Two-Dimensional Post-Traumatic Measurements of Orbital Floor Blowout Fractures Underestimate Defect Sizes Compared to Three-Dimensional Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
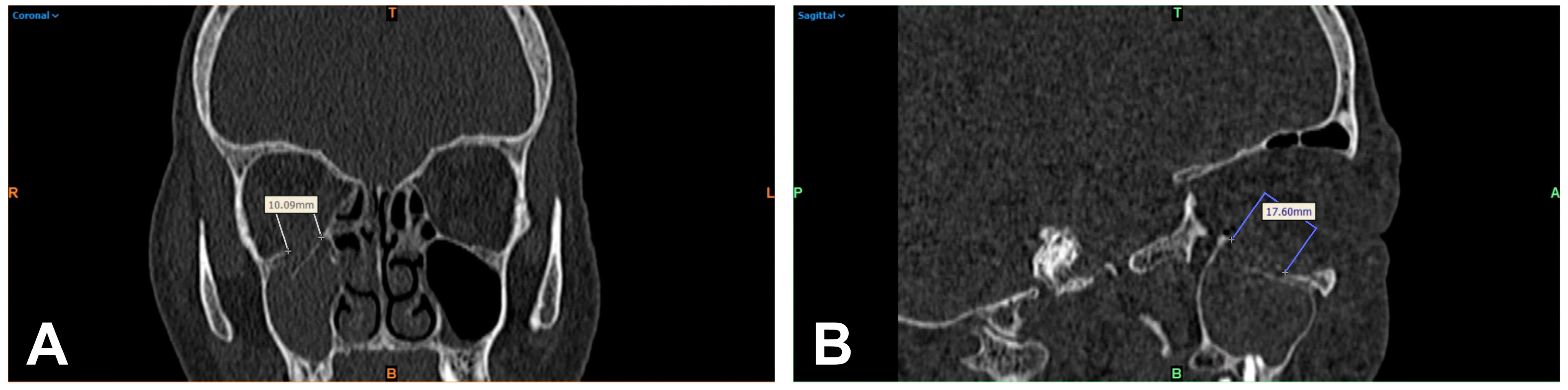
2.2. 2D-Based Measurement of Orbital Floor Fractures
2.3. 3D-Based Measurements of Orbital Floor Fractures
2.4. Statistical Analysis
3. Results
3.1. Characterization of the Patient Cohort
3.2. Comparison of Orbital Floor Defect Areas Based on 2D and 3D Measurements
3.3. Comparison of Processing Duration of 2D- and 3D-Based Assessments of Orbital Floor Defect Areas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellis, E., 3rd; El-Attar, A.; Moos, K.F. An analysis of 2067 cases of zygomatico-orbital fracture. J. Oral Maxillofac. Surg. 1985, 43, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Cornelius, C.P.; Groten, M.; Pröbster, L.; Pfannenberg, C.; Schwenzer, N. Orbital reconstruction with individually copy-milled ceramic implants. Plast. Reconstr. Surg. 1998, 101, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Gross, C.W. Facial fractures. Analysis of five years of experience. Arch. Otolaryngol. 1973, 97, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Lim, J.S.; Yoo, G.; Byeon, J.H. An analysis of pure blowout fractures and associated ocular symptoms. J. Craniofacial Surg. 2013, 24, 703–707. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, N. Clinical application of three-dimensional printing technology in craniofacial plastic surgery. Arch. Plast. Surg. 2015, 42, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Ellis, E., 3rd; Tan, Y. Assessment of internal orbital reconstructions for pure blowout fractures: Cranial bone grafts versus titanium mesh. J. Oral Maxillofac. Surg. 2003, 61, 442–453. [Google Scholar] [CrossRef]
- Grob, S.; Yonkers, M.; Tao, J. Orbital Fracture Repair. Semin. Plast. Surg. 2017, 31, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Ploder, O.; Klug, C.; Backfrieder, W.; Voracek, M.; Czerny, C.; Tschabitscher, M. 2D- and 3D-based measurements of orbital floor fractures from CT scans. J. Cranio-Maxillofac. Surg. 2002, 30, 153–159. [Google Scholar] [CrossRef]
- Sigron, G.R.; Rüedi, N.; Chammartin, F.; Meyer, S.; Msallem, B.; Kunz, C.; Thieringer, F.M. Three-Dimensional Analysis of Isolated Orbital Floor Fractures Pre- and Post-Reconstruction with Standard Titanium Meshes and “Hybrid” Patient-Specific Implants. J. Clin. Med. 2020, 9, 1579. [Google Scholar] [CrossRef]
- Smith, B.; Regan, W.F., Jr. Blow-out fracture of the orbit; mechanism and correction of internal orbital fracture. Am. J. Ophthalmol. 1957, 44, 733–739. [Google Scholar] [CrossRef]
- Jordan, D.R.; Allen, L.H.; White, J.; Harvey, J.; Pashby, R.; Esmaeli, B. Intervention within days for some orbital floor fractures: The white-eyed blowout. Ophthalmic Plast. Reconstr. Surg. 1998, 14, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Broyles, J.M.; Jones, D.; Bellamy, J.; Elgendy, T.; Sebai, M.; Susarla, S.M.; Vaca, E.E.; Tuffaha, S.H.; Manson, P.N.; Dorafshar, A.H. Pediatric Orbital Floor Fractures: Outcome Analysis of 72 Children with Orbital Floor Fractures. Plast. Reconstr. Surg. 2015, 136, 822–828. [Google Scholar] [CrossRef]
- Wolff, A.; Ohayon, C.; Emodi, O.; Sviri, G.E.; Shapira, V.; Rachmiel, A. Management of Pediatric Orbital Fractures. J. Craniofacial Surg. 2021, 32, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Shokri, T.; Alford, M.; Hammons, M.; Ducic, Y.; Sokoya, M. Management of Orbital Floor Fractures. Facial Plast. Surg. 2019, 35, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Hawes, M.J.; Dortzbach, R.K. Surgery on orbital floor fractures. Influence of time of repair and fracture size. Ophthalmology 1983, 90, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Felding, U.N.A. Blowout fractures—Clinic, imaging and applied anatomy of the orbit. Dan. Med. J. 2018, 65, B5459. [Google Scholar] [PubMed]
- Silverman, N.; Spindle, J.; Tang, S.X.; Wu, A.; Hong, B.K.; Shore, J.W.; Wester, S.; Levin, F.; Connor, M.; Burt, B.; et al. Orbital floor fracture with entrapment: Imaging and clinical correlations in 45 cases. Orbit 2017, 36, 331–336. [Google Scholar] [CrossRef]
- Bite, U.; Jackson, I.T.; Forbes, G.S.; Gehring, D.G. Orbital volume measurements in enophthalmos using three-dimensional CT imaging. Plast. Reconstr. Surg. 1985, 75, 502–508. [Google Scholar] [CrossRef]
- Manson, P.N.; Grivas, A.; Rosenbaum, A.; Vannier, M.; Zinreich, J.; Iliff, N. Studies on enophthalmos: II. The measurement of orbital injuries and their treatment by quantitative computed tomography. Plast. Reconstr. Surg. 1986, 77, 203–214. [Google Scholar] [CrossRef]
- Charteris, D.G.; Chan, C.H.; Whitehouse, R.W.; Noble, J.L. Orbital volume measurement in the management of pure blowout fractures of the orbital floor. Br. J. Ophthalmol. 1993, 77, 100–102. [Google Scholar] [CrossRef] [Green Version]
- Grove, A.S., Jr.; Tadmor, R.; New, P.F.; Momose, K.J. Orbital fracture evaluation by coronal computed tomography. Am. J. Ophthalmol. 1978, 85, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Khomutinnikova, N.E.; Durnovo, E.A.; Vyseltseva, Y.V.; Gorbatov, R.O. Digital Technologies in the Surgical Treatment of Post-Traumatic Zygomatico-Orbital Deformities. Sovrem. Tekhnologii Med. 2021, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Rahimov, C.R.; Ahmadov, S.G.; Rahimli, M.C.; Farzaliyev, I.M. Three-Dimensional Diagnosis in Orbital Reconstructive Surgery. Ann. Maxillofac. Surg. 2020, 10, 3–9. [Google Scholar] [CrossRef]
- Modabber, A.; Winnand, P.; Hölzle, F.; Ilgner, J.; Hackenberg, S. Current developments in primary and secondary surgical treatment of midface and periorbital trauma. HNO 2022, 70, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Schlittler, F.; Vig, N.; Burkhard, J.P.; Lieger, O.; Michel, C.; Holmes, S. What are the limitations of the non-patient-specific implant in titanium reconstruction of the orbit? Br. J. Oral Maxillofac. Surg. 2020, 58, e80–e85. [Google Scholar] [CrossRef]
- Kim, Y.C.; Min, K.H.; Choi, J.W.; Koh, K.S.; Oh, T.S.; Jeong, W.S. Patient-specific puzzle implant preformed with 3D-printed rapid prototype model for combined orbital floor and medial wall fracture. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 496–503. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Elgalal, M.; Loba, P.; Komuński, P.; Arkuszewski, P.; Broniarczyk-Loba, A.; Stefańczyk, L. Clinical application of 3D pre-bent titanium implants for orbital floor fractures. J. Cranio-Maxillofac. Surg. 2009, 37, 229–234. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Luo, T.Y.; Ma, J.; Dong, Z.; Cao, G.; Xu, J.K.; Liu, B.Y.; Zhang, Q.R.; Zhang, S.L. Application of Three-Dimensional Printing Technology in the Orbital Blowout Fracture Reconstruction. J. Craniofacial Surg. 2019, 30, 1825–1828. [Google Scholar] [CrossRef]
- Baek, W.I.; Kim, H.K.; Kim, W.S.; Bae, T.H. Comparison of Absorbable Mesh Plate versus Titanium-Dynamic Mesh Plate in Reconstruction of Blow-Out Fracture: An Analysis of Long-Term Outcomes. Arch. Plast. Surg. 2014, 41, 355–361. [Google Scholar] [CrossRef]
- Jarrahy, R.; Vo, V.; Goenjian, H.A.; Tabit, C.J.; Katchikian, H.V.; Kumar, A.; Meals, C.; Bradley, J.P. Diagnostic accuracy of maxillofacial trauma two-dimensional and three-dimensional computed tomographic scans: Comparison of oral surgeons, head and neck surgeons, plastic surgeons, and neuroradiologists. Plast. Reconstr. Surg. 2011, 127, 2432–2440. [Google Scholar] [CrossRef]
- Gunarajah, D.R.; Samman, N. Biomaterials for repair of orbital floor blowout fractures: A systematic review. J. Oral Maxillofac. Surg. 2013, 71, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, C.P.; Stiebler, T.; Mayer, P.; Smolka, W.; Kunz, C.; Hammer, B.; Jaquiéry, C.C.; Buitrago-Téllez, C.; Leiggener, C.S.; Metzger, M.C.; et al. Prediction of surface area size in orbital floor and medial orbital wall fractures based on topographical subregions. J. Cranio-Maxillofac. Surg. 2021, 49, 598–612. [Google Scholar] [CrossRef]
- Basta, M.N.; Rao, V.; Roussel, L.O.; Crozier, J.W.; Liu, P.Y.; Woo, A.S. Refining Indications for Orbital Floor Fracture Reconstruction: A Risk-Stratification Tool Predicting Symptom Development and Need for Surgery. Plast. Reconstr. Surg. 2021, 148, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Schouman, T.; Courvoisier, D.S.; Imholz, B.; Van Issum, C.; Scolozzi, P. Computational area measurement of orbital floor fractures: Reliability, accuracy and rapidity. Eur. J. Radiol. 2012, 81, 2251–2254. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.J.; Garcia, G.H.; Logani, S.C.; Murphy, M.L.; Sheth, B.P.; Seth, A.K. Orbital blow-out fractures: Correlation of preoperative computed tomography and postoperative ocular motility. Trans. Am. Ophthalmol. Soc. 1998, 96, 329–347, Discussion 347–353. [Google Scholar] [PubMed]
- Faisal, A.; Ng, S.C.; Goh, S.L.; Lai, K.W. Knee cartilage segmentation and thickness computation from ultrasound images. Med. Biol. Eng. Comput. 2018, 56, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Deveci, M.; Oztürk, S.; Sengezer, M.; Pabuşcu, Y. Measurement of orbital volume by a 3-dimensional software program: An experimental study. J. Oral Maxillofac. Surg. 2000, 58, 645–648. [Google Scholar] [CrossRef]
- Schönegg, D.; Wagner, M.; Schumann, P.; Essig, H.; Seifert, B.; Rücker, M.; Gander, T. Correlation between increased orbital volume and enophthalmos and diplopia in patients with fractures of the orbital floor or the medial orbital wall. J. Cranio-Maxillofac. Surg. 2018, 46, 1544–1549. [Google Scholar] [CrossRef]


| Category | MV (Range) | Total (n = 177) |
|---|---|---|
| Gender: | ||
| Female | - | 60 (33.9%) |
| Male | - | 117 (66.1%) |
| Age (years) | 49.10 (14–87) | - |
| Cause of fracture: | ||
| Rough offense | - | 35 (19.8%) |
| Fall | - | 66 (37.3%) |
| Sports accident | - | 14 (7.9%) |
| Traffic accident | - | 36 (20.3%) |
| Horse kick | - | 5 (2.8) |
| Other | - | 21 (11.9%) |
| Surgery after fracture (days) | 5.68 (0–22) | - |
| Surgery duration (minutes) | 109.84 (23–550) | - |
| Supply type: | ||
| PDS foil | - | 119 (67.2%) |
| Titanium mesh | - | 32 (18.1%) |
| PSI | - | 5 (2.8%) |
| Maxillary sinus balloon | - | 1 (0.6%) |
| Reposition only | - | 17 (9.6%) |
| Refusal of supply | - | 2 (1.1%) |
| Monocortical iliac crest | - | 1 (0.6%) |
| PDS foil thickness: (mm) | ||
| 0.15 | - | 86 (48.6%) |
| 0.25 | - | 91 (51.4%) |
| Inpatient stay (days) | 11.25 (3–61) | - |
| n = 177 | Measurement Method (mm2) | Difference (-Fold) | |
|---|---|---|---|
| 2D | 3D | ||
| Mean | 287.59 | 374.16 | 1.53 |
| Median | 267.12 | 351.90 | 1.35 |
| SD | 138.60 | 139.37 | 1.06 |
| Minimum | 10.00 | 90.46 | 0.63 |
| Maximum | 631.12 | 665.06 | 11.7 |
| p-value | <0.001 | - | |
| n = 177 | Processing Time (Minutes) | |
|---|---|---|
| 2D | 3D | |
| Mean | 11 | 20 |
| Minimum | 5.4 | 18.7 |
| Maximum | 13.2 | 26.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taxis, J.; Ungerboeck, L.; Gehrking, M.R.; Motel, C.; Wurm, M.; Eckert, A.W.; Spanier, G.; Nieberle, F.; Platz Batista da Silva, N.; Ludwig, N.; et al. Two-Dimensional Post-Traumatic Measurements of Orbital Floor Blowout Fractures Underestimate Defect Sizes Compared to Three-Dimensional Approaches. Tomography 2023, 9, 579-588. https://doi.org/10.3390/tomography9020047
Taxis J, Ungerboeck L, Gehrking MR, Motel C, Wurm M, Eckert AW, Spanier G, Nieberle F, Platz Batista da Silva N, Ludwig N, et al. Two-Dimensional Post-Traumatic Measurements of Orbital Floor Blowout Fractures Underestimate Defect Sizes Compared to Three-Dimensional Approaches. Tomography. 2023; 9(2):579-588. https://doi.org/10.3390/tomography9020047
Chicago/Turabian StyleTaxis, Juergen, Lena Ungerboeck, Mika R. Gehrking, Constantin Motel, Matthias Wurm, Alexander W. Eckert, Gerrit Spanier, Felix Nieberle, Natascha Platz Batista da Silva, Nils Ludwig, and et al. 2023. "Two-Dimensional Post-Traumatic Measurements of Orbital Floor Blowout Fractures Underestimate Defect Sizes Compared to Three-Dimensional Approaches" Tomography 9, no. 2: 579-588. https://doi.org/10.3390/tomography9020047





