Changes in Choroidal Circulation Hemodynamics Measured Using Laser Speckle Flowgraphy after a Cold Pressor Test in Young Healthy Participants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cold Pressor Test
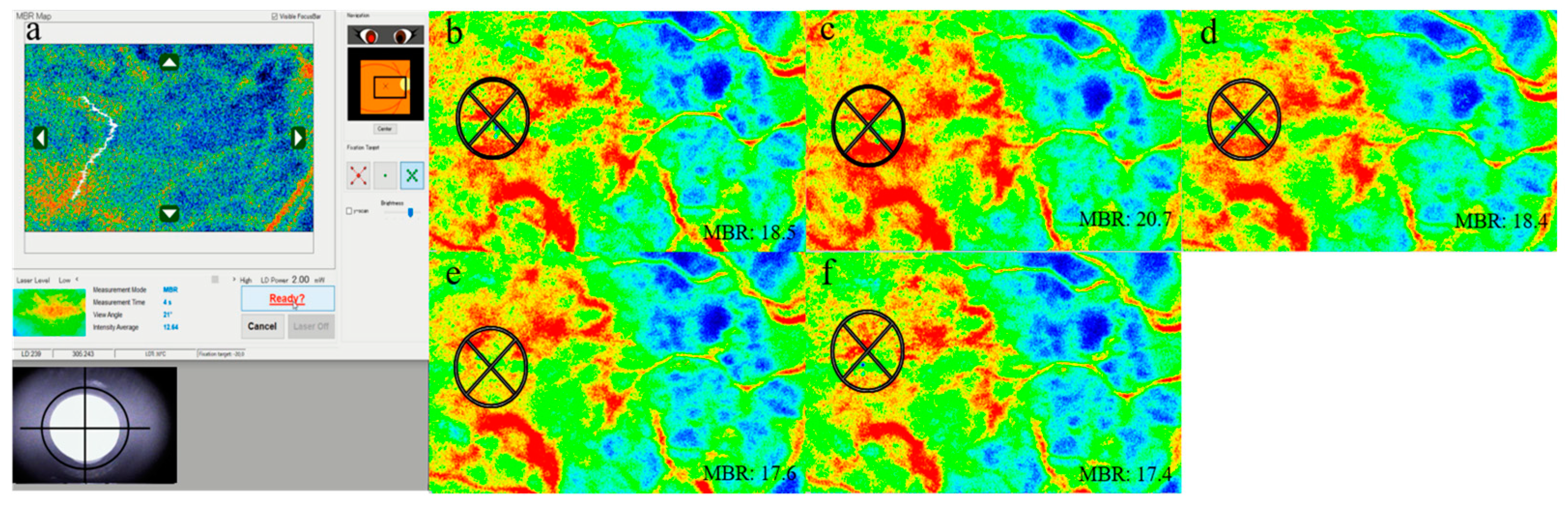
2.3. LSFG Measurement
2.4. Hemodynamics and IOP
2.5. Statistics
3. Results
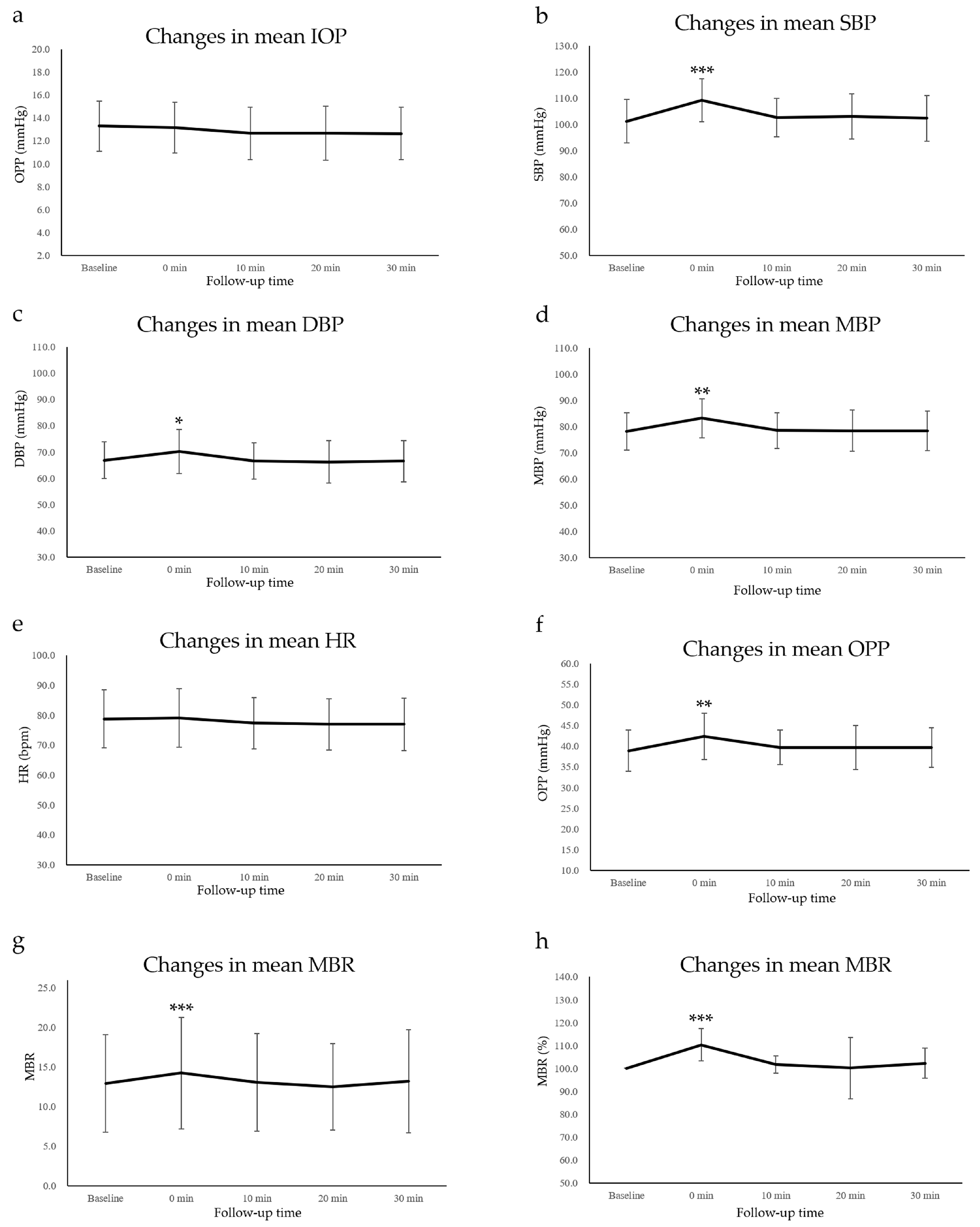
3.1. IOP and Systemic Factors
3.2. OPP Data
3.3. LSFG Data
3.4. Correlation between MBR and the Other Studied Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hines, E.A.; Brown, G.E. The cold pressor test for measuring the reactibility of the blood pressure: Data concerning 571 normal and hypertensive subjects. Am. Heart J. 1936, 11, 1–9. [Google Scholar] [CrossRef]
- Reiser, M.F.; Ferris, E.B., Jr. The nature of the cold pressor test and its significance in relation to neurogenic and humoral mechanisms in hypertension. J. Clin. Investig. 1948, 27, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korhonen, I. Blood pressure and heart rate responses in men exposed to arm and leg cold pressor tests and whole-body cold exposure. Int. J. Circumpolar Health 2006, 65, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.; Gómez, J.; Blanco, M.; Rodriguez, I. The cold pressor test: Pharmacological and therapeutic aspects. Am. J. Ther. 1997, 4, 34–38. [Google Scholar] [CrossRef]
- Budidha, K.; Kyriacou, P.A. Photoplethysmography for quantitative assessment of sympathetic nerve activity (SNA) during cold stress. Front. Physiol. 2018, 9, 1863. [Google Scholar] [CrossRef] [Green Version]
- Flügel-Koch, C.; May, C.A.; Lütjen-Drecoll, E. Presence of a contractile cell network in the human choroid. Ophthalmologica 1996, 210, 296–302. [Google Scholar] [CrossRef]
- Delaey, C.; Van De Voorde, J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000, 32, 249–256. [Google Scholar] [CrossRef]
- Jendzjowsky, N.G.; Steinback, C.D.; Herman, R.J.; Tsai, W.H.; Costello, F.E.; Wilson, R.J.A. Functional-optical coherence tomography: A non-invasive approach to assess the sympathetic nervous system and intrinsic vascular regulation. Front. Physiol. 2019, 10, 1146. [Google Scholar] [CrossRef]
- Okuno, T.; Sugiyama, T.; Kohyama, M.; Kojima, S.; Oku, H.; Ikeda, T. Ocular blood flow changes after dynamic exercise in humans. Eye 2006, 20, 796–800. [Google Scholar] [CrossRef] [Green Version]
- Kiel, J.W.; Shepherd, A.P. Autoregulation of choroidal blood blow in the rabbit. Investig. Ophthalmol. Vis. Sci. 1992, 8, 2399–2410. [Google Scholar]
- Polska, E.; Simader, C.; Weigert, G.; Doelemeyer, A.; Kolodjaschna, J.; Scharmann, O.; Schmetterer, L. Regulation of choroidal blood flow during combined changes in intraocular pressure and arterial blood pressure. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3768–3774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidl, D.; Garhofer, G.; Schmetterer, L. The complex interaction between ocular perfusion pressure and ocular blood flow—relevance for glaucoma. Exp. Eye Res. 2011, 93, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, R.; Hashimoto, Y.; Imabayashi, S.; Yoshitomi, T. Changes in choroidal thickness in healthy participants after induction of sympathetic hyperactivity using the cold pressor test. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 261, 585–587. [Google Scholar] [CrossRef]
- Aizawa, N.; Yokoyama, Y.; Chiba, N.; Omodaka, K.; Yasuda, M.; Otomo, T.; Nakamura, M.; Fuse, N.; Nakazawa, T. Reproducibility of retinal circulation measurements obtained using laser speckle flowgraphy-NAVI in patients with glaucoma. Clin. Ophthalmol. 2011, 5, 1171–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, M.; Saito, W.; Hashimoto, Y.; Yoshizawa, C.; Fujiya, A.; Noda, K.; Ishida, S. Macular choroidal blood flow velocity decreases with regression of acute central serous chorioretinopathy. Br. J. Ophthalmol. 2013, 97, 775–780. [Google Scholar] [CrossRef]
- Saito, M.; Saito, W.; Hirooka, K.; Hashimoto, Y.; Mori, S.; Noda, K.; Ishida, S. Pulse waveform changes in macular choroidal hemodynamics with regression of acute central serous chorioretinopathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6515–6522. [Google Scholar] [CrossRef] [Green Version]
- Saito, M.; Noda, K.; Saito, W.; Hirooka, K.; Hashimoto, Y.; Ishida, S. Increased choroidal blood flow and choroidal thickness in patients with hypertensive chorioretinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 233–240. [Google Scholar] [CrossRef]
- Iwase, T.; Ueno, Y.; Tomita, R.; Terasaki, H. Relationship between retinal microcirculation and renal function in patients with diabetes and chronic kidney disease by laser speckle flowgraphy. Life 2023, 13, 424. [Google Scholar] [CrossRef]
- Yanagida, K.; Iwase, T.; Yamamoto, K.; Ra, E.; Kaneko, H.; Murotani, K.; Matsui, S.; Terasaki, H. Sex-related differences in ocular blood flow of healthy subjects using laser speckle flowgraphy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4880–4890. [Google Scholar] [CrossRef] [Green Version]
- Riva, C.E.; Titze, P.; Hero, M.; Movaffaghy, A.; Petrig, B.L. Choroidal blood flow during isometric exercises. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2338–2343. [Google Scholar]
- Nagahara, M.; Tamaki, Y.; Tomidokoro, A.; Araie, M. In vivo measurement of blood velocity in human major retinal vessels using the laser speckle method. Investig. Ophthalmol. Vis. Sci. 2011, 52, 87–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaki, Y.; Araie, M.; Kawamoto, E.; Eguchi, S.; Fujii, H. Noncontact, two-dimensional measurement of retinal microcirculation using laser speckle phenomenon. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3825–3834. [Google Scholar]
- Haneda, M.; Hashimoto, Y.; Mishima, A.; Saito, D.; Yoshitomi, T. Changes in choroidal circulation hemodynamics during the menstrual cycle in young, healthy women. PLoS ONE 2022, 17, e0270501. [Google Scholar] [CrossRef] [PubMed]
| Post | Friedman Test (p Value) | Scheffe’s Paired Comparison (p Value) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 0 min | 10 min | 20 min | 30 min | 0 min | 10 min | 20 min | 30 min | ||
| IOP (mmHg) | 13.3 ± 2.2 | 13.2 ± 2.2 | 12.7 ± 2.3 | 12.7 ± 2.4 | 12.6 ± 2.3 | 0.232 | 0.991 | 0.783 | 0.613 | 0.374 |
| SBP (mmHg) | 101.2 ± 8.3 | 109.3 ± 8.2 *** | 102.6 ± 7.3 | 103.2 ± 8.6 | 102.4 ± 8.6 | <0.001 | <0.001 | 0.443 | 0.533 | 0.623 |
| DBP (mmHg) | 66.8 ± 7.0 | 70.2 ± 8.4 * | 66.6 ± 7.0 | 66.2 ± 8.0 | 66.5 ± 7.9 | <0.001 | 0.025 | 0.982 | 0.982 | 0.996 |
| MBP (mmHg) | 78.3 ± 7.1 | 83.2 ± 7.5 ** | 78.6 ± 6.8 | 78.5 ± 7.9 | 78.4 ± 7.6 | <0.001 | 0.005 | 0.983 | 0.994 | 0.998 |
| HR (bpm) | 78.8 ± 9.6 | 79.1 ± 9.8 | 77.3 ± 8.5 | 77.0 ± 8.6 | 76.9 ± 8.7 | 0.565 | 0.999 | 0.970 | 0.815 | 0.951 |
| OPP (mmHg) | 38.9 ± 5.0 | 42.3 ± 5.6 ** | 39.7 ± 4.2 | 39.7 ± 5.4 | 39.7 ± 4.8 | 0.001 | 0.004 | 0.776 | 0.901 | 0.931 |
| MBR | 12.9 ± 6.2 | 14.2 ± 7.0 *** | 13.1 ± 6.2 | 12.5 ± 5.5 | 13.2 ± 6.5 | <0.001 | <0.001 | 0.894 | 0.969 | 0.987 |
| MBR (%) | 100.0 ± 0.0 | 110.3 ± 7.1 *** | 101.6 ± 3.8 | 100.2 ± 13.4 | 102.3 ± 6.6 | <0.001 | <0.001 | 0.894 | 0.969 | 0.987 |
| Macular MBR | ||
|---|---|---|
| Coefficient | p Value | |
| IOP | −0.196 | 0.421 |
| SBP | 0.732 | <0.001 |
| DBP | 0.196 | 0.420 |
| MBP | 0.545 | 0.015 |
| HR | −0.009 | 0.704 |
| OPP | 0.495 | 0.030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imabayashi, S.; Hashimoto, Y.; Ishimaru, Y.; Umemoto, R.; Chiyozono, M.; Yamanokuchi, T.; Yoshitomi, T. Changes in Choroidal Circulation Hemodynamics Measured Using Laser Speckle Flowgraphy after a Cold Pressor Test in Young Healthy Participants. Tomography 2023, 9, 790-797. https://doi.org/10.3390/tomography9020064
Imabayashi S, Hashimoto Y, Ishimaru Y, Umemoto R, Chiyozono M, Yamanokuchi T, Yoshitomi T. Changes in Choroidal Circulation Hemodynamics Measured Using Laser Speckle Flowgraphy after a Cold Pressor Test in Young Healthy Participants. Tomography. 2023; 9(2):790-797. https://doi.org/10.3390/tomography9020064
Chicago/Turabian StyleImabayashi, Sakurako, Yuki Hashimoto, Yumi Ishimaru, Rino Umemoto, Miho Chiyozono, Toshitaka Yamanokuchi, and Takeshi Yoshitomi. 2023. "Changes in Choroidal Circulation Hemodynamics Measured Using Laser Speckle Flowgraphy after a Cold Pressor Test in Young Healthy Participants" Tomography 9, no. 2: 790-797. https://doi.org/10.3390/tomography9020064
APA StyleImabayashi, S., Hashimoto, Y., Ishimaru, Y., Umemoto, R., Chiyozono, M., Yamanokuchi, T., & Yoshitomi, T. (2023). Changes in Choroidal Circulation Hemodynamics Measured Using Laser Speckle Flowgraphy after a Cold Pressor Test in Young Healthy Participants. Tomography, 9(2), 790-797. https://doi.org/10.3390/tomography9020064





