.22 | 15.73 | ||||||||
| II | 7.71 | 4.26 | 5.37 | 2.09 | 2.92 | 3.81 | 7.13 | 53.36 | ||||||||
| III | 10.03 | 6.24 | 7.23 | 2.68 | 3.78 | 4.42 | 13.10 | 90.47 | ||||||||
| IV | 10.70 | 7.16 | 7.24 | 2.71 | 4.32 | 6.24 | 16.24 | 124.06 | ||||||||
| Age | 0.522 | 0.449 | 0.309 | 0.396 | 0.273 | 0.334 | 0.531 | 0.334 | ||||||||
| <44 | 10.38 | 5.90 | 7.41 | 2.79 | 4.17 | 5.45 | 8.83 | 76.88 | ||||||||
| ≥44 | 8.64 | 4.98 | 6.15 | 2.36 | 3.15 | 3.94 | 10.06 | 64.47 | ||||||||
| HER2 status | 0.371 | 0.741 | 0.509 | 0.386 | 0.364 | 0.296 | 0.610 | 0.923 | ||||||||
| Negative | 10.26 | 5.81 | 7.38 | 2.81 | 3.96 | 5.07 | 8.83 | 75.17 | ||||||||
| Positive | 7.99 | 4.68 | 5.58 | 2.09 | 3.04 | 3.92 | 10.67 | 61.55 | ||||||||
| Size (mm) | 0.814 | 0.597 | 0.753 | 0.834 | 0.513 | 0.916 | 0.067 | 0.025 | ||||||||
| <26 | 9.32 | 4.96 | 6.90 | 2.61 | 3.84 | 4.58 | 6.61 | 36.96 | ||||||||
| ≥26 | 9.63 | 5.84 | 6.65 | 2.53 | 3.48 | 4.77 | 11.92 | 99.51 | ||||||||
| Grading | 0.491 | 0.430 | 0.731 | 0.709 | 0.406 | 0.315 | 0.351 | 0.240 | ||||||||
| 2 | 8.43 | 5.09 | 6.19 | 2.32 | 3.11 | 3.89 | 8.50 | 75.20 | ||||||||
| 3 | 9.92 | 5.57 | 7.01 | 2.67 | 3.87 | 5.01 | 9.85 | 68.59 | ||||||||
| N+ | 0.168 | 0.336 | 0.309 | 0.181 | 0.057 | 0.051 | 0.002 | 0.014 | ||||||||
| Yes | 10.23 | 5.36 | 7.31 | 2.85 | 4.25 | 5.35 | 12.87 | 97.61 | ||||||||
| No | 9.06 | 5.47 | 6.42 | 2.39 | 3.26 | 4.25 | 4.25 | 28.20 | ||||||||
| BRCA mutation | 0.025 | 0.154 | 0.016 | 0.018 | 0.058 | 0.278 | 0.338 | 0.069 | ||||||||
| Yes | 12.60 | 6.71 | 9.30 | 3.49 | 4.64 | 5.49 | 11.52 | 108.00 | ||||||||
| No | 8.04 | 4.83 | 5.59 | 2.14 | 3.19 | 4.30 | 8.50 | 53.12 | ||||||||
| Ki-67 status | 0.002 | 0.003 | 0.003 | 0.002 | 0.002 | 0.006 | 0.960 | 0.190 | ||||||||
| <40 | 6.79 | 3.75 | 4.75 | 1.80 | 2.64 | 3.39 | 9.51 | 52.33 | ||||||||
| ≥40 | 12.32 | 7.19 | 8.88 | 3.38 | 4.71 | 6.03 | 9.40 | 89.62 | ||||||||
| ER status | 0.007 | 0.015 | 0.004 | 0.003 | 0.012 | 0.017 | 0.300 | 0.653 | ||||||||
| <80 | 7.03 | 4.01 | 4.82 | 1.82 | 2.79 | 3.53 | 7.90 | 64.23 | ||||||||
| ≥80 | 12.07 | 6.92 | 8.81 | 3.32 | 4.55 | 5.88 | 10.94 | 77.14 | ||||||||
| PR status | 0.022 | 0.050 | 0.025 | 0.023 | 0.033 | 0.029 | 0.185 | 0.749 | ||||||||
| <50 | 4.25 | 2.56 | 2.87 | 1.10 | 1.83 | 2.07 | 8.66 | 59.43 | ||||||||
| ≥50 | 10.38 | 5.94 | 7.43 | 2.82 | 3.96 | 5.13 | 14.13 | 72.42 | ||||||||
| M+ | 0.275 | 0.301 | 0.453 | 0.439 | 0.142 | 0.065 | 0.691 | 0.319 | ||||||||
| Yes | 14.17 | 8.25 | 9.23 | 3.51 | 5.98 | 8.74 | 12.05 | 133.35 | ||||||||
| No | 9.25 | 5.28 | 6.64 | 2.52 | 3.53 | 4.47 | 9.32 | 67.30 |
| BRCA without Mutation | BRCA with Mutation | p-Value | |
|---|---|---|---|
| AJCC Stage | 0.218 | ||
| I | 5 (12.2%) | 2 (4.9%) | |
| II | 13 (31.7%) | 4 (9.7%) | |
| III | 3 (7.3%) | 5 (12.2%) | |
| IV | 7 (17.1%) | 2 (4.9%) | |
| Age | 0.053 | ||
| <42 | 14 (34.1%) | 6 (14.7%) | |
| ≥42 | 14 (34.1%) | 7 (17.1%) | |
| HER2 status | 0.084 | ||
| Negative | 16 (39.0%) | 11 (26.8%) | |
| Positive | 12 (29.3%) | 2 (4.9%) | |
| Size (mm) | 0.173 | ||
| <26 | 15 (36.6%) | 4 (9.7%) | |
| ≥26 | 13 (31.7%) | 9 (22.0%) | |
| Grading | 0.552 | ||
| 2 | 9 (22.0%) | 3 (7.3%) | |
| 3 | 19 (46.3%) | 10 (24.4%) | |
| N+ | 0.034 | ||
| Yes | 14 (34.1%) | 11 (26.8%) | |
| No | 14 (34.1%) | 2 (4.9%) | |
| Ki67 status | 0.265 | ||
| <41 | 16 (39.0%) | 5 (12.2%) | |
| ≥41 | 12 (29.3%) | 8 (19.5%) | |
| ER status | 0.818 | ||
| <50 | 14 (34.1%) | 6 (14.7%) | |
| ≥50 | 14 (34.1%) | 7 (17.1%) | |
| PR status | 0.391 | ||
| <50 | 23 (56.1%) | 12 (29.3%) | |
| ≥50 | 5 (12.2%) | 1 (2.4%) | |
| M+ | 0.322 | ||
| Yes | 2 (4.9%) | 0 (0.0%) | |
| No | 26 (63.4%) | 13 (31.7%) |
| Single Analysis | BRCA and Semiquantitative Parameters | |||
|---|---|---|---|---|
| PFS | OS | PFS | OS | |
| SUVmax | 0.520 | 0.854 | 0.161 | 0.174 |
| SUVmean | 0.545 | 0.854 | 0.366 | 0.288 |
| SUVlbm | 0.977 | 0.854 | 0.376 | 0.174 |
| SUVbsa | 0.977 | 0.854 | 0.376 | 0.174 |
| S-L | 0.947 | 0.854 | 0.388 | 0.174 |
| S-BP | 0.806 | 0.919 | 0.707 | 0.319 |
| MTV | 0.034 | 0.117 | 0.199 | 0.479 |
| TLG | 0.008 | 0.151 | 0.028 | 0.438 |
| BRCA | 0.047 | 0.024 | ||
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| p-Value | HR (95% CI) | p-Value | HR (95% CI) | |
| PFS | ||||
| BRCA status | 0.797 | 1.19 (0.30–4.76) | ||
| Age | 0.858 | 1.12 (0.30–4.19) | ||
| Stage | 0.012 | 14.45 (1.81–114.87) | 0.059 | 8.09 (0.92–70.49) |
| Size | 0.386 | 1.84 (0.46–7.32) | ||
| Grading | 0.678 | 1.39 (0.29–6.66) | ||
| HER2 status | 0.390 | 0.50 (0.11–2.40) | ||
| Ki-67 expression | 0.563 | 1.47 (0.39–5.46) | ||
| ER expression | 0.862 | 1.12 (0.30–4.16) | ||
| PR expression | 0.557 | 1.60 (0.33–7.67) | ||
| Therapy | 0.121 | 3.46 (0.72–16.53) | ||
| N+ | 0.107 | 5.52 (0.69–43.73) | ||
| SUVmax | 0.523 | 1.53 (0.41–5.70) | ||
| SUVmean | 0.548 | 1.49 (0.40–5.55) | ||
| SUVlbm | 0.977 | 0.98 (0.26–3.64) | ||
| SUVbsa | 0.977 | 0.98 (0.26–3.64) | ||
| S-L | 0.947 | 0.95 (0.26–3.54) | ||
| S-BP | 0.806 | 0.84 (0.23–3.14) | ||
| MTV | 0.053 | 4.73 (0.98–22.75) | ||
| TLG | 0.024 | 10.87 (1.36–86.32) | 0.152 | 4.94 (0.56–43.63) |
| OS | ||||
| BRCA status | 0.668 | 1.83 (0.12–28.94) | ||
| Age | 0.850 | 0.76 (0.05–12.16) | ||
| Stage | 0.949 | 0.82 (0.34–2.92) | ||
| Size | 0.949 | 0.80 (0.31–3.78) | ||
| Grading | 0.958 | 0.95 (0.12–3.68) | ||
| HER2 status | 0.957 | 0.99 (0.24–3.07) | ||
| Ki-67 expression | 0.982 | 0.97 (0.06–15.29) | ||
| ER expression | 0.953 | 0.92 (0.06–14.51) | ||
| PR expression | 0.968 | 0.12 (0.01–2.07) | ||
| Therapy | 0.949 | 0.80 (0.41–3.65) | ||
| N+ | 0.952 | 1.21 (0.40–3.92) | ||
| SUVmax | 0.855 | 1.29 (0.08–20.42) | ||
| SUVmean | 0.855 | 1.29 (0.08–20.42) | ||
| SUVlbm | 0.855 | 1.29 (0.08–20.42) | ||
| SUVbsa | 0.855 | 1.29 (0.08–20.42) | ||
| S-L | 0.855 | 1.29 (0.08–20.42) | ||
| S-BP | 0.919 | 1.15 (0.07–18.21) | ||
| MTV | 0.948 | 0.79 (0.29–2.75) | ||
| TLG | 0.948 | 0.79 (0.28–2.75) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dondi, F.; Albano, D.; Bellini, P.; Camoni, L.; Treglia, G.; Bertagna, F. Relationship between Baseline [18F]FDG PET/CT Semiquantitative Parameters and BRCA Mutational Status and Their Prognostic Role in Patients with Invasive Ductal Breast Carcinoma. Tomography 2022, 8, 2662-2675. https://doi.org/10.3390/tomography8060222
Dondi F, Albano D, Bellini P, Camoni L, Treglia G, Bertagna F. Relationship between Baseline [18F]FDG PET/CT Semiquantitative Parameters and BRCA Mutational Status and Their Prognostic Role in Patients with Invasive Ductal Breast Carcinoma. Tomography. 2022; 8(6):2662-2675. https://doi.org/10.3390/tomography8060222
Chicago/Turabian StyleDondi, Francesco, Domenico Albano, Pietro Bellini, Luca Camoni, Giorgio Treglia, and Francesco Bertagna. 2022. "Relationship between Baseline [18F]FDG PET/CT Semiquantitative Parameters and BRCA Mutational Status and Their Prognostic Role in Patients with Invasive Ductal Breast Carcinoma" Tomography 8, no. 6: 2662-2675. https://doi.org/10.3390/tomography8060222
APA StyleDondi, F., Albano, D., Bellini, P., Camoni, L., Treglia, G., & Bertagna, F. (2022). Relationship between Baseline [18F]FDG PET/CT Semiquantitative Parameters and BRCA Mutational Status and Their Prognostic Role in Patients with Invasive Ductal Breast Carcinoma. Tomography, 8(6), 2662-2675. https://doi.org/10.3390/tomography8060222







